Abstract
In transformation of Streptococcus pneumoniae DNA enters the cell as single-strand fragments and integrates into the chromosome by homologous recombination. Deletions and insertions of a few hundred base pairs frequently stop the recombination process of a donor strand. In this work we took advantage of such interruptions of recombination to compare the transformation efficiencies of the segments 5′- and 3′-ward from a deletion. The deletion was created in the center of a fragment of the ami locus, and sites around the deletion were labeled by a frameshift generating a restriction site. Heteroduplexes were constructed containing two restriction sites on one strand and two different ones on the complementary strand. ami+ bacteria were transformed with such heteroduplexes. ami− transformants were isolated and individually underwent amplification of the transformed ami region. We have obtained two kinds of amplification products: short when the deletion was integrated, long when recombination stops at the deletion. Each long fragment was tested by the four restriction enzymes to detect which strand and which side of the deletion had recombined. We found that 80% of the cuts were located 5′ to the deletion, showing that, in vivo, the 5′ side is strongly favored by recombination. Further results suggest that exchanges occurring from 5′ to 3′ relative to the donor strand are more efficient than in the opposite direction, thus accounting for the 5′ preference.
The discovery in the 1940s that in Streptococcus pneumoniae, a pathogenic and transformable bacterium, genes are made of DNA (1) opened the modern field of molecular genetics. In pneumococcal transformation, double-strand DNA binds to the membrane, where it becomes nicked, then breaks (see ref. 2 for a review). Soon it was suggested that donor DNA is converted to single-stranded segments entering linearly into the bacterium (3, 4). Additional studies have shown that while one strand enters the cell from a 3′ end, the complementary strand is degraded to oligonucleotides with the opposite polarity (5, 6). The weight average size of entering fragments was estimated to be about 6,000 bases (7, 8). DNA is recovered immediately after the entry as an eclipse complex, without transforming activity and coated by a single-strand-binding protein, which accumulates during competence (9). It has been estimated that half of the incoming DNA integrates into the chromosome by homologous recombination and that up to 10% of the pneumococcal genome may be exchanged by recombination (10). Still in S. pneumoniae, it was later found that donor mutations are removed at the heteroduplex stage by a mismatch-repair system (11, 12). It was recognized that this system, called Hex, also corrects mismatches resulting from DNA replication errors (13). The Hex system depends on two genes, hexA and hexB (14), and repairs mainly single-base mismatches (see ref. 15 for a review). A mismatch-repair system was later reported in other bacteria, in yeast, and in humans. In humans it plays a major role in preventing certain types of cancers (16). Although transformation of pneumococcus was the first process of genetic exchange described among bacteria (17), the mechanism of recombination between the donor and the recipient DNA is less documented than the entry of DNA or the mismatch repair system. A few genes involved in recombination have now been characterized in pneumococcus. The first gene described was recP, encoding a transketolase whose function in the recombination process remains unknown (18, 19). The gene encoding for the RecA protein has been characterized (20). It belongs to a competence-inducible operon, and recombination is abolished in recA-null mutants. Another gene, mmsA, which is homologous to the DNA–RNA helicase encoded by recG of Escherichia coli, was reported to be involved in recombination and viability of pneumococcus (21). To investigate the process of heteroduplex formation between the chromosome and a donor DNA, we have used an approach based on the disruption of this process by long heterologous sequences. We previously found that deletions and insertions of a few hundred base pairs are excluded from the heteroduplex structure, suggesting that in vivo the donor/recipient strand exchange is frequently interrupted at regions of high sequence divergence (22). These exclusion events explain both the low efficiency of transformation and the excess of wild-type recombinants observed when long heterologous sequences are involved in two-point crosses. The aim of this work was to analyze at the molecular level the recombination of a donor strand carrying a long deletion. In particular, the strategy was designed to compare the transformation efficiencies for the DNA regions located 3′ and 5′ to the deletion. Our results confirm that strand exchange often stops at the deletion, and they indicate that the 5′ region is most frequently integrated. This study suggests that strand exchange does not initiate at a free end and then extends mostly from 5′ to 3′ relative to the donor strand polarities.
MATERIALS AND METHODS
Pneumococcal Strains and Transformation Procedures.
All recipient strains derive from R6, a nonencapsulated strain derived from Avery’s R36A strain (1). R800 is our wild-type strain, mismatch-repair proficient, hex+ (23). A hexA− strain and a hexB− isogenic strain were constructed for this study by transforming R800 with plasmids carrying a deletion insertion in either the hexA or the hexB gene coupled with resistance to erythromycin. Strain R317 is hexA− and recA+(Ind−), genotype indicating that the competence-specific induction of recA is abolished (24). Plasmids used were pSP11hexAΔ and pSP41hexBΔ, which derive from pSP11 and pSP41 (25, 26). Erythromycin-resistant clones were transformed with DNA carrying the low-efficiency marker ami6 and the high-efficiency one str41. The number of transformants was equal for the two markers, confirming the hex-null phenotype of the erythromycin-resistant clones. Competence was induced by adding synthetic competence-stimulating peptide (25 ng/ml) to precompetent cells (20). After 10-min induction at 37°C, DNA was added. Cells were incubated for 20 min at 30°C to allow DNA uptake and for 90 min at 37°C for the phenotypic expression and then plated on agar medium for the selection of transformant colonies. Antibiotic concentrations were the following: 5 μM methotrexate; 2 μg/ml erythromycin; 200 μg/ml streptomycin.
DNA Manipulations and Heteroduplex Constructions.
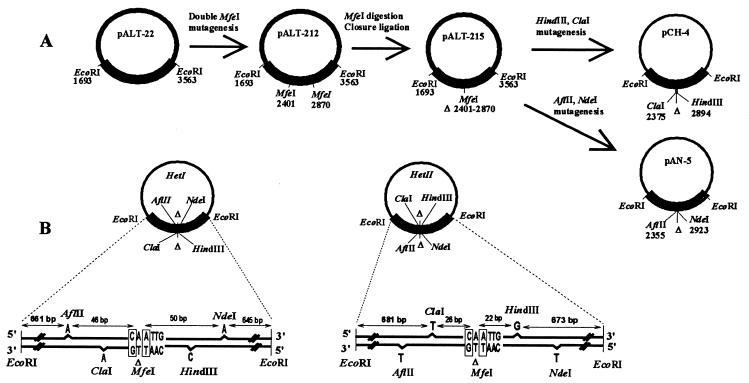
A 1870-bp EcoRI fragment of the ami locus was previously cloned in M13 mp11 (22). We subcloned it into the pALTER-1 vector for in vitro mutagenesis (Promega), generating the pALT-22 recombinant plasmid (Fig. 1A). On the basis of the wild-type sequence of the ami operon (27), we have chosen six mutagenic oligonucleotides for introducing restriction sites as indicated (Fig. 1A). Heteroduplexes I and II (Fig. 1B) were prepared and purified in M13 mp11 as described (28).
Figure 1.
Mutagenesis and heteroduplex structure. (A) In a first round of oligonucleotide mutagenesis, two MfeI sites were created at positions 2401 and 2870 (position numbers of the cut sites) on the ami insert of pALT-22, leading to pALT-212. The MfeI site at 2401 was generated by an A-to-C substitution at position 2401. The MfeI site at 2870 was generated by a T-to-A substitution at position 2872. pALT-212 was then digested with MfeI and ligated in diluted conditions to close the vector. The new plasmid, pALT-215, contains a deletion of 469 bp in the center of the ami fragment. By oligonucleotide mutagenesis, ClaI and HindIII sites were created at positions 2375 and 2894 (position numbers of the undeleted original are retained). Both are single nucleotide additions, G and T, respectively. Another round of mutagenesis was done to generate an AflII site at position 2355 and an NdeI site at position 2923, both being the addition of one A. To produce large amounts of single-stranded (SS) DNA, we subcloned the mutagenized EcoRI inserts from the pALT vectors to M13 mp11. We obtained two plasmids: pCH4, which contains ClaI and HindIII sites, and pAN5, which contains AflII and NdeI sites. Each DNA was checked at each step by restriction analysis; SS pAN5 and SS pCH4 were sequenced to check sites, insert orientation, and the absence of additional mutation on the insert. (B) Replicative form (RF) of pCH4 was hybridized with SS pAN5 to generate the heteroduplex I (HetI); RF pAN5 was hybridized with SS pCH4 to generate HetII. Sequence structures of the EcoRI inserts in HetI and HetII are represented. Each added base is indicated below the site it generates. The MfeI sequence created by ligating the MfeI ends to introduce the 469-bp deletion (Δ) is indicated. Within this sequence, boxed bases are the mutagenized positions 2401 and 2872. Distances from the restriction sites to the EcoRI ends and to the boxed bases are indicated.
Colony PCR and Digestions.
The ami region amplified by PCR is located between positions 1726 (primer A) and 3229 (primer B). Primer sequences are as follows. A, 3′-GGGTTAGGAGTCTACTTCG; and B, 3′-CGGTACCGAGCAAAGTTCT. Bacteria from 2-day-old methotrexate-resistant (Mtxr) colonies were pipetted and introduced in 50 μl of the following mix: 50 mM Tris⋅HCl (pH 9), 20 mM (NH4)2SO4, 2.5 mM MgSO4, 25 pmol of primer A, 25 pmol of primer B, 0.1 mM each of the four dNTPs. One unit of Hot Tub DNA polymerase (Amersham) was added tube by tube. Conditions of DNA amplification were the following: 94°C, 3 min; 94°C, 30 sec; 50°C, 3 min; 72°C, 2 min and 30 sec for 30 cycles; then 72°C, 10 min. Three microliters of each PCR product was introduced into the cup of a microtitration plate and incubated 4 hr at 37°C with 1 unit of enzyme AflII, NdeI, HindIII, ClaI, or MfeI. Digestion products were analyzed on a 1% agarose gel.
RESULTS
Strategy for Molecular Analysis of Recombination Polarity.
To determine a possible polarity of strand exchange, our strategy was based on the observation that a deletion of a few hundred base pairs frequently stops the integration of a donor strand into the chromosome (22). The question addressed was, Which region flanking the deletion, 5′ or 3′, integrates into the chromosome before the exchange is blocked?
A deletion of 469 bp was generated in the center of a cloned EcoRI fragment of the ami locus in which mutations confer resistance to methotrexate (29) (Fig. 1A). Regions on both sides of the deletion were marked by the addition of one base pair to generate a restriction site. These side-marking frameshifts (SMFs) enable the selection of Mtxr transformants and further screening by restriction site analysis. SMFs were introduced close to the deletion to ensure strong linkage with it and to keep most of the homology 3′ and 5′ of the SMFs congruent with the homology 3′ and 5′ of the deletion. AflII and NdeI sites were created at ≈50 bp on the left and on the right of the deletion, respectively, leading to the pAN5 vector (Fig. 1A). HindIII and ClaI sites were created at ≈25 bp from the deletion, leading to the pCH4 vector (Fig. 1A). The two distances, 50 and 25 bp, were envisaged for studying a possible effect of the proximity of the deletion on the recombination of neighbor sites. Identifying 5′ and 3′ regions of a single strand requires discrimination of the complementary strands. This can be fulfilled by the use of donor heteroduplex DNAs (30, 31). Heteroduplex I (HetI) was constructed by hybridizing single-stranded pCH4 with linearized double-stranded pAN5 (Fig. 1B). The reciprocal hybridization generated HetII. One heteroduplex therefore carries four SMFs, each one reflecting one side of the deletion for a given strand (Fig. 1B). The assumption was as follows. Heteroduplexes should transform ami+ strains to the Mtxr phenotype if strand exchange integrates the deletion and also if it stops, excluding the deletion but integrating one SMF. The two classes of events should be distinguished by a PCR analysis: a deleted locus will lead to a short PCR product, an undeleted locus to a wild-type-sized PCR product. Digesting the second class of products with the four restriction enzymes separately should reveal what SMF has transformed, thus indicating what strand segment, 5′ or 3′ to the deletion, was integrated.
Exclusion of Deletions and Preponderance of the 5′ Side.
Transformations were carried out with plasmid heteroduplexes that are closed, circular, and 8,600 bp long. On the basis of a previous study (32), the average number of cuts per molecule should be about 1.3. As the vector part is 7,200 bp and the insert 1,400 bp, entering strands should be mostly full-length molecules, broken within the vector. This ensures maximal length of homology, identical 3′ and 5′ of the deletion, which is important in comparing recombination on both sides. Recipient strains were ami+ and were hex− to avoid repair of donor SMFs, which could interfere with the recombination process. Mtxr transformants were selected, and the region located between bases 1726 and 3229 was amplified. Of 448 Mtxr transformants, 147 led to a short band and 301 to a long band (Table 1). Short bands were ≈1,000 bp long, and should correspond to completed exchanges integrating the deletion into the chromosome. Indeed, each digested short band carried a pair of SMFs of the same strand, either AflII and NdeI or HindIII and ClaI. On average, the transformation efficiency of the cloned deletion was 0.33, and no difference between HetI and HetII was observed (Table 1).
Table 1.
Analysis of the transformants obtained with circular heteroduplexes, in hex− recipient strains
| DNA | Transformants-PCR products
|
Cut patterns of the long bands
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal cuts
|
Proximal cuts
|
|||||||||
| Total | Short | Long | Δ TE* | AflII | NdeI | 5′, % | HindIII | ClaI | 5′, % | |
| HetI | 231 | 73 | 158 | 0.32 | 70 (5′) | 21 (3′) | 77 | 58 (5′) | 9 (3′) | 87 |
| HetII | 217 | 74 | 143 | 0.34 | 20 (3′) | 63 (5′) | 76 | 11 (3′) | 49 (5′) | 82 |
Transformation efficiency of the deletion—i.e., the ratio short bands/total.
Long bands were 1,500 bp long, which is the wild-type size of the amplified region. They must correspond to integrations of just one region flanking the deletion, including the SMF to account for the Mtxr phenotype. This was confirmed by digesting each long band with the four restriction enzymes (Fig. 2). Interestingly, cuts corresponded mainly to sites located 5′ to the deletion (Fig. 2 and Table 1). This unbalanced distribution of the 5′ and the 3′ cuts could not be attributed to site bias, because exchanging HetI with HetII reversed the sites preference, not the 5′ preponderance (Table 1). Proximal cuts (HindIII and ClaI) were recovered less than distal ones (AflII and NdeI): 127 and 174 cuts, respectively. The deletion might partially prevent integration of DNA in its close vicinity, as previously postulated (22). In addition, the 5′/3′ ratio of cuts was greater for proximal than for distal sites: 5.35/1 and 3.25/1, respectively. Adding all the results, we found 240 cuts 5′ and 61 cuts 3′, which means that on average, 80% of the exchanges blocked at the deletion have integrated the segment localized 5′ to the deletion. One part of the transformations was carried out in a hexA− strain and the other part in a hexB− strain, otherwise isogenic (data not shown). No difference concerning the deletion efficiency or the 5′/3′ polarity was found between the two backgrounds, ruling out a possible effect of HexA, the mismatch-binding protein, on the heteroduplex stability.
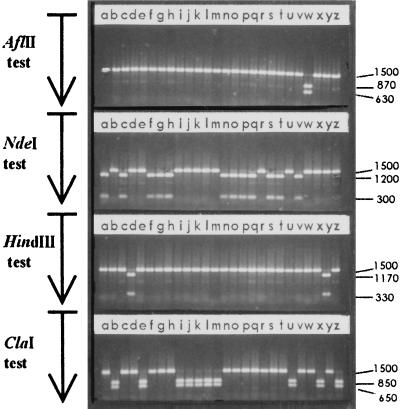
Figure 2.
Example of analysis of the long bands. Twenty-six long bands of 1,500 bp corresponding to 26 independent Mtxr colonies obtained by transformation of a hexA− strain with cloned HetII were digested with AflII, NdeI, HindIII, and ClaI separately, and then the products migrated simultaneously on the same agarose gel. On hetII, AflII and HindIII are located 3′ to the deletion, whereas ClaI and NdeI are located 5′ to the deletion. Uncut band and digestion-product lengths are indicated in bp. The sample introduced in lanes r, resistant to the four enzymes, is likely to correspond to a spontaneous Mtxr mutant. Other bands are sensitive to just one enzyme—e.g., lanes w indicate a band sensitive only to AflII.
Free Ends Limit the 5′ Preponderance.
From cloned fragments, entering segments are mostly flanked by vector sequences. To study recombination without any influence of such terminal heterologous sequences, plasmids were digested with EcoRI prior to transformation. Of 303 Mtxr transformants colonies, 38 led to short amplification products. This transformation efficiency of the deletion, 0.13, is significantly lower than with the cloned DNA (0.33). The 303 transformants actually correspond to 163 transformations with purified heteroduplex inserts, and to 140 transformations with a mix of heteroduplex insert and vector. The deletion efficiency was 0.07 for the former class and 0.19 for the latter, suggesting that the deletion transforms more efficiently when heterologous DNA is present.
Again, digestion of the long bands revealed that 5′ was the major side recovered (Table 2). Adding all the results, we found 149 cuts on 5′ and 71 on 3′—i.e., 67.7% of 5′. However, whereas the 5′/3′ cuts ratio was about 4/1 with circular cloned heteroduplexes, it was close to 2/1 with vector-free heteroduplexes. The mechanism of DNA entry during transformation renders this observation surprising. Linear fragments are randomly broken and shortened upon entry, but because of the 3′ → 5′ polarity of strand penetration, this shortening is located at the 3′ end of entering strands. Less homology in this region should affect specifically homologous recombination of segment 3′-ward from the deletion, increasing the preponderance of 5′ regions. The observed decrease of 5′ preponderance might be a consequence of free homologous ends, modifying recombination parameters.
Table 2.
Cuts of the long bands obtained with vector-free heteroduplexes
| DNA | Long bands | Distal cuts
|
Proximal cuts
|
||||
|---|---|---|---|---|---|---|---|
| AflII | NdeI | 5′, % | HindIII | ClaI | 5′, % | ||
| HetI | 107 | 47 (5′) | 30 (3′) | 61 | 23 (5′) | 7 (3′) | 77 |
| HetII | 113 | 23 (3′) | 35 (5′) | 60 | 11 (3′) | 44 (5′) | 80 |
Bypass of the MfeI Mismatch.
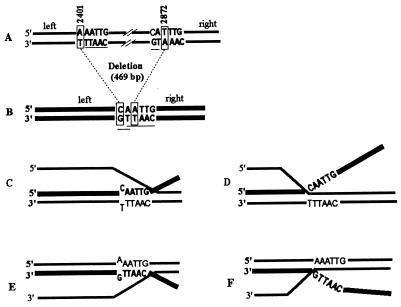
The deletion was generated by cutting and splicing two MfeI sites created by an A-to-C substitution at position 2401 and a T-to-A substitution at 2872. Strand exchanges occurring on the left of the deletion should be blocked at 2401, and those occurring on the right should be blocked at 2872. However, within the spliced MfeI site, five bases are homologous beyond the changed base at 2401 and two bases are homologous beyond the changed base at 2872 (Fig. 3 A and B). We took advantage of this situation to examine precisely where the exchange is interrupted: at the change, excluding the MfeI site (Fig. 3 D and F), or beyond the change, integrating it (Fig. 3 C and E). In the former case long bands will be resistant to MfeI, whereas in the latter they will be cut by MfeI. Of 47 long bands, corresponding to exchanges blocked on the right of the deletion, none was sensitive to MfeI. We can assume that 2 bp is a very short homology which renders the substitution at 2872 the actual point of sequence divergence where right-side exchanges are blocked. Of 111 long bands corresponding to left-side exchanges, 22 were sensitive to Mfe1. This result indicates that 5 bp is a sufficient homology for left-side exchanges to bypass the single mismatch at 2401. Among the 111 long bands, 80 were characterized as 5′ recombinants, and 31 as 3′ recombinants. Interestingly, the 22 MfeI-sensitive bands were all 5′ recombinants, as if exchanges involving the 3′ half were unable to bypass the MfeI mismatch at 2401. We must point out that the mismatch created at position 2401 is A/G when the strand 3′ to the deletion recombines, but C/T when the complementary 5′ segment recombines (Fig. 3 C and E). An easier incorporation of C/T than A/G in the heteroduplex structure might explain the bias observed in the MfeI bypass independent of strand polarities. This hypothesis is nevertheless unlikely. C/T is reported to be more destabilizing for the helical structure than A/G (33, 34) and should be harder to bypass. In addition, indirect effects of Hex on the bypass of A/G and C/T are excluded because experiments were carried out in a hexA− background. Another mechanism of correction, A/G-specific, was found in pneumococcus (31, 35). However, it requires a specific sequence environment and, by changing A/G mismatches to C⋅G pairs would favor the fixation of MfeI from A/G, not from C/T mismatches as observed.
Figure 3.
Sequence context and heteroduplex structure at the deletion. (A) Wild-type sequence. Lettering correspond to near-MfeI sites. Boxed base pairs are the two positions further mutagenized to generate MfeI sites. (B) Sequence of HetI or HetII at the deletion. Lettering indicates the MfeI spliced site. Boxed base pairs are the mutagenized positions. The 5 homologous bases beyond the change at 2401 and the 2 homologous bases beyond the change at 2872 are underlined. (C–F) Heteroduplex structures that may occur when a donor strand from HetI or HetII integrates into a wild-type chromosome and is blocked at the deletion. Only exchanges on the left side are represented. They are characterized as long bands carrying either AflII or ClaI sites (5′ and 3′, respectively, with HetI as donor; 3′ and 5′, respectively, with HetII as donor). Thick line, donor deleted strand; thin line, wild-type recipient. (C and E) Donor strand pairs until the 5 homologous bases beyond the MfeI substitution. (D and F) Donor strand is excluded at the MfeI change in spite of the 5 homologous bases.
The Action of Hex Partially Masks the Polarity of Recombination in the Experimental System.
SMFs are 1-bp additions, which should undergo excision by the Hex mismatch repair system (36). To test a possible effect of Hex on the polarity of recombination, the hex+ strain R800 was transformed with plasmid heteroduplexes I and II. Two major differences were found relative to a hex− background (Table 3). First, HindIII was mainly recovered, whether located 3′ or 5′ to the deletion, and second, the proportion of short bands obtained with HetI was very low. The first observation suggested that donor/recipient heteroduplexes generated by the HindIII sequence could escape the action of Hex, while those generated by AflII, NdeI, and ClaI could not. Supporting this idea was the fact that HindIII generates a +A or a +T, depending on the donor strand, whereas AflII, NdeI, and ClaI lead to a +G or a +C (Fig. 1B). To test such a marker effect, we have transformed R800 with long bands previously characterized. Such DNAs are homoduplex, without deletion, and just carry one of the four frameshifts. Bands containing HindIII sites yielded on average 7 to 8 times as many transformants as those carrying AflII, NdeI, or ClaI sites. These results suggested that single additions are not repaired identically by Hex. In spite of this marker effect favoring HindIII, a polarized mechanism was still evident, because the preponderance of HindIII sites was maximal with HetII—i.e., when located 5′ to the deletion (Table 3). Combining the advantage conferred to 5′ segments with the fact that HindIII specifically escapes the action of Hex should account for the transformation efficiencies of the deletion (Δ TE), observed with HetI and HetII (Table 3). The efficient integration of a deletion into the chromosome should require that both the 5′ and the 3′ flanking regions recombine efficiently. Hex-sensitive markers reduce the integration of segments carrying them by inducing their excision. If such markers are located 3′ to the deletion, integrations of 3′ segments should be very rare, and consequently the Δ TE very low. Such a behavior was observed with HetI, which carries non-HindIII sites on 3′ segments and HindIII on a 5′ one. If Hex-sensitive markers are located 5′ to the deletion, excision of the 5′ segments should be partly compensated by their 5′ location and the Δ TE should be reasonably high. This result was observed with HetII, which carries non-HindIII sites on 5′ segments and HindIII on a 3′ one.
Table 3.
Analysis of the transformants obtained with circular heteroduplexes in a hex+ background
| DNA | Transformants-PCR products
|
Cut patterns of the long bands
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Distal cuts
|
Proximal cuts
|
|||||||||
| Total | Short | Long | Δ TE | AflII | NdeI | 5′, % | HindIII | ClaI | 5′, % | |
| HetI | 30 | 1 | 29 | 0.03 | 2 (5′) | 0 (3′) | 100 | 27 (5′) | 0 (3′) | 100 |
| HetII | 39 | 14 | 25 | 0.36 | 1 (3′) | 2 (5′) | 67 | 17 (3′) | 5 (5′) | 23 |
DISCUSSION
The tools developed in this study allow a molecular approach to understand the recombination pathway occurring in transformation. The results confirm that a donor fragment carrying a long deletion recombines mostly on one side of the deletion, which means that the exchange is interrupted at the deletion. However, the 469-bp deletion is recovered among transformants with a reasonably high efficiency. On a cloned fragment, the deletion has an efficiency of 0.33, whereas on a free and purified fragment it has an efficiency of 0.07. Because in the latter situation the fragment undergoes degradation and, of course, shortening by entry breaks, we suggest that the less homology that flanks a deletion, the more frequent is the interruption of exchange. Strengthening this idea are two observations. First, mixing the linearized fragment with vector DNA increases the deletion efficiency: heterologous DNA, although unconnected to the insert, could compete for nucleases, leading to longer insert sequences. Second, the efficiency of a deletion similar in size but carried on chromosomal DNA is 0.35 (22), which is greater than 0.07. As donor chromosomal DNAs lead to entering segments of about 6,000 bp—i.e., longer than what is expected with a donor fragment of 1,400 bp, we can speculate that the longer the fragment, the higher the efficiency of the deletion it carries. Interestingly, efficiencies of the deletion on the chromosome and on the cloned fragment—i.e., bound and protected by the vector—are quite similar: 0.35 and 0.33, respectively. This result suggests that only ≈700 bp of homologous DNA on both sides enables a long deletion to transfer with a maximal efficiency.
Besides confirming that deletion blocks the recombinational exchange, the tools developed here allow one to characterize what segment flanking the deletion, 5′or 3′, is integrated before the block. The analysis has shown that the two regions integrate unequally: markers located 5′ to the deletion are mostly recovered. Such an imbalance between the 5′ and the 3′ segments strongly suggests that the mechanism of strand exchange is polarized from 5′ to 3′. Bias due to opposite strand efficiencies are excluded, since the markers surrounding the deletion were in the center of the same segment, just 50 or 100 bp apart. In addition, the analysis was carried out in a hex− background, excluding any effect of the mismatch-repair system on the transformation efficiencies of the markers 5′ and 3′ to the deletion. Variations in the transformation efficiencies of markers were soon reported in pneumococcus, leading to the concept of the mismatch-repair system (11). Opposite strands of the same markers were also reported to have very different transformation efficiencies (4). However, such marker effects could not be attributable to a polarized mechanism of recombination. Experiments were carried out in hex+ backgrounds, and there was no correspondence between markers and polar ends of DNA. In the present work high and low efficiencies of transformation are just correlated with the 5′ and 3′ localizations of markers.
This polar effect seems characteristic of the in vivo recombination process. It is observed with cloned or free DNA, with sites located at various distances from the deletion, in hexA− or hexB− backgrounds. In a hex+ background we first detected a HindIII effect rather than a polar effect. This result suggests that a single base addition may sometimes escape the action of Hex, contrary to what was proposed previously (36). The localized environment, like a HindIII palindromic sequence, might influence the structure of heteroduplexes corresponding to single base additions and their further recognition by Hex. However, in spite of this mismatch repair effect, the 5′ preponderance was still evident in the hex+ background. Such a 5′ advantage might reflect invasion of the chromosome from a 5′ end, and/or a greater proficiency of strand exchanges occurring from 5′ to 3′, relative to the donor polarities. The first hypothesis is unlikely, because the 5′ preference is observed with cloned DNA as donor. Plasmids being broken mainly within the vector, the entering segments should be ended by vector DNA. This situation renders unlikely any advantage to a free homologous end. Rather, the 5′ preference might reflect a more efficient extension of strand exchange from 5′ to 3′. The transformants blocked at the deletion but carrying the terminal MfeI site strengthen this hypothesis: they all correspond to integrations of a segment 5′ to the deletion. Exchanges extending from 5′ to 3′ might include the loose MfeI mismatch because of a high proficiency. Those extending from 3′ to 5′ might easily block at the MfeI mismatch that is flanked by just 5 bp of homology. Relative to the polarity of DNA entry, which is 3′ to 5′, our result is surprising. First in the cell, 3′ ends should be coated earlier with single-stranded DNA-binding protein (SSB) and RecA, and should invade the chromosome more quickly, progressing 5′-ward. This model, however, assumes that 3′ free ends are homologous, which is not the case with circular heteroduplexes as DNA donor. Interestingly, the use of vector-free heteroduplexes as donor led to an increased proportion of 3′ cuts. This observation might indeed support a greater invasiveness of free homologous 3′ ends. However, 5′ cuts are still preponderant whether donor DNA is vector-ended or free. We propose that heteroduplexes initiate mostly from paranemic junctions—i.e., not from a DNA end—then extend from 5′ to 3′ relative to the donor polarities (Fig. 4).
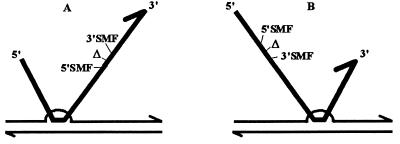
Figure 4.
Model accounting for the preponderance of 5′ site recombinants. (A) The initial junction involves the segment 5′ to the deletion. (B) The initial junction involves the segment 3′ to the deletion. Lengths of homology lying 5′ and 3′ to the deletion being identical, A and B junctions should be equally frequent. Extension of the joint heteroduplex from 5′ to 3′, with respect to the donor strand, is detrimental to incorporation of 3′ SMFs which are beyond the deletion in A and 5′ to the junction in B. Although rare, because of the small distances, junctions occurring within the segment lying between the SMFs and the deletion should invert the SMF preferences. Thin line, recipient strand; thick line, donor strand; Δ, deletion; 3′SMF, side-marking frameshift located 3′ to the deletion; 5′SMF, side-marking frameshift located 5′ to the deletion.
The polarity observed in vivo is likely to reflect a balance involving different DNA-processing pathways. We can imagine a better unraveling of the displaced strand in the 5′-to-3′ direction because of chromosomal supercoiling constraints or a greater degradation of the unrecombined tails from 5′ to 3′. However 5′ to 3′ is usually reported to be the polarity of exchanges catalyzed in vitro by the E. coli RecA protein (see ref. 37 for a review). Here, the use of a recA-null mutant was not possible because transformation is abolished in such strains (20). As an alternative, we have carried out transformations in strain R317 (24). This strain is hexA− and carries a recA gene uncoupled from its competence-inducible promoter. Competence develops normally in R317, but RecA remains at a basal level and transformability is reduced about 20-fold (24). Among Mtxr transformants (Table 4), the deletion shared an average frequency of 0.12—i.e., 1/3 relative to a recA+ background. Integration of a long deletion seems therefore specifically affected in R317, suggesting a recombination pathway requiring high levels of RecA. The 5′ preponderance was mostly unchanged by the low RecA level (Table 4). We propose that either the RecA depletion affects recombination of 5′ and 3′ segments similarly or that the 5′/3′ polarity depends on a set of functions among which RecA might not be the main actor.
Table 4.
Analysis of the transformants obtained with circular heteroduplexes in R317: recA+(Ind−), hex−
| DNA | Transformants-PCR products
|
Cut patterns of the long bands
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Short | Long | Δ TE | AflII | NdeI | HindII | ClaI | 5′, % | |
| HetI | 88 | 11 | 77 | 0.12 | 36 (5′) | 5 (3′) | 28 (5′) | 8 (3′) | 83 |
| HetII | 30 | 4 | 26 | 0.13 | 1 (3′) | 13 (5′) | 2 (3′) | 10 (5′) | 88 |
Acknowledgments
We thank Drs. Bernard Martin and Jean-Pierre Claverys for providing plasmid pSP11hexAΔ, plasmid pSP41hexBΔ, and the strain R317. We are very grateful to Dr. Sanford A. Lacks for helpful comments on the manuscript. This work was supported by the Centre National de la Recherche Scientifique and by the Université Paul Sabatier.
ABBREVIATIONS
- Mtxr
methotrexate-resistant
- SMF
side-marking frameshift
References
- 1.Avery O T, MacLeod C M, McCarty M. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacks S A. In: Genetic Recombination. Kucherlapati R, Smith G R, editors. Washington, DC: Am. Soc. Microbiol.; 1988. pp. 43–86. [Google Scholar]
- 3.Lacks S. J Mol Biol. 1962;5:119–131. doi: 10.1016/s0022-2836(62)80067-9. [DOI] [PubMed] [Google Scholar]
- 4.Gabor M, Hotchkiss R D. Proc Natl Acad Sci USA. 1966;56:1441–1448. doi: 10.1073/pnas.56.5.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Méjean V, Claverys J P. Mol Gen Genet. 1988;213:444–448. doi: 10.1007/BF00339614. [DOI] [PubMed] [Google Scholar]
- 6.Méjean V, Claverys J P. J Biol Chem. 1993;268:5594–5599. [PubMed] [Google Scholar]
- 7.Lacks S, Greenberg B. J Mol Biol. 1976;101:255–275. doi: 10.1016/0022-2836(76)90376-4. [DOI] [PubMed] [Google Scholar]
- 8.Morrison D A, Guild W R. J Bacteriol. 1972;112:1157–1168. doi: 10.1128/jb.112.3.1157-1168.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morrison D A, Baker M, Mannarelli B. In: Transformation 1978. Glover S W, Butler L O, editors. Oxford: Cotswold; 1979. pp. 43–52. [Google Scholar]
- 10.Lacks S, Greenberg B, Carlson K. J Mol Biol. 1967;29:327–347. [Google Scholar]
- 11.Ephrussi-Taylor H, Gray T C. J Gen Physiol. 1966;49:211–231. doi: 10.1085/jgp.49.6.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mejean V, Claverys J P. Mol Gen Genet. 1984;197:467–471. doi: 10.1007/BF00329944. [DOI] [PubMed] [Google Scholar]
- 13.Tiraby G, Fox M S. Proc Natl Acad Sci USA. 1973;70:3541–3545. doi: 10.1073/pnas.70.12.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Claverys J-P, Prats H, Vasseghi H, Gherardi M. Mol Gen Genet. 1984;196:91–96. doi: 10.1007/BF00334098. [DOI] [PubMed] [Google Scholar]
- 15.Claverys J P, Lacks S A. Microbiol Rev. 1986;50:133–165. doi: 10.1128/mr.50.2.133-165.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fishel R, Lescoe M K, Rao M R S, Copeland N G, Jenkins N A, Garber J, Kane M, Kolodner R. Cell. 1993;75:1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- 17.Griffith F. J Hyg. 1928;27:113–159. doi: 10.1017/s0022172400031879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee D K, Morrison D A. J Bacteriol. 1988;170:630–637. doi: 10.1128/jb.170.2.630-637.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reizer J, Reizer A, Bairoch A, Saier M H., Jr Res Microbiol. 1993;144:341–347. doi: 10.1016/0923-2508(93)90191-4. [DOI] [PubMed] [Google Scholar]
- 20.Martin B, Garcia P, Castanié M-P, Claverys J P. Mol Microbiol. 1995;15:367–379. doi: 10.1111/j.1365-2958.1995.tb02250.x. [DOI] [PubMed] [Google Scholar]
- 21.Martin B, Scharples G J, Humbert O, Lloyd R G, Claverys J P. Mol Microbiol. 1996;19:1035–1045. doi: 10.1046/j.1365-2958.1996.445975.x. [DOI] [PubMed] [Google Scholar]
- 22.Pasta F, Sicard M A. Microbiology. 1996;142:695–705. doi: 10.1099/13500872-142-3-695. [DOI] [PubMed] [Google Scholar]
- 23.Lefèvre J-C, Claverys J-P, Sicard A M. J Bacteriol. 1979;138:80–86. doi: 10.1128/jb.138.1.80-86.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mortier-Barriere I, de Saizieu A, Claverys J P, Martin B. Mol Microbiol. 1998;27:159–170. doi: 10.1046/j.1365-2958.1998.00668.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin B, Prats H, Claverys J P. Gene. 1985;34:293–303. doi: 10.1016/0378-1119(85)90138-6. [DOI] [PubMed] [Google Scholar]
- 26.Prats A, Martin B, Claverys J P. Mol Gen Genet. 1985;200:482–489. doi: 10.1007/BF00425735. [DOI] [PubMed] [Google Scholar]
- 27.Alloing G, Trombe M C, Claverys J P. Mol Microbiol. 1990;4:633–644. doi: 10.1111/j.1365-2958.1990.tb00632.x. [DOI] [PubMed] [Google Scholar]
- 28.Lu A-L, Clark S, Modrich P. Proc Natl Acad Sci USA. 1983;80:4639–4643. doi: 10.1073/pnas.80.15.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trombe M-C, Laneelle G, Sicard A M. J Bacteriol. 1984;158:1109–1114. doi: 10.1128/jb.158.3.1109-1114.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claverys J-P, Roger M, Sicard A M. Mol Gen Genet. 1980;178:191–201. doi: 10.1007/BF00267229. [DOI] [PubMed] [Google Scholar]
- 31.Pasta F, Sicard M A. Mutat Res. 1994;315:113–122. doi: 10.1016/0921-8777(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 32.Lacks S A. J Bacteriol. 1979;138:404–409. doi: 10.1128/jb.138.2.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werntges H, Steger G, Riesner D, Fritz H J. Nucleic Acid Res. 1986;14:3773–3790. doi: 10.1093/nar/14.9.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhommerig S A, van Genderen M H, Buck H M. Biopolymers. 1991;31:1087–1094. doi: 10.1002/bip.360310908. [DOI] [PubMed] [Google Scholar]
- 35.Sicard M, Lefèvre J C, Mostachfi P, Gasc A-M, Sarda C. Genetics. 1985;110:557–568. doi: 10.1093/genetics/110.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gasc A M, Sicard A M. Mol Gen Genet. 1986;203:269–273. doi: 10.1007/BF00333965. [DOI] [PubMed] [Google Scholar]
- 37.Kowalczykowski S C, Dixon D A, Eggleston A K, Lauder S D, Rehrauer W M. Microbiol Rev. 1994;58:401–465. doi: 10.1128/mr.58.3.401-465.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]