Abstract
GsMTx4, a cationic hydrophobic peptide isolated from tarantula venom, is a specific inhibitor of stretch-activated channels (SACs). Here we show that the toxin also affects the membrane motor of outer hair cells at low doses. The membrane motor of outer hair cells is based on prestin, a member of the SLC26 family of membrane proteins, and directly uses electrical energy available at the plasma membrane. It is considered to be an essential part of the “cochlear amplifier,” which increases the sensitivity, tuning, and dynamic range of the mammalian ear. The toxin shifts the operating point of the motor. The saturating value of the voltage shift is (26±1)mV, capable of significantly reducing the performance of the cochlear amplifier. The dissociation constant is (3.1±0.6)μM, about 5 fold higher than that for SACs.
Keywords: membrane capacitance, operating point, prestin
Introduction
Electromotility of outer hair cells (OHCs) (1–3) directly uses electrical energy available at the plasma membrane. It is critical for ear function, as illustrated by the hearing loss of mice without normal prestin (4), a member of the SLC26 family of membrane proteins, which is essential for electromotility (5). This motility is fast and can respond in the auditory range of the frequency (6, 7).
Hyperpolarization induces the cylindrical cell’s elongation and depolarization induces shortening. The amplitude is between 4 to 5% of the total length (3). These changes are associated with charge transfer across the membrane (8), which gives rise to nonlinear membrane capacitance (NLC) with bell shaped voltage dependence (9, 10).
Tarantula toxin GsMTx4, a cationic hydrophobic polypeptide, has been identified as a specific blocker of stretch-activated cation channels (SACs) (11). This property is attributed to the toxin’s effect on the interface between the channel and the lipid bilayer (12). Thus it is of interest to find whether or not another class of membrane proteins is also sensitive to this toxin.
Here we examine the effect of GsMTx4 on the OHC motor by monitoring the membrane capacitance and the amplitude of mechanical cell displacement in the whole-cell recording configuration.
Materials and Methods
Cell Preparation
The method for preparing isolated outer hair cells has been described earlier (13). Briefly, bullas were obtained from guinea pigs (in accordance with the protocol NINDS/NIDCD 1061-02). The organ of Corti was dissociated from opened cochleas by teasing with a fine needle under a dissection microscope. Dispase (Worthington) treatment (1 mg/ml for 10–20 min at 21°C) was used before mechanical isolation. The strips of organ of Corti thus obtained were triturated three times gently with a plastic pipette and placed in a chamber mounted on an inverted microscope. Isolated outer hair cells with the normal shape were chosen for experiments. The cell length ranged between 40 μm and 75 μm. Red blood cells were also collected from guinea pigs.
Media and Extracellular Perfusion
The intracellular medium consisted of 140 mM CsCl, 2 mM CaCl2, 5 mM EGTA, and 10 mM Cs-HEPES. The extracelluar medium contained 140 mM NaCl, 5 mM CsCl, 2mM MgCl2, 1 mM CaCl2, 2 mM CoCl2, 10 mM Na-HEPES, and about 10 mM glucose, which was used to adjust the osmolarity to 300 mOsm/kg. The pH of both media was adjusted to 7.4. These channel blocking media were intended to facilitate capacitance measurements.
Tarantula toxin GsMTx4 was purchased from Peptide International (Louisville, KY). Chlorpromazine (CPZ) and trinitrophenol (TNP) were obtained from Sigma. Each of these chemicals was dissolved in the external medium and put in a perfusion pipette. Perfusion was controlled by a solenoid valve and a pressure reservoir.
Membrane Capacitance Measurement
Experiments were performed on isolated outer hair cells in the whole-cell recording configuration. The membrane capacitance was determined by the capacitive current elicited by voltage jumps. The voltage dependence of the capacitance was usually determined with a pair of ascending (10 mV steps from −135 mV to +35 mV) and descending (−10 mV steps from +35 mV to −135 mV) staircase voltage waveforms. The holding potential was −75 mV. The sampling interval of the data acquisition was 10 μs. The pipette resistance was between 2.5 and 4.5 MΩ when filled with the intracellular medium. The access resistance in the whole-cell configuration was between 8 and 12 MΩ. The membrane resistance Rm was somewhat dependent on the membrane potential and was between 200 and 800 MΩ. The membrane potential dependence of the capacitance obtained was compensated for the voltage drop. A patch amplifier (Axopatch 200B, Axon Instruments) was used for whole-cell voltage clamp experiments. A train of voltage pulses was generated with an ITC-16 interface (Instrutech, Mineola, NY) by the Igor program (WaveMetrics, Lake Oswego, OR) with a software module created by R. J. Bookman’s laboratory at the University of Miami (http://chroma.med.miami.edu). To concisely describe the bell-shaped voltage dependence of the capacitance Cm we fit our data with a function,
| (1) |
| (2) |
Eq. 1 has a peak value Cpk + Clin at V=Vpk. The charge q determines the sharpness of the peak. The quantities kB and T are respectively Boltzmann’s constant and the temperature.
Cell Displacement
Images of the cells during the whole-cell voltage clamp experiments were captured and stored in a DVD recorder (model RDR-GX7, Sony). These images were then transferred to a computer with an image grabber card (Scion, Frederick, MD) and analyzed off-line using a computer program (NIH Image, W. Rasband, NIMH). The resolution was 4.04 pixels per μm. Length changes ΔL of the cells were determined with a previously developed macro (14). The data obtained were fit with a Boltzmann function,
| (3) |
where L0 is the length of the cell at the holding potential and ΔLmax is the maximum voltage-dependent length change. The function B(V) is defined by Eq. 2.
Results
Effects of toxin perfusion
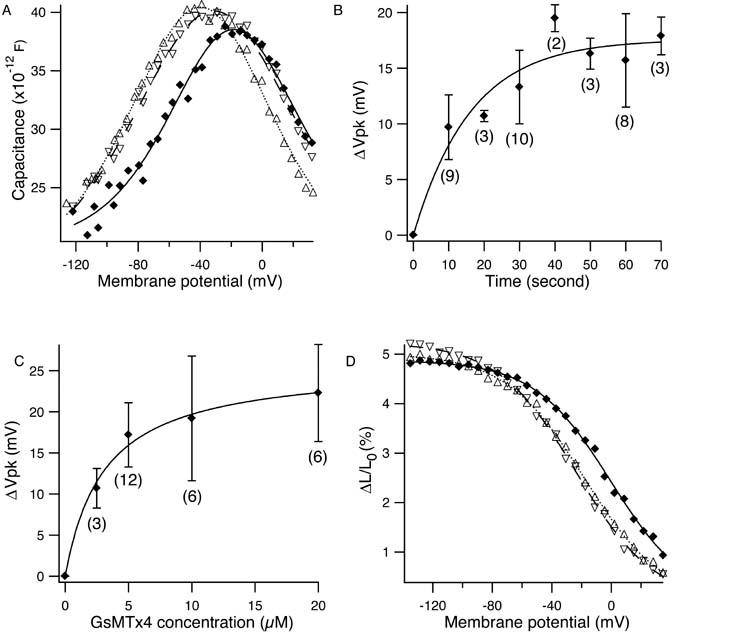
Tarantula toxin GsMTx4 shifted the membrane potential dependence of the motor without significantly affecting the peak height and the steepness of the nonlinear capacitance. At 5 μM concentration, the toxin shifted the peak of the nonlinear capacitance from (−39.8 ± 0.4) mV to (−18.4 ± 0.8) mV (Fig. 1A). Throughout this paper, mean values are accompanied by standard deviations. Wash-out returned the peak to (−31.2 ± 0.5) mV. The time course of the shift had a time constant of (17.6 ± 1.6) s at toxin concentration of 5 μM (Fig. 1B). The apparent dissociation constant obtained from the mass action law was (3.1 ± 0.6) μM. The saturating voltage shift was (26 ± 1) mV. The amplitude of length change, which was about 5% in the control condition, was not affected by the toxin (Fig. 1D). The cell length at the holding potential of −75 mV did not changed by perfusion with 10 μM GsMTx4 beyond 0.6 %, the standard deviation of length determination.
Figure 1.
Effects of GsMTx4 on the outer hair cell motor. (A) Effect on the membrane capacitance. Control (Δ), 5 μM GsMTx4 (♦), and recovery (∇). Parameter values are (mean±SD): for control, q = (0.84±0.04) e (electronic charge), Clin = (17.8 ± 0.1) pF, Vpk = (−39.8 ± 0.4) mV; for 5 μM GsMTx4, q =(0.93±0.05) e, Clin = (20.1±0.1) pF, Vpk = (−18.4±0.8) mV; and for recovery, (0.74 ± 0.04) e, Clin = (17.1 ± 0.1) pF, Vpk = (−31.2 ± 0.5) mV. (B) The time course of voltage shift. The fit with a single exponential gives the time constant of (17.6 ± 1.6) s. (C) Dose response. The curve fit with the mass action law gives the dissociation constant of (3.1±0.6) μM. The maximal shift is (26 ± 1) mV. (D) Effect on voltage dependent axial strain. Control (Δ), 20 μM GsMTx4 (♦), and wash-out (∇). Error bars show standard deviations. The number of data points are shown in the parentheses. The holding potential is −75 mV.
The reported dissociation constant of this toxin to SACs is 0.63 μM, about 1/5 of our value for the hair cell motor (11).
Interaction with chlorpromazine and trinitrophenol
To examine the nature of the effect of the toxin on the membrane motor’s nonlinear capacitance (NLC), we briefly perfused outer hair cells bathed with the toxin with the standard external medium with and without another charged hydrophobic ions chlorpromazine (CPZ) and trinitrophenol (TNP), which have been reported to interact with lipid bilayers (15).
The cells bathed with 5 μM GsTMx4 showed a shift in the voltage dependence of the NLC of (−11.0 ± 1.6) mV with 100 μM TNP perfusion and a shift of (3.9 ± 1.8) mV with 100 μM CPZ (Table 1). These shifts were reversed on termination of perfusion. Perfusion with the plain external medium did not produce any shifts.
Table 1.
Effect of ion substitution
| bath medium (μM) | perfusate (μM) | on-shift mV | off-shift mV | N |
|---|---|---|---|---|
| GsMTx4(5) | TNP(100) | −11.0 ± 1.6 | 9.7 ± 3.4 | 4 |
| GsMTx4(5) | CPZ(100) | 3.9 ± 1.8 | −2.7 ± 1.4 | 3 |
| CPZ(100) | TNP(100) | −12.6 ± 4.8 | 16.1 ± 6.8 | 5 |
The shifts given were measured 2 or 3 min. after the onset and the termination of the perfusion. The holding potential was 0 mV.
The effect of 5 μM GsTMx4 resembled that of 100 μM CPZ. Not only did 100 μM CPZ shift the voltage dependence of the capacitance by 17 mV, but also perfusing 100 μM TNP on outer hair cells bathed in 100 μM CPZ led to a negative shift in the voltage dependence (Table 1).
Effects on erythrocytes
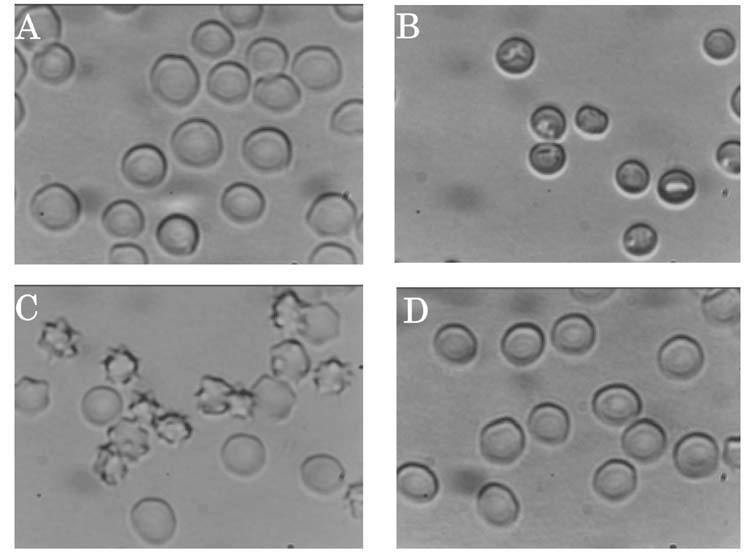
Because the toxin and CPZ showed similar effects, we tested the effects of GsMTx4 on the shape of erythrocytes, using CPZ and TNP as positive controls (Fig. 2). With 100 μM CPZ they were transformed into cups. With 500 μM TNP, they were crenated. These results were consistent with earlier reports (15). However, 5 μM GsMTx4 did not show a noticeable effect on the shape of erythrocytes.
Figure 2.
Effects of amphipathic ions on erythrocyte morphology. (A) control, (B) 100 μM CPZ, (C) 500 μM TNP, and (D) 5 μM GsMTx4.
Discussion
The tarantula toxin GsMTx4 shifts the voltage dependence of the outer hair cell motor up to 26 mV in the positive direction. This shift is expected to reduce the efficiency of the cochlear amplifier. The dissociation constant of 3.1 μM could be rather specific even though it is still 5 fold higher than that for SACs. It is 1/10th of the value for CPZ, which is about 30 μM (16).
The effect of the toxin, which is cationic, is similar to that of CPZ, a cationic amphipath. Both are counteracted by TNP, an anionic amphipath. In addition, CPZ induces only minor shifts on cells which have been incubated in the toxin. These observations indicate that the mechanism of the toxin’s action on the membrane motor could be similar to that of CPZ, which is thought to act through bending the lipid bilayer by entering the more negatively charged inner leaflet owing to electric interaction (16).
Thus one would expect that the toxin induces shape changes in erythrocytes in a manner similar to CPZ. However, that is not the case. One possible explanation for the difference could be that erythrocyte membrane differs from the plasma membrane of outer hair cells. However, as with erythrocytes, the inner leaflet of OHC membrane is also rich in phosphatidylserine and more negatively charged than the outer leaflet (17).
An alternative explanation is that unlike CPZ the toxin may have an affinity for the outer hair cell motor in a manner similar to SACs while it also has electric interaction with charged amphipaths. Although the interaction of GsMTx4 with SACs is considered specific, it is not of the key-and-keyhole type because an isomer is equally effective (12). Thus it has been suggested that this molecule prefers the interface between the channel and lipid bilayer. Even though the effect of the toxin on the membrane motor may not be as specific, the toxin may still have a significant effect at the interface between the membrane motor and lipid bilayer.
Acknowledgments
We thank Drs. Richard Chadwick and Ronald Petralia for comments. This work is supported by the Intramural Research Program of the NIH, NIDCD.
References
- 1.Brownell W, Bader C, Bertrand D, Ribaupierre Y. Evoked mechanical responses of isolated outer hair cells. Science. 1985;227:194–196. doi: 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- 2.Kachar B, Brownell WE, Altschuler R, Fex J. Electrokinetic shape changes of cochlear outer hair cells. Nature. 1986;322:365–368. doi: 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- 3.Ashmore JF. A fast motile response in guinea-pig outer hair cells: the molecular basis of the cochlear amplifier. J Physiol (Lond) 1987;388:323–347. doi: 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liberman MC, Gao J, He DZ, Wu X, Jia S, Zuo J. Prestin is required for electromotility of the outer hair cell and for the cochlear amplifier. Nature. 2002;419:300–304. doi: 10.1038/nature01059. [DOI] [PubMed] [Google Scholar]
- 5.Zheng J, Shen W, He DZZ, Long KB, Madison LD, Dallos P. Prestin is the motor protein of cochlear outer hair cells. Nature. 2000;405:149–155. doi: 10.1038/35012009. [DOI] [PubMed] [Google Scholar]
- 6.Dallos P, Evans B. High-frequency motility of outer hair cells and the cochlear amplifier. Science. 1995;267:2006–2009. doi: 10.1126/science.7701325. [DOI] [PubMed] [Google Scholar]
- 7.Frank G, Hemmert W, Gummer AW. Limiting dynamics of high-frequency electromechanical transduction of outer hair cells. Proc Natl Acad Sci USA. 1999;96:4420–4425. doi: 10.1073/pnas.96.8.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashmore JF. Forward and reverse transduction in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. Neurosci Res Suppl. 1990;12:S39–S50. doi: 10.1016/0921-8696(90)90007-p. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Sacchi J. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J Neurophysiol. 1991;11:3096–3110. doi: 10.1523/JNEUROSCI.11-10-03096.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iwasa KH. Effect of stress on the membrane capacitance of the auditory outer hair cell. Biophys J. 1993;65:492–498. doi: 10.1016/S0006-3495(93)81053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF, Baumgarten CM, Sachs F. Identification of a peptide toxin from grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suchyna TM, Tape SE, Koeppe RE, Andersen OS, Sachs F, Gottlieb PA. Bilayer-dependent inhibition of mechanosensitive channels by neuroactive peptide enantiomers. Nature. 2004;430:235–240. doi: 10.1038/nature02743. [DOI] [PubMed] [Google Scholar]
- 13.Dong XX, Ospeck M, Iwasa KH. Piezoelectric reciprocal relationship of the membrane motor in the cochlear outer hair cell. Biophys J. 2002;82:1254–1259. doi: 10.1016/S0006-3495(02)75481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwasa KH, Adachi M. Force generation in the outer hair cell of the cochlea. Biophys J. 1997;73:546–555. doi: 10.1016/S0006-3495(97)78092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheetz MP, Singer SJ. Equilibrium and kinetic effects of drugs on the shapes of human erythrocytes. J Cell Biol. 1976;70:193–203. doi: 10.1083/jcb.70.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lue AJC, Zhao HB, Brownell WE. Chlorpromazine alters outer hair cell electromotility. Otolaryngol Head Neck Surg. 2001;125:71–76. doi: 10.1067/mhn.2001.116446. [DOI] [PubMed] [Google Scholar]
- 17.Shi XX, Gillespie P, Nuttall AL. Phosphatidylserine exposure on the outer leaf of cochlear hair cell plasma membrane is induced by in vitro sodium loading. Assoc Res Otolaryngol Abstract. 2005;28:134. [Google Scholar]