Abstract
The intravenous glucose tolerance test (I.V.G.T.T.) was used to diagnose chemical diabetes during pregnancy in 180 women, 50 of whom subsequently received chlorpropamide therapy in a daily dosage of 100 mg; the remainder had no drug therapy.
Preliminary work showed the I.V.G.T.T. to be reproducible in the second and third trimesters but not in the puerperium in normal pregnancy. Though intravenous glucose tolerance deteriorates between the second and third trimesters in women with no features of diabetes, a significant improvement occurs after a course of chlorpropamide in a daily dosage of 100 mg during pregnancy in chemical diabetes, but this treatment did not enhance the rate of return to normal glucose tolerance post partum.
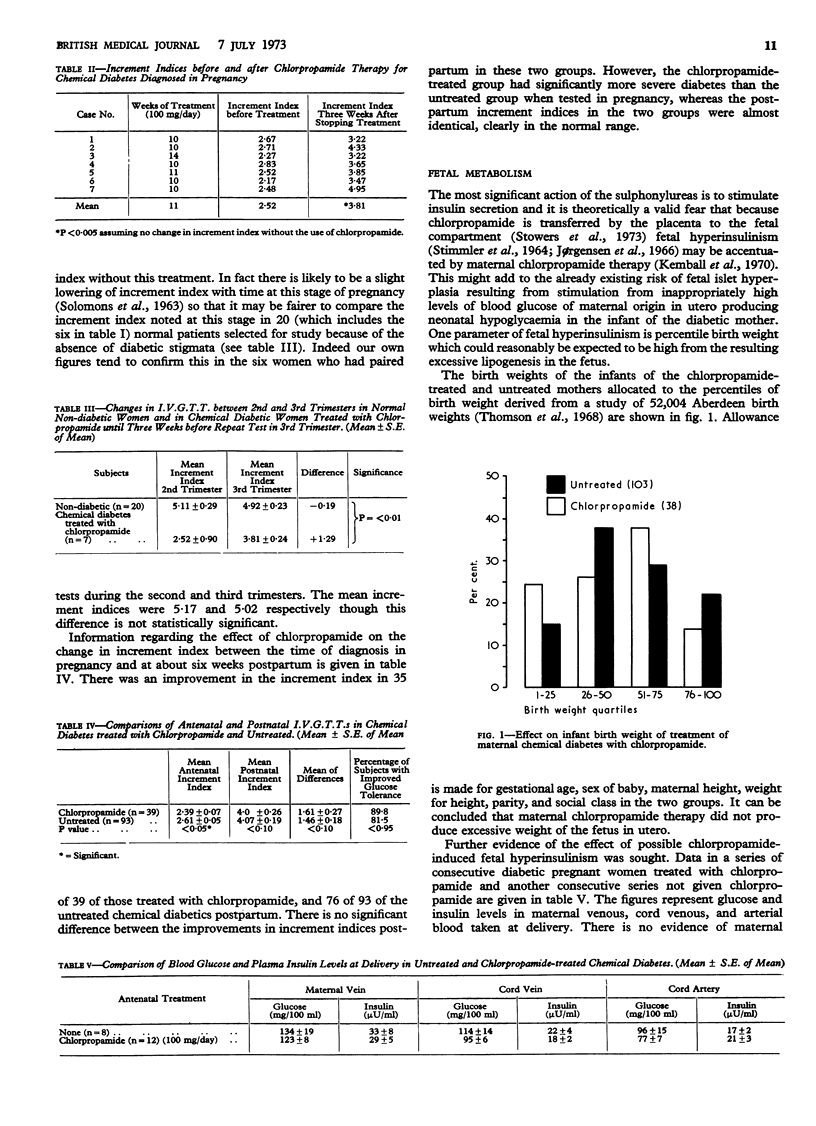
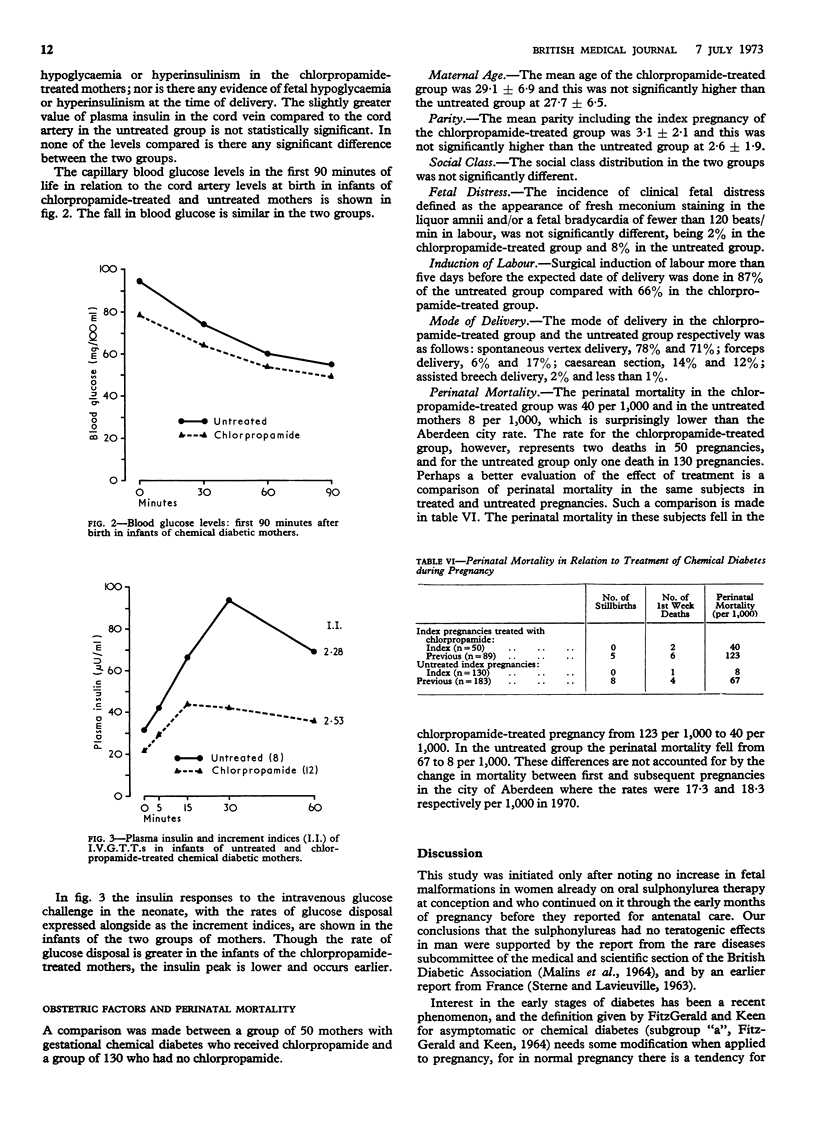
Plasma glucose and insulin studies showed no evidence of hypoglycaemia or hyperinsulinism in the mother at delivery or in the newborn when chlorpropamide had been used compared with a group receiving no such treatment. In the infants of the chlorpropamide-treated mothers there was a suggestion of an increased rate of glucose disposal in response to a glucose challenge, but no increase in birth weight.
There were two fetal deaths in the 50 pregnancies of mothers treated with chlorpropamide, one being due to a mistaken premature delivery and the other to a diaphragmatic hernia. Thus chlorpropamide in a dose of 100 mg a day has been shown to reverse chemical diabetes diagnosed and treated in pregnancy without apparent risk to the fetus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HALES C. N., RANDLE P. J. Immunoassay of insulin with insulin-antibody precipitate. Biochem J. 1963 Jul;88:137–146. doi: 10.1042/bj0880137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen K. R., Deckert T., Pedersen L. M., Pedersen J. Insulin, insulin antibody and glucose in plasma of newborn infants of diabetic women. Acta Endocrinol (Copenh) 1966 May;52(1):154–167. doi: 10.1530/acta.0.0520154. [DOI] [PubMed] [Google Scholar]
- Kemball M. L., McIver C., Milner R. D., Nourse C. H., Schiff D., Tiernan J. R. Neonatal hypoglycaemia in infants of diabetic mothers given sulphonylurea drugs in pregnancy. Arch Dis Child. 1970 Oct;45(243):696–701. doi: 10.1136/adc.45.243.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINS J. M., COOKE A. M., PYKE D. A., FITZGERALD M. G. SULPHONYLUREA DRUGS IN PREGNANCY. Br Med J. 1964 Jul 18;2(5402):187–187. doi: 10.1136/bmj.2.5402.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALINS J. M., FITZGERALD M. G. CHILDBEARING PRIOR TO RECOGNITION OF DIABETES; RECOLLECTED BIRTH WEIGHTS AND STILLBIRTH RATE IN BABIES BORN TO PARENTS WHO DEVELOPED DIABETES. Diabetes. 1965 Apr;14:175–178. doi: 10.2337/diab.14.4.175. [DOI] [PubMed] [Google Scholar]
- SOLOMONS E., SILVERSTONE F. A., POSNER N. A. Obstetric factors suggesting diabetes evaluated by the rapid intravenous glucose tolerance test. Obstet Gynecol. 1963 Jul;22:50–55. [PubMed] [Google Scholar]
- STERNE J., LAVIEUVILLE M. [Clinical research on the effects of oral antidiabetics on the fetus]. Presse Med. 1963 Jun 22;71:1547–1549. [PubMed] [Google Scholar]
- STIMMLER L., BRAZIE J. V., O'BRIEN D. PLASMA-INSULIN LEVELS IN THE NEWBORN INFANTS OF NORMAL AND DIABETIC MOTHERS. Lancet. 1964 Jan 18;1(7325):137–138. doi: 10.1016/s0140-6736(64)92223-8. [DOI] [PubMed] [Google Scholar]
- Sutherland H. W., Stowers J. M., McKenzie C. Simplifying the clinical problem of glycosuria in pregnancy. Lancet. 1970 May 23;1(7656):1069–1071. doi: 10.1016/s0140-6736(70)92751-0. [DOI] [PubMed] [Google Scholar]


