Abstract
To determine how murine memory and naive T cells differ, we generated large numbers of long-lived memory CD8+ T cells and compared them to naive cells expressing the same antigen-specific receptor (T cell receptor; TCR). Although both populations expressed similar levels of TCR and CD8, on antigen stimulation in vitro memory T cells down-regulated their TCR faster and more extensively and secreted IFN-γ and IL-2 faster than naive T cells. Memory cells were also larger, and when freshly isolated from mice they contained perforin and killed target cells without having to be restimulated. They further differed from naive cells in requiring IL-15 for proliferation and in having a greater tendency to undergo apoptosis in vitro. On antigen stimulation in vivo, however, they proliferated more rapidly than naive cells. These findings suggest that, unlike naive T cells, CD8 memory T cells are intrinsically programmed to rapidly express their effector functions in vivo without having to undergo clonal expansion and differentiation.
Following an initial response to antigen, some lymphocytes acquire altered properties and persist for prolonged periods. The collective behavior of these cells, termed immunologic memory, constitutes one of the cardinal features of the vertebrate adaptive immune system (reviewed in refs. 1–3). For B lymphocytes the distinguishing properties between the cells responsible for memory (memory B cells) and their precursors (naive B cells) have long been known (4, 5). In response to antigenic stimulation, memory B cells secrete high-affinity IgG (or IgE or IgA) rather than the low-affinity Abs (IgM and IgG) produced during the primary B cell response (6). These differences result from Ig gene class switching, somatic hypermutation of Ig variable gene segments, and selection of B cells expressing high-affinity Ig receptors by antigen during the primary response to antigen (7–9). The antigen-stimulated generation of memory B cells is largely responsible for the effectiveness of natural infection and vaccines in conferring immunity against many microbial pathogens.
Although memory T cells are known to exist (10–20), the properties that distinguish them from their naive precursors are only known to a limited extent. A major obstacle preventing a more complete characterization of memory T cells has been their limited availability and the heterogeneity of the memory T cells expressing different T cell receptors (TCRs) in most model systems. In this study we modified an adoptive transfer protocol (21) by using recombination activating gene-1 (RAG1)-deficient mice as transfer recipients. Because they lack endogenous lymphocytes, a substantial number of memory T cells (3–4 × 106/mouse) were generated after adoptive transfer of CD8 TCR transgenic T cells followed by immunization with antigenic peptide. Functional comparisons of the resulting CD8 memory T cells with their naive counterparts revealed some unique properties that may underlie the characteristic memory T cell response.
MATERIALS AND METHODS
Peptides, CTL Clones, and Mice.
The T cells we are concerned with are CD8+ cells that express the transgenic αβ TCR of a cytotoxic T lymphocyte (CTL) clone known as 2C (22, 23). The 2C TCR recognizes SIYRYYGL (called SYRGL) in association with Kb (syngeneic) and QLSPFPFDL (called QL9) in association with Ld (allogeneic) (24, 25). CD8+ 2C CTL clones (2C88 and L3.100) were maintained by weekly stimulation with irradiated P815 (Ld+) cells. RAG1-deficient (RAG1−/−) mice, backcrossed to C57BL/6 (B6) mice for 13 generations, were used between 3 to 10 weeks of age as adoptive transfer recipients. Also used as recipients were B6 female mice, 4 weeks of age, from The Jackson Laboratory. Naive donor T cells were from lymph nodes of 2C TCR transgenic mice on the RAG1−/− background (2C/RAG).
Adoptive Transfer and Immunization.
More than 95% of lymph node cells in 2C/RAG mice express the 2C TCR. About 0.5–1.0 × 106 of these cells (CD25−CD69−CD44−) were injected i.v. into nonirradiated RAG1−/− recipient mice. To generate memory cells, recipients were immunized 3 days later with 50 μg SYRGL in Freund’s adjuvant at three s.c. sites: the base of the tail and the scruff of the neck (complete adjuvant) and the right forepaw (incomplete adjuvant). To transfer naive and memory cells into B6 mice, each recipient received 1 × 105 2C lymph node cells (naive cells from 2C/RAG donors and memory cells from RAG1−/− recipients 1 month or more after immunization), and recipients were immunized as above.
Abs, Intracellular Staining, and Flow Cytometry.
Abs to CD8, CD25, CD69, CD44, CD62L (L-selectin), Ly-6C, Fas, Fas ligand, cytolytic T lymphocyte-associated antigen 4 (CTLA-4), and IL-2 receptor β (IL-2Rβ) were purchased as conjugates from PharMingen, as were Annexin V, streptavidin-phycoerythrin (S:PE), and strepavidin-allophycocyanin (S:APC). Anti-CD11A [lymphocyte function-associated antigen 1 (LFA-1)] Ab was conjugated with FITC. Clonotypic Ab 1B2, specific for the 2C TCR, was conjugated to biotin. FITC-labeled anti-BrdUrd Ab was from Becton Dickinson. Cells were stained in the presence of 3 μg/ml anti-FcR Ab in PBS containing 0.1% BSA and 0.1% NaN3 and analyzed by flow cytometry, collecting 10,000–400,000 live cells per sample. 2C cells having DNA-incorporated BrdUrd were stained with Abs to the 2C TCR and CD8, fixed, permeabilized, and then stained with anti-BrdUrd Ab (26). To detect intracellular perforin, cells were incubated with 10% mouse serum in PBS (to block FcR), stained with biotinylated 1B2 followed by S:PE, fixed in 75% alcohol, permeabilized in PBS containing 1% paraformaldehyde and 0.01% Tween-20, and incubated with ascites fluid (1:500 dilution) containing rat anti-mouse perforin Ab (P1–8, 27) followed with FITC-labeled goat anti-rat Ab (Kirkegaard & Perry Laboratories, Gaithersburg, MD).
Purification of T Cells by Magnetic Sorting.
Unless otherwise indicated, magnetically purified memory and naive cells and 2C CTL clones (all 1B2+CD8+) were used in in vitro assays. Cells from lymph nodes and spleens were pretreated with anti-FcR Ab and then incubated with anti-CD8α Ab-labeled microbeads (Militenyi Biotec, Alburn, CA) by using 2 beads/cell, and isolated on a SuperMACS cell sorter (Militenyi Biotec). The purity of the eluted cells was typically 40–70% (memory cells), 90% (naive cells), and 99% (2C clones). The procedure did not activate naive or memory cells, as indicated by negative results for expression of CD25, CD69, and CD44 over the next 3 days and for [3H]thymidine incorporation after culturing for 72 hr.
T Cell Activation, Cytokine Secretion, and Proliferation in Vitro.
Approximately 5 × 105 purified 1B2+CD8+ naive or memory cells were incubated (37°C) with 1 × 10−8 M SYRGL and a 2.5- to 5-fold excess of irradiated syngeneic B6 splenocytes in 1 ml in 48-well flat-bottom plates. The addition of excess B6 splenocytes not only provided antigen-presenting cells (APC) but also helped to minimize any effects of varying numbers of contaminating cells in various purified preparations. After 3, 6, 12, 24, 48, and 72 hr, samples were removed and separated into cell pellets to detect activation markers and supernatants to measure secreted IL-2 and IFN-γ. Cell proliferation was measured by [3H]thymidine incorporation in 96-well flat-bottom plates by using 1 × 105 memory or naive cells, a 2.5-fold excess of irradiated B6 splenocytes, and various concentrations of SYRGL. After 48 hr, 1 μCi (1 Ci = 37 GBq) of [3H]thymidine (NEN) was added and cells were harvested 16 hr later.
Cytolytic Assays.
51Cr labeled T2-Kb target cells and SYRGL were incubated with naive or memory cells or CTL clone L3.100 in round bottom wells of 96-well plates. After 6 hr 51Cr in supernatants was counted. Except for sextuplet wells to determine spontaneous and maximum 51Cr release, all samples were assayed in triplicate. Specific lysis was calculated as follows: [(experimental counts − spontaneous counts)/(maximum counts − spontaneous counts)] × 100.
RESULTS
Expansion, Survival, and Surface Phenotype of Adoptively Transferred 2C Cells in RAG1−/− Recipients.
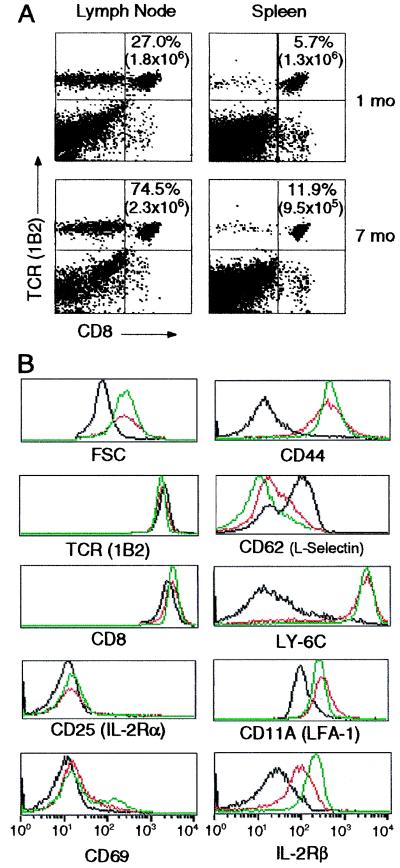
Five days after immunizing RAG1−/− recipients, the 2C cells in draining lymph nodes were 70 times more abundant than in the corresponding lymph nodes of nonimmunized controls. In the immunized mice, nearly all of these cells had become CD44+ and CD25+, indicating their activation by antigen (data not shown). One month later, the total number of 2C cells had decreased to ≈3 × 106 cells/recipient, and they amounted to about 30% of lymph node cells and 5% of splenocytes (Fig. 1A). After 7 months, the total number of CD8+ 2C cells remained essentially unchanged; they still exceeded the number of naive cells initially transferred and amounted to 50–75% of lymph node cells and 10% of splenocytes. Antigen-primed 2C cells expressed similar levels of TCR and CD8 as naive cells and were negative for activation markers CD25 and CD69 (Fig. 1B). However, they were larger, their level of the IL-2Rβ subunit was greater and increased over time, and they uniformly expressed the characteristic surface phenotype of memory T cells: high levels of CD44, CD11A, and Ly-6C, and low levels of CD62L. They are therefore referred to as memory cells. For all subsequent functional analyses, memory cells were taken from RAG−/− recipients at least 1 month after immunization.
Figure 1.
Persistence and surface phenotype of antigen-stimulated 2C cells. (A) Persistence. One and 7 months after immunization, lymph node and spleen cells of RAG1−/− recipients were stained with Abs to the 2C TCR and CD8. The percentages and total numbers (in parentheses) of 1B2+CD8+ T cells are shown. In the transferred naive cell population, 5–25% of the 1B2+ cells were CD8− and CD4−; these cells persisted after the recipients were immunized. (B) Surface phenotype. Lymph node cells from naive 2C/RAG donors and immunized RAG1−/− recipients were stained with Abs to 2C TCR, CD8, and other cell-surface proteins. Cell size (forward light scatter, FSC) and expression of various markers were gated on live 1B2+CD8+ cells. Naive cells (black); memory cells at 1 month (red) and 7 months (green).
Memory 2C Cells Undergo More Rapid Activation Than Naive Cells.
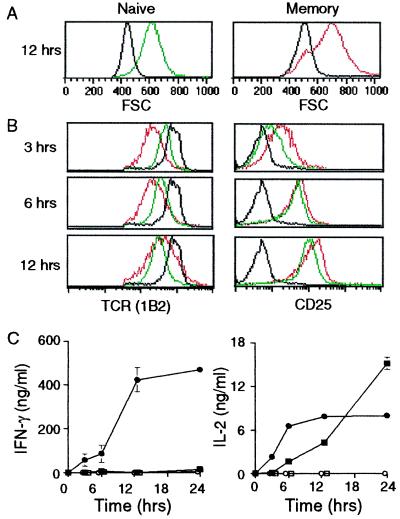
To compare the functional properties of naive and memory cells, 2C cells were isolated by magnetic sorting from donor and immunized recipient mice, respectively. They were then incubated with SYRGL using irradiated B6 splenocytes as APC. After 3, 6, 12 and 24 hr incubation, cells were analyzed by flow cytometry and supernatants were examined for secreted cytokines. Both memory and naive cells were activated to express CD69 (data not shown) and to increase in size (became “blastic”) by 12 hr (Fig. 2A), but the 2C TCR was down-regulated much faster and more extensively on memory cells (Fig. 2B). About 75% of the TCR molecules on memory cells had disappeared by 3 hr and they had largely returned to their normal level by 12 hr, whereas on naive cells the lowest level was not observed until 12 hr. The CD25 levels were also considerably higher on memory cells at 3 hr, but after 6 and 12 hr the increase was about the same on both cell populations (Fig. 2B). Without peptide stimulation, neither memory nor naive cells secreted IFN-γ or IL-2, but addition of peptide led memory cells to respond much faster: by 12 hr they secreted 40-times more IFN-γ than naive cells (Fig. 2C). IL-2 was also initially secreted faster by memory cells (Fig. 2C), but its production by these cells leveled off at ≈12 hr, probably because of extensive cell death (see below).
Figure 2.
Memory cells respond more rapidly to antigen than naive cells. Purified memory and naive 2C cells were incubated with antigen, removed after 3, 6, and 12 hr, and stained with Abs to 2C TCR, CD8, and CD25. Forward light scatter (FSC) and expression of TCR and CD25 were gated on live CD8+ cells. No antigen added (black); memory cells plus antigen (red); naive cells plus antigen (green). (A) FSC. (B) Surface levels of TCR and CD25. (C) Secretion of IFN-γ and IL-2. Culture supernatants were assayed by ELISA for IFN-γ and IL-2. The symbols are as follows: memory cells with antigen (●) or without antigen (○); naive cells with antigen (■) or without antigen (□).
Freshly Isolated CD8 Memory T Cells Are Cytolytic.
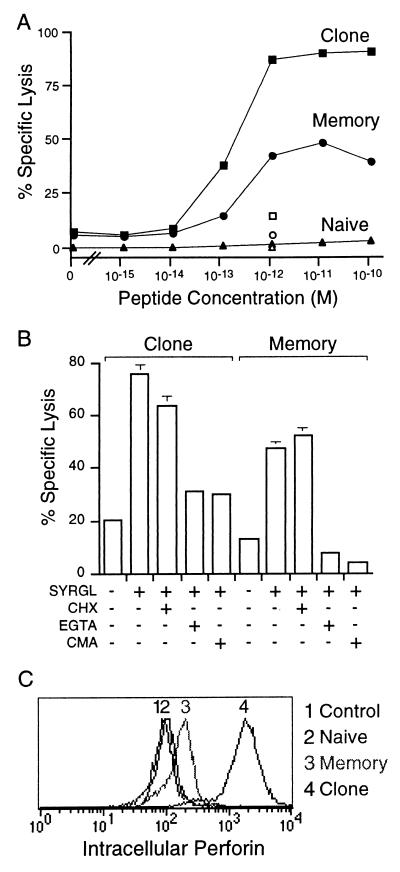
To evaluate the cytolytic activity of 2C memory and naive cells, we examined them when freshly removed from lymph nodes and spleen and purified by magnetic sorting. That the purification procedure did not influence the results was indicated by the finding that cells of 2C clone L3.100 had the same cytolytic activity whether subjected to magnetic sorting or not. Memory cells, but not naive cells, were cytolytic (Fig. 3A). Although maximum lysis of target cells by memory cells was less than that of a potent 2C CTL clone (L3.100), the same concentration of SYRGL peptide (1–2 × 10−13 M) was required for half-maximal killing by both memory cells and the cultured clone. To determine whether memory cells already had the capacity to kill in vivo or had acquired cytolytic capability during the course of the 6-hr ex vivo assay, the assay was performed in the presence of cycloheximide. This inhibitor of protein synthesis had no effect (Fig. 3B), indicating that all the proteins required for cytolysis were already present in memory cells in vivo. In support of this evidence, perforin could be detected in freshly isolated memory cells (Fig. 3C). Moreover, the cytolytic activity of memory cells, like that of the CTL clone, was inhibited by concanamycin, an inhibitor of perforin activity (36), and by EGTA, which inhibits degranulation (Fig. 3B). Together, these findings show that memory CD8 T cells can kill target cells in vivo by perforin–granule exocytosis without requiring restimulation by antigen.
Figure 3.
Memory 2C cells are cytolytic. (A) Comparison of cytolytic activities of memory and naive cells and 2C CTL clone. Purified cells were added at a 20:1 T cell to target cell ratio to 51Cr-labeled T2-Kb cells with various concentrations of SYRGL. 1B2 Ab to the 2C TCR was added at 60 μg/ml to verify that lysis was TCR dependent (open symbols). (B) The effect of various inhibitors on cytolytic activity of memory cells. Cytolytic assays were as in A, but before the addition of target cells and peptide, T cells were incubated with cycloheximide (CHX, 20 μg/ml) or concanamycin A (CMA, 2 mM) for 2.5 hr or with EGTA (4 mM) for 20 min. Inhibitors were present throughout the assay. (C) Memory cells express perforin. Memory cells, naive cells, and a 2C CTL clone (2C88) were stained with 1B2, fixed, permeabilized, and stained intracellularly with an anti-perforin Ab (P1–8). Intracellular perforin of 1B2+ cells is shown. Control refers to memory cells stained only with the secondary Ab. Peak 1, control; peak 2, naive cells; peak 3, memory cells; and peak 4, CTL clone 2C88.
Proliferation of Memory Cells in Vitro Requires Special Conditions.
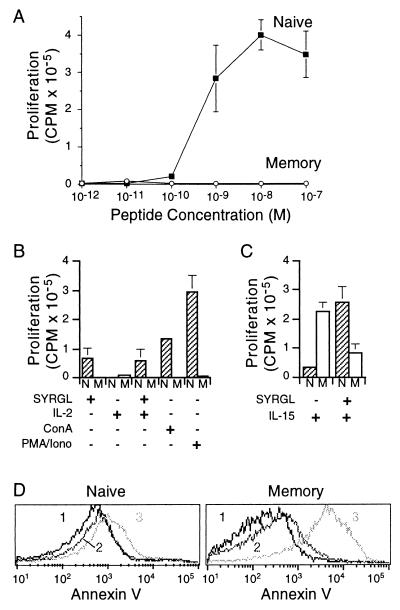
To compare the proliferative responses of memory and naive cells, equal numbers of purified cells were titrated with SYRGL by using irradiated B6 splenocytes as APC. Naive cells proliferated vigorously, with a half-maximal response at about 5 × 10−10 M SYRGL (Fig. 4A). However, memory cells did not proliferate appreciably at any peptide concentration tested. They also failed to proliferate in response to phorbol 12-myristate 13-acetate (PMA) plus ionomycin, or Con A, or IL-2, or IL-2 plus SYRGL, or when syngeneic antigen (SYRGL-Kb on B6 splenocytes) was replaced with a potent allogeneic antigen (QL9-Ld on BALB/c splenocytes) (Fig. 4B and data not shown). These results were particularly surprising because in response to antigen memory 2C cells became blastic and rapidly expressed CD25 and secreted IL-2 and IFN-γ. The failure to proliferate could not be attributed to a suppressive factor or to the purification procedure because (i) naive cells alone and a 1:1 mixture of naive plus memory cells proliferated to the same extent in response to antigen and (ii) unpurified memory cells also failed to proliferate (data not shown). It was only when IL-15 was added that memory cells proliferated (Fig. 4C). When peptide was added in addition to IL-15, [3H]thymidine incorporation was markedly reduced, perhaps because many antigen-stimulated memory cells died before they could proliferate (see below). The naive cells, in contrast, responded only weakly to IL-15 alone, but their response was strongly enhanced by the combination of IL-15 and peptide.
Figure 4.
Proliferation of memory and naive cells in vitro. (A) Comparison of the proliferation of purified memory and naive cells by [3H]thymidine incorporation. (B and C) Proliferation under different conditions. Cells were stimulated with SYRGL in the presence or absence of IL-2 (100 units/ml) or human recombinant IL-15 (100 ng/ml), or with Con A (2 μg/ml) or PMA plus ionomycin (25 ng/ml and 0.5 μM, respectively). Naive cells (N) are shown in hatched columns and memory cells (M) in open columns. (D) Apoptosis of memory cells following antigen stimulation. Memory cells were incubated with SYRGL and irradiated B6 splenocytes as in Fig. 2. After 3-hr, cells were stained with Abs to the 2C TCR, CD8, and with Annexin V. The Annexin V expression was gated on 1B2+CD8+, propidium iodide-negative cells. Peak 1, unstimulated cells at time zero; peak 2, unstimulated cells at 3 hr; and peak 3, antigen-stimulated cells at 3 hr.
To investigate further the curious proliferative behavior of memory 2C cells in vitro, memory and naive cells were incubated with SYRGL plus B6 splenocytes and examined by flow cytometry after 3, 6, 12, 24, 48, and 72 hr. No differences were detected in the expression of Fas, Fas ligand, and cytolytic T lymphocyte-associated antigen 4 (CTLA-4) (data not shown). However, when stained with Annexin V, which binds to phosphotidylserine translocated to the outer membrane of apoptotic cells (28), the majority of memory cells were Annexin V-positive (and propidium iodide-negative) 3 hr after stimulation, whereas most naive cells remained negative at all time points analyzed (Fig. 4D). Consistent with Annexin V staining, >85% of memory cells died within the first 24 hr after stimulation and very few were still alive at 72 hr.
Memory T Cell Proliferation in Vivo.
Approximately 1 × 105 memory or naive cells were injected i.v. into nonirradiated B6 mice, and 3 days later the recipients were immunized with SYRGL in Freund’s adjuvant. Draining lymph nodes of memory and naive cell recipients contained detectable 2C cells only if the recipients had been immunized (Fig. 5A), indicating antigen-stimulated expansion of both cell populations in vivo. To verify that the apparent increase in memory cell number was a result of proliferation and not a redistribution of cells, the recipients were treated with BrdUrd before and after immunization. After 3 and 5 days, almost all 1B2+CD8+ cells were BrdUrd positive, confirming that memory (as well as naive) cells had proliferated (Fig. 5A and data not shown). The total number of 2C cells recovered on day 3 was about 3-fold lower in recipients of memory cells than in recipients of naive cells (3 × 104 vs. 1 × 105, P < 0.005, Fig. 5B), but by day 5, the total number of 2C cells was about the same in recipients of either cell type. Moreover, at day 5, the intensity of BrdUrd staining (geometric mean fluorescence values) was actually higher in the proliferating memory cell population (150 vs. 100, P < 0.025, Fig. 5A), suggesting that whereas fewer memory cells initially divided, those that did proliferated more rapidly than naive cells.
Figure 5.
Proliferation of memory T cells in vivo. (A) Memory or naive cells were transferred into nonirradiated B6 recipients and immunized 3 days later. Mice were injected i.p. with BrdUrd 5 hr before immunization and were maintained on BrdUrd in drinking water. Three and 5 days following immunization, cells from draining lymph nodes (axial, lateral, and inguinal) were stained with Abs to TCR, CD8, and BrdUrd to determine the number and percentage of 1B2+CD8+ cells that incorporated BrdUrd (see arrow). Specificity of the BrdUrd stain is shown by its inhibition by 0.1 mM soluble BrdUrd (+sBrdUrd). The plots shown are for recipients on day 5 after immunization. (B) Percentages of 2C CD8+ cells (Left) and total numbers (Right) in lymph nodes 3 and 5 days after immunization. The symbols are as follows: naive recipients with immunization (□) or without immunization (■); memory recipients with immunization (○) or without immunization (●). Each symbol represents an individual mouse. Horizontal lines are mean values.
DISCUSSION
To characterize the functional properties of memory T cells it is useful to have available for comparison adequate numbers of homogeneous naive, memory, and effector cells, all expressing the same TCR. To this end we used transgenic mice expressing the 2C TCR as donors and modified the adoptive transfer procedure developed by Kearney et al. (21) by using RAG1−/− instead of normal mice as recipients. Based on the numbers of memory cells generated in immunized recipients of 2C cells and on previous reports (21, 29), we estimate that at least 10 times more memory cells are produced in RAG1−/− recipients than in normal recipients, probably because antigen-activated T cells could expand in the RAG1−/− mice without competition from other lymphocytes. Because both the donor and recipient mice were RAG1−/−, all memory cells expressed the same TCR and could be isolated without contamination by other T cells.
Memory cells uniformly expressed high levels of CD44, CD11A and Ly-6C, and low levels of CD62L as variably reported previously (16, 17, 29, 30). They were larger and responded faster than naive cells to antigen, especially in down-regulating TCR and secreting IFN-γ (Figs. 1 and 2). Because the levels of TCR and CD8 coreceptor on both populations were similar, this difference suggests that memory and naive cells differ quantitatively, and perhaps qualitatively, in the components needed for TCR endocytosis (31). In contrast to effector cells (18, 19, 32), memory cells did not express CD25 or CD69 or secrete IFN-γ or IL-2 in the absence of antigen stimulation (Figs. 1 and 2), indicating that they were not chronically stimulated by persistent antigen. Antigen persistence was also most unlikely because SYRGL peptide could be detected in serum only for the first 2 days after immunization but not thereafter (<1 × 10−13 M, data not shown). In addition, the memory cells survived for at least 6 weeks after transfer into nonirradiated, nonimmunized B6 recipients (data not shown). However, like effector cells, the memory cells contained perforin and were able to lyse target cells without antigenic restimulation and also in the presence of a protein synthesis inhibitor (Fig. 3). Even 1 year after initial immunization, the memory cells were able to lyse target cells ex vivo (data not shown). Thus, in contrast to the notion that the cytolytic activity of memory cells requires antigen induction (2, 3), memory cells are poised to immediately lyse target cells in vivo.
Despite their rapid responses to TCR-mediated stimulation, memory 2C cells proved surprisingly incapable of proliferating in vitro in response to antigens (syngeneic and allogeneic), or PMA plus ionomycin, or Con A (Fig. 4). The defect was not because of induction of anergy, for unlike anergic T cells (33), memory 2C cells could respond to antigen by expressing CD25 and secreting IL-2 (Figs. 1 and 2). They also did not secrete IL-10 on antigen stimulation (data not shown), as reported for tolerized T cells (34). The only factor that provoked memory cell proliferation in vitro was recombinant IL-15 (Fig. 4), consistent with the up-regulation of the IL-15 receptor on memory cells (increased expression of the IL-2Rβ but not α subunit; Fig. 1B and ref. 35). Memory cells also proliferated in vivo in response to antigenic peptide (in adjuvant) (Fig. 5). It may be that a factor, possibly IL-15, is required for memory cell proliferation and that it was absent from the in vitro assays but was available in the immunized mice, perhaps because of an “adjuvant” effect on dendritic cells and macrophages. In the absence of this factor in vitro, activation of memory 2C cells appears to have led to apoptosis (Fig. 4D). Although the underlying mechanisms remain to be elucidated, taken together the observations show that naive and memory 2C cells differ profoundly in the conditions required for proliferation and in susceptibilities to apoptosis.
The functional properties of memory cells point to the advantages these cells have over naive cells in providing protection against infections with viruses or other intracellular microbes. If infection is initiated when memory cells are already present (as a result, say, of previous infection with the same organism or vaccination) these cells are capable of immediately mounting a cytolytic attack on infected cells and secreting IFN-γ and IL-2. Naive cells, in contrast, have to undergo proliferation and differentiation into effector cells, a process that often takes many days during which infection progresses. And even though memory cells appear to have a greater propensity to undergo antigen-driven cell death, the surviving cells can proliferate and probably maintain the memory population. These properties suggest that current efforts to develop vaccines for eliciting CD8 T cell responses should focus on immunization strategies and immunogens that enhance the generation and survival of memory T cells.
Acknowledgments
We thank Drs. D. Loh, D. Kranz, S. Tonegawa, and J. Delaney for 2C transgenic mice and RAG1−/− mice; K. Okumura and H. Yagita for anti-perforin Ab; D. Kranz for anti-CD11A Ab; and P. Henkart and members of the Chen and Eisen laboratories for helpful discussion and technical assistance, particularly C. McKinley . This work was supported in part by National Institutes of Health Grants AI44478 (to J.C.) and AI44477 and CA60686 (to H.N.E.). B.K.C. was supported by a National Institutes of Health training grant (AI07463) and S.S. by Mitsubishi Chemical Corporation.
ABBREVIATIONS
- TCR
T cell receptor
- IL-2R
IL-2 receptor
- RAG1
recombination activating gene-1
- APC
antigen presenting cells
- CTL
cytotoxic T lymphocyte
- PMA
phorbol 12-myristate 13-acetate
References
- 1.Zinkernagel R M, Bachmann M F, Kündig T M, Oehen S, Pircher H, Hengartner H. Annu Rev Immunol. 1996;14:333–367. doi: 10.1146/annurev.immunol.14.1.333. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 3.Sprent J. Curr Opin Immunol. 1997;9:371–379. doi: 10.1016/s0952-7915(97)80084-2. [DOI] [PubMed] [Google Scholar]
- 4.Eisen H N. Harvey Lect. 1966;60:1–34. [PubMed] [Google Scholar]
- 5.Steiner L A, Eisen H N. J Exp Med. 1967;126:1185–1205. doi: 10.1084/jem.126.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kocks C, Rajewsky K. Annu Rev Immunol. 1989;7:537–539. doi: 10.1146/annurev.iy.07.040189.002541. [DOI] [PubMed] [Google Scholar]
- 7.Weigert M G, Cesari I M, Yonkovich S J, Cohn M. Nature (London) 1970;228:1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- 8.Griffiths G M, Berek C, Kaartinen M, Milstein C. Nature (London) 1984;312:271–275. doi: 10.1038/312271a0. [DOI] [PubMed] [Google Scholar]
- 9.Rajewsky K. Nature (London) 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- 10.Swain S L. Immunity. 1994;1:543–552. doi: 10.1016/1074-7613(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 11.Hou S, Hyland L, Ryan K W, Portner A, Doherty P C. Nature (London) 1994;369:652–654. doi: 10.1038/369652a0. [DOI] [PubMed] [Google Scholar]
- 12.Lau L L, Jamieson B D, Somasundaram T, Ahmed R. Nature (London) 1994;369:648–652. [Google Scholar]
- 13.Sprent J, Tough D F. Science. 1994;265:1395–1400. doi: 10.1126/science.8073282. [DOI] [PubMed] [Google Scholar]
- 14.Bruno L, Kirberg J, von Boehmer H. Immunity. 1995;2:37–43. doi: 10.1016/1074-7613(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 15.McHeyzer-Williams M G, Davis M M. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 16.Pihlgren M, Dubois P M, Tomkoiak M, Sjogren T, Marvel J. J Exp Med. 1996;184:2141–2151. doi: 10.1084/jem.184.6.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmermann C, Brduscha-Riem K, Blaser C, Zinkernagel R M, Pircher H. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanchot C, Lemonnier F A, Pérarnau B, Freitas A A, Rocha B. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- 19.Lalvani A, Brookes R, Hambleton S, Britton W J, Hill A V S, McMichael A J. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markiewicz M A, Girao C, Opferman J T, Sun J, Hu Q, Agulnik A A, Bishop C E, Thompson C B, Ashton-Rickardt P G. Proc Natl Acad Sci USA. 1998;95:3065–3070. doi: 10.1073/pnas.95.6.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kearney E R, Pape K A, Loh D Y, Jenkins M K. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 22.Kranz D M, Sherman D H, Sitkovsky M V, Pasternack M S, Eisen H N. Proc Natl Acad Sci USA. 1984;81:573–577. doi: 10.1073/pnas.81.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russell J H, Loh D Y. Nature (London) 1988;335:271–274. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 24.Sykulev Y, Brunmark A, Tsomides T J, Kageyama S, Jackson M, Peterson P A, Eisen H N. Proc Natl Acad Sci USA. 1994;91:11487–11491. doi: 10.1073/pnas.91.24.11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Udaka K, Wiesmuller K H, Kienle S, Jung G, Walden P. J Immunol. 1996;157:670–678. [PubMed] [Google Scholar]
- 26.Tough D F, Sprent J. J Exp Med. 1994;179:1127–1135. doi: 10.1084/jem.179.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawasaki A, Shinkai Y, Kuwana Y, Furuya A, Iigo Y, Hanai N, Itoh S, Yagita H, Okumura K. Int Immunol. 1990;2:677–684. doi: 10.1093/intimm/2.7.677. [DOI] [PubMed] [Google Scholar]
- 28.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 29.Kedl R M, Mescher M F. J Immunol. 1998;161:674–683. [PubMed] [Google Scholar]
- 30.Walker P R, Ohteki T, Lopez J A, MacDonald H R, Maryanski J L. J Immunol. 1995;155:3443–3452. [PubMed] [Google Scholar]
- 31.Viola A, Lanzavecchia A. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]
- 32.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J D, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz R H. Curr Opin Immunol. 1997;9:351–357. doi: 10.1016/s0952-7915(97)80081-7. [DOI] [PubMed] [Google Scholar]
- 34.Tanchot C, Guillaume S, Delon J, Bourgeois C, Franzke A, Sarkhan A, Trautmann A, Rocha B. Immunity. 1998;8:581–590. doi: 10.1016/s1074-7613(00)80563-4. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Sun S, Hwang I, Tough S F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 36.Kataoka T, Togashi K, Takayama H, Takaku K, Nagai K. Immunology. 1997;91:493–500. doi: 10.1046/j.1365-2567.1997.00257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]