Abstract
We compared the efficiency of transduction by an HIV-1-based lentiviral vector to that by a Moloney murine leukemia virus (MLV) retroviral vector, using stringent in vitro assays of primitive, quiescent human hematopoietic progenitor cells. Each construct contained the enhanced green fluorescent protein (GFP) as a reporter gene. The lentiviral vector, but not the MLV vector, expressed GFP in nondivided CD34+ cells (45.5% GFP+) and in CD34+CD38− cells in G0 (12.4% GFP+), 48 hr after transduction. However, GFP could also be detected short-term in CD34+ cells transduced with a lentiviral vector that contained a mutated integrase gene. The level of stable transduction from integrated vector was determined after extended long-term bone marrow culture. Both MLV vectors and lentiviral vectors efficiently transduced cytokine-stimulated CD34+ cells. The MLV vector did not transduce more primitive, quiescent CD34+CD38− cells (n = 8). In contrast, stable transduction of CD34+CD38− cells by the lentiviral vector was seen for over 15 weeks of extended long-term culture (9.2 ± 5.2%, n = 7). GFP expression in clones from single CD34+CD38− cells confirmed efficient, stable lentiviral transduction in 29% of early and late-proliferating cells. In the absence of growth factors during transduction, only the lentiviral vector was able to transduce CD34+ and CD34+CD38− cells (13.5 ± 2.5%, n = 11 and 12.2 ± 9.7%, n = 4, respectively). The lentiviral vector is clearly superior to the MLV vector for transduction of quiescent, primitive human hematopoietic progenitor cells and may provide therapeutically useful levels of gene transfer into human hematopoietic stem cells.
Gene therapy using human hematopoietic stem cells (HSC) has great theoretical appeal as an approach to many genetic and acquired diseases affecting the hematopoietic and immune systems. However, progress in the field has been blocked by the fact that levels of gene transfer into human long-term repopulating cells are too low for any likely therapeutic benefit (1–5). The reason for the disappointingly low levels of transduction is believed to lie in certain incompatible features of the vectors used and the HSC that they target. Vectors for hematopoietic gene therapy have until now been based on the Moloney murine leukemia virus (MLV) and are thus unable to infect and integrate into nondividing cells (6). Most HSC are in a quiescent state (7), are relatively slow to respond to stimulation (8–12), and, when induced to divide, tend to lose long-term repopulating capacity (12–17). In addition, the relative paucity of viral receptors on the surface of HSC may limit binding of virus and further prevent efficient gene transfer (18, 19).
Recent incremental improvements in MLV retroviral-mediated gene transfer into HSC have been achieved by using gibbon ape leukemia virus (GALV) pseudotypes, “mobilized bone marrow,” recombinant fibronectin support, new cytokines (Flt-3 ligand, thrombopoietin), and manipulation of cell cycle kinetics (14, 20–23). Combinations of these techniques have resulted in modest, yet significant, increases in gene marking in primate stem cell transplant models. However, higher levels of gene transduction of HSC are likely to be needed for applications to many genetic diseases and AIDS.
Recent reports show that vectors derived from the HIV-1 lentivirus can transduce a variety of nondividing human cells, including neurons, macrophages, hepatocytes, and cardiac myocytes (24–32). The nuclear localization signals present in HIV allow entry of virus through the intact nuclear membrane of nondividing cells (33). Pseudotyping of lentivirus vector with the vesicular stomatitis virus (VSV) envelope G glycoprotein allows virus particles to bind nonspecifically to membrane phospholipid of target cells rather than relying on specific receptor binding (34). Thus, lentiviral vectors pseudotyped with VSV offer a potential solution to the dual problems of quiescence and low viral receptor expression inherent in transduction of HSC with MLV.
We show that lentiviral vectors, but not MLV vectors, can transduce nondivided hematopoietic progenitors and CD34+CD38− cells in G0 cell cycle status. Using stringent long-term culture (LTC) and single-cell assays, we show that lentiviral vectors are able to provide efficient, stable transduction in primitive, quiescent human progenitors normally resistant to transduction with MLV.
MATERIALS AND METHODS
Production and Characterization of Vectors.
The MLV retroviral vector, MLV-Neo-CMV-GFP (35), and the lentiviral vector, pHR′-CMV-GFP (24, 27), were constructed as described and contained the enhanced green fluorescent protein (GFP; CLONTECH) reporter gene with the internal human cytomegalovirus (CMV) immediate-early promoter. The plasmid pHIT60 (36) was used to express the MLV gag-pol proteins. The plasmid pCMVΔR8.91 (28) was used to express HIV-1 gag, pol, tat, and rev proteins to package lentiviral vectors without the accessory genes vif, vpu, vpr, and nef. An integration-defective lentiviral vector was generated as described (24, 26). The plasmid pMD.G (24) was used to express the VSV envelope G glycoprotein from the CMV immediate-early promoter.
The lentiviral vector, the integrase-defective lentiviral vector, and the MLV vector were all pseudotyped with the VSV envelope (lenti/VSV, lenti(int−)/VSV, and MLV/VSV, respectively). VSV-pseudotyped vectors were produced by transient three-plasmid transfection as previously described (24) with 2 μg of the pMD.G envelope plasmid and 10 μg of the various packaging and vector plasmids. Sodium butyrate (Sigma) induction was performed as described (36). Preparations of VSV-pseudotyped vectors were concentrated by ultracentrifugation (37). Another MLV vector, MND-GFP-SV40-Neo, was produced in a GALV pseudotype (MLV/GALV) from the stable packaging cell line PG13 (38).
Titers of all vector preparations were determined by transducing 293 cells (American Type Culture Collection) with serial dilutions of vector supernatants, followed by fluorescence-activated cell sorting (FACS) analysis 2 days later. Initial titers were 0.5 × 106 to 10 × 106 infectious units (i.u.)/ml for the lenti/VSV, lenti(int−)/VSV, MLV/VSV, and MLV/GALV vectors. After ultracentrifugation, the titers of the VSV pseudotyped lentiviral and MLV vectors were 1–15 × 108 i.u./ml.
All lentiviral vector preparations were tested for the presence of replication-competent retrovirus (RCR) by infection of phytohemagglutinin (PHA)-stimulated human peripheral blood mononuclear cells, followed by culture for 2 weeks and then assay of culture medium for p24 gag by ELISA (Coulter). No vector preparations contained detectable RCR.
Cell Sources and Isolation.
Mononuclear cells from fresh bone marrow and umbilical cord blood were obtained as previously described under protocols approved by the Committee on Clinical Investigations (39). FACS was performed on a FACSVantage [Becton Dickinson Immunocytometry Systems (BDIS)] using Lysys II software (BDIS). CD34+CD38− cells were defined as previously described (39). CD34+ cells were defined as either cells with high CD34 expression alone, or in some experiments cells with high CD34 and CD38 expression (CD34+CD38+), as previously described (39).
Multiparameter Cell Cycle Analysis.
CD34+ cells (3.4 × 106) were transduced in 6 ml of X-vivo 15 (BioWhittaker) containing 0.5 ng/ml interleukin (IL)-3 and 25 units/ml Flt-3 ligand in flasks coated with the recombinant fibronectin fragment CH-296 (Takara Shuzo, Otsu, Shiga, Japan). Viral supernatants were added daily to the cells on 3 consecutive days. lenti/VSV transduction was performed with the supernatant concentration at 1 × 107 i.u./ml [equivalent multiplicity of infection (moi) = 18] each day. MLV/GALV transduction was performed with the supernatant concentration at 5 × 105 i.u./ml (moi = 0.9) each day. Mock-infected (nontransduced) controls were handled exactly the same, but with no vector supernatants added to the CD34+ cells.
Cell cycle activity and transgene expression were analyzed 24 hr after the third addition of virus by employing a modification of a previously described flow cytometric procedure (40). The revised protocol allows the use of an additional fluorochrome and is hence referred to as five-color SID (surface, intracellular, DNA) staining. Specifically, cells were labeled with anti-CD34-biotin (Coulter), streptavidin-Red613 (GIBCO/BRL), and anti-CD38-APC (BDIS; APC is allophycocyanin). Cells were then fixed in 0.5% formaldehyde (Polysciences) and permeabilized with 0.1% Triton X-100 (Amersham), Ki-67-PE (Dako; PE is phycoerythrin) was added, and finally 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes) was added (2 μM) to stain for DNA content. Analysis was performed on a Becton Dickinson FACSVantage flow cytometer. DNA level was measured by excitation of DAPI from the 350-nm line, PE and Red613 were excited by the 488-nm line, and APC by the 633-nm line. Transduction of primitive cells was detected by the presence of GFP (488-nm laser). The nuclear antigen Ki-67 was used as a marker of cell cycle entry (41, 42) and was used with DAPI to delineate G0 and G1 populations and cycling (S, G2, M) stages.
Analysis of Transgene Expression in Nondivided Cells.
CD34+ cells (1–2 × 106) were incubated in 2 ml of diluent with the red fluorescent membrane marker PKH26 (final concentration 2 × 10−6 M; Sigma) for 1.5 min at room temperature. Then 10% fetal calf serum (FCS; Summit Biotechnology, Fort Collins, CO) was added to block further adsorption of dye and the cells were washed four times. A narrow band of viable PKH26-bright (nondivided) cells was isolated by FACS after overnight culture on CH-296 and immediately transduced once with lenti/VSV, lenti(int−)/VSV, or MLV/GALV on CH-296 in X-vivo 15 with 2.5 ng/ml IL-3, 8.25 units/ml IL-6, and 12.5 ng/ml Steel factor (SF). After 24 hr, cells were washed twice and incubated in the absence of vector for a further 24 hr on fresh CH-296 in the same culture conditions. A total of 48 hr after transduction, cells were washed and incubated with anti-CD34-APC (BDIS). Cells were analyzed by FACS for simultaneous PKH26, GFP, and CD34 expression. PKH26 fluorescence in nondivided cells remained identical between the first isolation and the time of final analysis. The width of the PKH26 band set for each generation was identical.
Transduction of Hematopoietic Cells Before LTC.
Hematopoietic cell transductions were performed in plates coated with CH-296. CD34+ cells (1–10 × 104 per plate) were transduced in 2–4 ml of diluent in 35-mm plates (Costar). CD34+CD38− cells (3–30 × 102 per well) were transduced in 200 μl of X-vivo 15 in 96-well plates (Costar). Transductions were performed with lenti/VSV, lenti(int−)/VSV, and MLV/VSV, using equivalent supernatant concentrations of 3–15 × 106 i.u./ml (moi = 1,000–3,000). Transductions with MLV/GALV were performed with supernatant concentrations of 5–18 × 105 i.u./ml (moi = 100–300).
Transductions carried out in the presence of growth factors (5 ng/ml IL-3, 16.5 units/ml IL-6, and 25 ng/ml SF) were performed with one addition of viral supernatant per day for either 1 day or for 3 consecutive days. As there was no significant difference in the results with the two protocols, the data were grouped together. Transductions carried out in the absence of growth factors were performed with one addition of viral supernatant for 12 hr immediately after isolation. After transduction, cells were placed in LTC for serial analysis.
Analysis of LTCs.
CD34+CD38− cells were cultured long-term (approximately 100 days) on irradiated allogeneic human bone marrow stroma in long-term bone marrow culture (LTBMC) medium (39). Every 2–3 weeks, nonadherent cells were analyzed by FACS for transgene expression or were plated in methylcellulose medium to measure colony-forming units (CFU) (39). CFU generated from the LTC were individually analyzed for GFP expression by using a fluorescent microscope, and they were isolated from the methylcellulose and analyzed by polymerase chain reaction (PCR) for detection of vector DNA, as described below.
Clonal Analysis of Transduced Single CD34+CD38− Cells.
CD34+ cells were transduced with lenti/VSV, MLV/VSV, or MLV/GALV on CH-296 in the presence of IL-3, IL-6, and SF for 1 day. After transduction, cells were washed three times and incubated with anti-CD34-PE (BDIS) and anti-CD38-APC. Single CD34+CD38− cord blood cells were isolated and plated in each well of a 96-well plate by FACS using the automated cell deposition unit (ACDU) device on the FACSVantage and grown on irradiated human stroma in LTBMC medium. Wells were observed every 7 days for the first appearance of clonal proliferation as described (39). GFP expression of clones was assessed by fluorescence microscopy and also by FACS analysis.
Detection of Vector DNA.
The presence of vector sequences in extracted DNA from bulk LTC was determined by using semiquantitative DNA PCR and Southern blot analysis for GFP. A clone of 293 cells with a single integrated copy of the pHR′-CMV-GFP vector was used to construct a standard curve for GFP normalized against human β-actin (D.B.K., unpublished work). Vector DNA in individual CFU was measured by PCR of whole cell lysate followed by Southern blot detection of GFP transgene.
Statistical Analyses.
Statistical analyses of vector-transduced CD34+ and CD34+CD38− cells used a two-sample paired Student’s t test assuming unequal variances as the number of experiments varied. Vector expression in clones from single CD34+CD38− cells was analyzed by using a paired two-sample Student’s t test.
RESULTS
Transduction of CD34+CD38− Cells in G0 by Lentiviral Vectors.
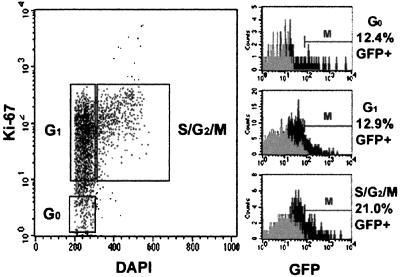
To determine if an HIV-1-based lentiviral vector pseudotyped with VSV was capable of transducing CD34+CD38− cells in G0 phase of the cell cycle, five-color SID staining was performed. Cells were transduced in conditions designed to minimize cell cycling. 12.4% of CD34+CD38− cells in G0, 12.9% in G1, and 21% in S/G2/M phase were GFP+ 24 hr after exposure to lenti/VSV, showing similar levels of transduction in cells at different stages of the cell cycle (Fig. 1). In contrast, exposure to MLV/GALV resulted in barely detectable levels of GFP expression in CD34+CD38− cells in G0 and G1 phase. However, once the cells entered S/G2/M phase, GFP expression from MLV/GALV was detectable in 7.2% of CD34+CD38− cells. These findings indicate that transgene expression in noncycling CD34+CD38− cells is possible with lentiviral vectors but not with MLV vectors.
Figure 1.
Lentiviral vector expression in CD34+CD38− cells defined according to cell cycle status. Cell cycle analysis of cells derived from the CD34+CD38− gate (not shown) and simultaneous GFP expression of G0, G1, and S/G2/M populations are shown. Black histogram = transduced cells, gray histogram = nontransduced cells (negative control). Percent GFP+ was calculated by subtracting the negative control cells falling within the marker region. A second experiment yielded similar results.
Transduction of Nondivided CD34+ Cells by Lentiviral Vectors.
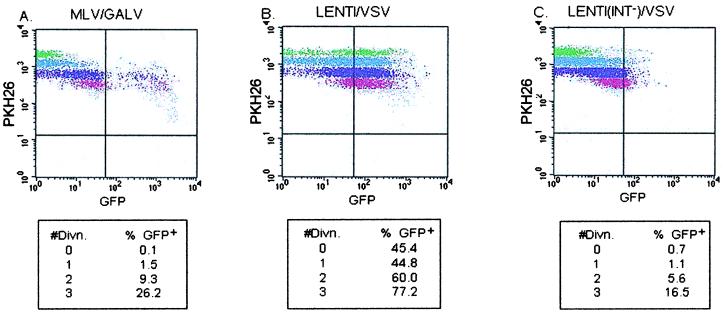
To determine if lentiviral vectors were capable of transducing hematopoietic progenitors prior to cell division, CD34+ cells were stained with PKH26. MLV/GALV was unable to transduce CD34+ cells prior to cell division, but it was able to transduce cells after the first, second, and third divisions at progressively increasing levels (Fig. 2A). In contrast, both nondivided and divided populations of CD34+ cells transduced with lenti/VSV expressed GFP at high levels, with nondivided, first, second, and third divisions at 45.5%, 44.8%, 56%, and 77.2% GFP+, respectively (Fig. 2B).
Figure 2.
Vector expression in nondivided and divided CD34+ cells. PKH26 fluorescence is brightest in nondivided cells and decreases with each cell division. Cells that retain their original level of PKH26 fluorescence have not divided. Shown is GFP expression of four generations of CD34+-gated cells. Each generation is represented by a different color in the dot plots, green indicating nondivided cells. (A) MLV/GALV. (B) lenti/VSV. (C) lenti(int−)/VSV. (Lower) Percent GFP+ cells is shown for each generation.
To determine whether GFP expression indicated integration of lentivirus vector, CD34+ cells were also transduced with lenti(int−)/VSV. As shown in Fig. 2C, lenti(int−)/VSV resulted in 0.7% GFP expression in nondivided CD34+ cells. In a second experiment (not shown), 38.5% of nondivided cells were GFP+. The average level of GFP expression from the integrase-defective vector was significantly lower than that expressed from the wild-type vector. These results imply that early scoring of transgene expression in lenti/VSV-transduced cells can detect pseudotransduction and/or transient expression from nonintegrated vector DNA, and in the absence of other data may not be fully predictive of stable transduction.
To determine the stability of GFP expression from lenti/VSV- and lenti(int−)/VSV-transduced CD34+ cells, nondivided CD34+ cells were isolated and analyzed after a further 7 days of in vitro culture. Although the nondivided CD34+ cells transduced by lenti/VSV were originally 45.5% GFP+, expression fell to 3.7% at day 7. GFP was not detected in lenti(int−)/VSV-transduced CD34+ cells after 7 days of culture. Comparable data were obtained in a second experiment.
Transduction of CD34+ Cells by Lentiviral Vectors.
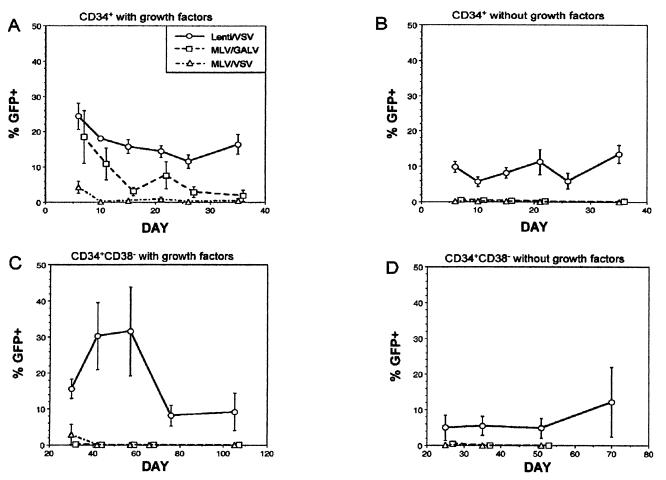
Since GFP protein was detectable in CD34+ cells exposed to nonintegrating lentiviral vector, long-term assays were required to evaluate stable integration of lentiviral vectors in human hematopoietic cells. As shown in Fig. 3A, all three vectors (lenti/VSV, MLV/GALV, and MLV/VSV) were able to transduce CD34+ cells, a largely cycling, committed progenitor population, in the presence of growth factors. At 6 days after transduction (in most cases with a single exposure to virus), GFP expression with lenti/VSV was 24.4 ± 3.7% (n = 11), with MLV/GALV it was 18.5 ± 7.4% (n = 6), and with MLV/VSV it was 4.3 ± 1.7% (n = 5) (P = 0.25 for MLV/GALV and P = 0.0003 for MLV/VSV, compared with lenti/VSV). The reason for the low efficiency of transduction of CD34+ cells with MLV/VSV, despite high titers on 293 cells, is unclear but may be inefficient MLV vector processing after endocytosis of the VSV-pseudotyped particle.
Figure 3.
Vector expression in transduced CD34+ and CD34+CD38− cells. FACS analysis was performed on LTC of transduced cells at the time points indicated. Mean ± SEM for all experiments is shown. For all panels, the legend of vectors is as follows: lenti/VSV = solid line with ○; MLV/GALV = dashed line with □, and MLV/VSV = broken-dashed line with ▵. (A) CD34+ cells transduced with growth factors by lenti/VSV (n = 11), MLV/GALV (n = 6), and MLV/VSV (n = 5). (B) CD34+ cells transduced in the absence of growth factors by lenti/VSV (n = 11), MLV/GALV (n = 8), and MLV/VSV (n = 7). (C) CD34+CD38− cells transduced with growth factors by lenti/VSV (n = 7), MLV/GALV (n = 4), and MLV/VSV (n = 4). (D) CD34+CD38− cells transduced in the absence of growth factors by lenti/VSV (n = 4), MLV/GALV (n = 4), and MLV/VSV (n = 2).
Only lenti/VSV produced relatively stable GFP expression (16.4 ± 2.8%) over 5 weeks of culture. GFP expression from cells transduced by either MLV/VSV or MLV/GALV fell to approximately 1% by 5 weeks (P = 0.002 for MLV/GALV and P = 0.003 for MLV/VSV, compared with lenti/VSV). CD34+ cells exposed to lenti(int−)/VSV showed no GFP expression in LTC.
Growth factors (e.g., IL-3, IL-6, and SF) are routinely used to induce CD34+ cell cycling to achieve MLV transduction but may also induce differentiation and loss of long-term engrafting capacity (43, 44). Therefore, we determined whether lentiviral vectors could transduce CD34+ cells in the absence of growth factors with brief (12-hr) exposure to virus. As shown in Fig. 3B, the lentiviral vector, but not the MLV vectors, was able to transduce CD34+ cells in these conditions. At 6 days after transduction, GFP expression with lenti/VSV was 9.9 ± 1.6% (n = 11), with MLV/GALV it was 0.6 ± 0.2% (n = 8), and with MLV/VSV it was 0.2 ± 0.1% (n = 7) (P = 0.0002 for MLV/GALV and P = 0.0001 for MLV/VSV, compared with lenti/VSV). Once again, lenti/VSV produced stable GFP expression of 13.5 ± 2.5% for 5 weeks of culture, whereas the two MLV vectors produced no long-term expression (P = 0.0004 for either MLV/GALV or MLV/VSV, compared with lenti/VSV).
Transduction of CD34+CD38− Cells by Lentiviral Vectors.
We next studied lentiviral transduction of CD34+CD38− cells, a more primitive progenitor population almost entirely in G0 which contains HSC and is relatively resistant to MLV vector transduction (39, 40). As shown in Fig. 3C, the lentiviral vector was able to efficiently transduce CD34+CD38− cells at levels similar to the level for CD34+ cells, while the MLV vectors produced low to undetectable levels of GFP early in culture. At 30 days after transduction, GFP expression with lenti/VSV was 15.6 ± 2.7% (n = 7), with MLV/GALV it was 0.1 ± 0% (n = 4), and with MLV/VSV it was 2.9 ± 2.8% (n = 4) (P = 0.002 for MLV/GALV and P = 0.01 for MLV/VSV, compared with lenti/VSV). Only lenti/VSV produced stable GFP expression (9.2 ± 5.2%) in extended LTC (ELTC) (P = 0.03 for either MLV/GALV or MLV/VSV, compared with lenti/VSV). Semiquantitative PCR analysis of DNA from nonadherent cells from LTC demonstrated that the lentiviral vector had efficiently transduced primitive progenitors (0.2–2.4 vector copies per cell at weeks 6–8) and confirmed the absence of vector DNA in LTC from CD34+CD38− cells exposed to MLV.
ELTC-initiating cells (ELTC-IC) are a subpopulation of CD34+CD38− cells that are quiescent and pluripotent and proliferate late in culture, generating CFU beyond 60 days and forming cobblestone areas after 30 days (11, 39, 45). As shown in Table 1, the ability of lenti/VSV to transduce ELTC-IC was confirmed by the presence of GFP+ CFU at 6, 8, and 10 weeks of ELTC. PCR of individual CFU arising after 6 weeks of LTC confirmed the presence of the transgene in lentiviral vector-transduced CD34+CD38− cells. Thus, lentiviral vectors and not MLV vectors are able to transduce ELTC-IC (11).
Table 1.
GFP transgene expression and vector DNA detection from lenti/VSV-transduced CFU
| Exp. | Week | % GFP+ | % DNA+ |
|---|---|---|---|
| 1 | 8 | 35 (6/17) | 47 (8/17) |
| 2 | 6 | 50 (17/34) | 91 (31/34) |
| 10 | 96 (46/48) | 94 (45/48) |
CFU arising from LTC at weeks 6–10 were analyzed for GFP expression and vector DNA. In parentheses is the number of GFP+ or DNA+ CFU/the total number of CFU.
To provide the most stringent test of transduction of quiescent cells, CD34+CD38− cells were briefly exposed to virus in the absence of growth factors. As shown in Fig. 3D, only lenti/VSV (n = 4) was able to transduce CD34+CD38− cells without growth factor stimulation (5 ± 3.5% at 25 days) with persistent GFP expression late in culture (12.2 ± 9.7% at 10 weeks). As expected, both MLV/GALV (n = 4) and MLV/VSV (n = 2) were unable to transduce CD34+CD38− cells without growth factors. Again, semiquantitative PCR analysis of DNA from nonadherent cells from LTC demonstrated the high transduction efficiency of CD34+CD38− cells by lentiviral vectors (0.4–1.3 copies per cell at weeks 7–10) and confirmed the absence of vector DNA in CD34+CD38− cells transduced by MLV.
Clonal Analysis of CD34+CD38− Cells Transduced by Lentiviral Vectors.
To analyze the stable transduction of clonogenic CD34+CD38− cells on a single-cell level, CD34+CD38− cells were isolated after one exposure to virus and plated in individual wells. New colonies that appeared each week were scored for GFP expression by fluorescent microscopy and FACS analysis. Late-appearing clones (those appearing after 4 weeks in culture) are the equivalent of ELTC-IC (9, 11). As shown in Table 2, MLV/GALV was able to transduce 2% (2/124) of the total cells that formed colonies, all of which appeared within the first 2 weeks (2/80, or 3%) and thus were generated from early proliferating cells. MLV/VSV was unable to transduce any of the CD34+CD38− cells. In contrast, lenti/VSV was able to transduce 29% (83/285) of the total clones that formed colonies, with comparable transduction efficiencies for early and late-appearing clones. Thus, lenti/VSV provided efficient stable transduction of both proliferating and quiescent primitive CD34+CD38− cells.
Table 2.
Clonal analysis of GFP transgene expression in single transduced CD34+CD38− cells
| Week | MLV/GALV | MLV/VSV | lenti/VSV |
|---|---|---|---|
| 1 and 2 | 2/80 | 0/105 | 49/172 |
| 3 | 0/31 | 0/48 | 25/92 |
| 4 | 0/11 | 0/7 | 8/18 |
| 5 | 0/2 | 0/3 | 1/3 |
| Total | 2/124 (2%)* | 0/163 (0%)† | 83/285 (29%) |
Analysis of GFP expression in clones from single CD34+CD38− cells grown in cobblestone area-forming cell assay. Shown is the number of GFP+ colonies/the total number of colonies (n = 2). ∗, P = 0.03; and
, P = 0.04 compared to lenti/VSV.
DISCUSSION
A major technical problem revealed in all clinical gene therapy trials using MLV vectors has been the ability of MLV to efficiently transduce mature committed human hematopoietic progenitor cells but not pluripotent long-term repopulating HSC (46). Transduction of HSC is necessary to achieve enduring production of genetically corrected hematopoietic and lymphoid cells in a clinical setting. The data presented here demonstrate that lentiviral vectors pseudotyped with the VSV envelope are able to transduce a hematopoietic progenitor population qualitatively different from that transduced by MLV retroviruses. Although both MLV and lentiviral vectors efficiently transduced CD34+ progenitors stimulated to divide during transduction, only lentiviruses could transduce more primitive, quiescent progenitors. The most stringent test of this ability was successful transduction of ELTC-IC, a subpopulation of CD34+CD38− cells that divide late in culture despite continuous cytokine stimulation. The demonstration of the transgene in CFU arising after 60 days of ELTC, and in late-appearing clones derived from single CD34+CD38− cells, proved the stable integration of the lentiviral vector into CD34+CD38− ELTC-IC.
Previous reports on the transduction of human hematopoietic progenitors with lentiviral vectors have used short-term functional assays or immunophenotypic definitions as surrogate markers of HSC (25, 30). These approaches can result in misleading conclusions. Short-term assays of CD34+ cells (e.g., CFU) measure mature progenitors, most of which are cycling and divide rapidly with growth factor stimulation. These cells are readily transduced by MLV vectors, and they have little or no long-term engrafting ability (6, 47–50). Although immunophenotypic definitions have been helpful for the enrichment of HSC, populations such as CD34+CD38− cells are functionally heterogeneous, particularly with respect to cytokine responsiveness and their ability to be transduced (11, 51). By studying CD34+CD38− cells in ELTC, we were able to measure a subpopulation of slowly dividing cells that possess other primitive characteristics expected of HSC, namely tremendous generative capacity (11) and pluripotentiality (45). It is likely that ELTC-IC are a population similar if not identical to the long-term repopulating CD34+CD38− cells measured by two in vivo assays of human HSC, the beige–nude–xid (bnx) and non-obese-diabetic/severe combined immune deficient (NOD/SCID) xenograft models (15, 52). CD34+CD38− cells that repopulate bnx and NOD/SCID mice are also highly resistant to transduction with MLV.
A second problem with using short-term assays for the assessment of stable lentiviral transduction is that transient expression can occur from nonintegrated lentiviral vectors. Pseudotransduction, particularly when using VSV pseudotyped vectors, can also result in transient detection of marker protein (37). This potential for artifact from nonintegrated lentivirus was clearly shown in our studies by short-term transgene expression in up to 38% of nondividing cells with an integrase-defective lentiviral vector, although the average expression level of the transgene was significantly lower than with wild-type vector. The integrase-defective vector was unable to produce stable long-term expression, suggesting that integration and not nuclear localization limits stable transduction of cells prior to cell division. Only integration of vectors into the target cell genome will allow the permanent and enduring clinical benefit desired in clinical trials of HSC gene therapy.
In this report we compared lentivirus with the MLV/GALV retroviral vector in all assays of transduction, as MLV has long been the gold standard in vector technology for HSC. The moi used for lenti/VSV was higher than for MLV/GALV based on titers obtained with short-term assay of 293 cells. However, pseudotransduction and/or transient expression in 293 cells may result in inaccurately high titers with lenti/VSV. It is therefore difficult to directly compare vectors based on moi. Uchida et al. (31) compared lentiviral vectors to MLV vectors in short-term assays and in clonal assay and showed stable integration of lentivirus into CD34+Thy-1+CD38−/lo cells. This study and our own provide the most compelling evidence to date of the superiority of lentiviral vectors pseudotyped with VSV over MLV-based vectors.
The finding that CD34+CD38− cells can be transduced even in the absence of growth factor stimulation and after only brief exposure to lentivirus confirmed that lentiviruses can transduce primitive, quiescent progenitors. Currently, several days of ex vivo stimulation are required to induce cycling for successful transduction of progenitors with MLV, after which much of the long-term repopulating ability is lost (12–14, 16, 17). The ability to transduce HSC without growth factor stimulation and to minimize the time that HSC spend ex vivo has obvious advantages for preservation of stem cell function. Studies analyzing gene transfer into long-term repopulating cells of large animals and using xenograft models to study human long-term repopulating cells will provide further information on the advantages that lentiviruses offer for HSC transduction. Third-generation, self-inactivating HIV-1-based vectors (32) are currently under study with biosafety issues in mind. The findings presented here strongly suggest that lentiviruses may provide the technical leap needed to achieve therapeutic levels of gene transfer into human HSC and justify further intensive investigation into this vector strategy.
Acknowledgments
We thank Inder M. Verma for helpful discussion throughout this project and Lora Barsky and David Bockstoce for flow cytometry. This work was supported by National Institutes of Health Grants R01DK54567, 1P50HL54850, and 5P01CA59318, and a Leukemia Society of America Translational Research Award (G.M.C.). S.S.C. is the recipient of a fellowship from the Childrens Hospital Los Angeles Research Institute. R.S. is the recipient of a Childrens Hospital Los Angeles career fellowship award. D.B.K. is the recipient of an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
ABBREVIATIONS
- MLV
Moloney murine leukemia virus
- GFP
enhanced green fluorescent protein
- LTC
long-term culture
- ELTC
extended LTC
- ELTC-IC
ELTC-initiating cell
- VSV
vesicular stomatitis virus
- GALV
gibbon ape leukemia virus
- i.u.
infectious unit
- moi
multiplicity of infection
- CFU
colony-forming units
- HSC
hematopoietic stem cell
- FACS
fluorescence-activated cell sorting
- IL
interleukin
- SF
Steel factor
- DAPI
4′,6-diamidino-2-phenylindole
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Bordignon C, Notarangelo L D, Nobili N, Ferrari G, Casorati G, Panina P, Mazzolari E, Maggioni D, Rossi C, Servida P, et al. Science. 1995;270:470–475. doi: 10.1126/science.270.5235.470. [DOI] [PubMed] [Google Scholar]
- 2.Kohn D B, Weinberg K I, Nolta J A, Heiss L N, Lenarsky C, Crooks G M, Hanley M E, Annett G, Brooks J S, El-Koureiy A, et al. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kohn D B, Hershfield M S, Carbonaro D, Shigeoka A, Brooks J S, Smogorzewska E M, Barsky L W, Chan R, Burotto F, Annett G, et al. Nat Med. 1998;4:775–780. doi: 10.1038/nm0798-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dunbar C E, Cottler-Fox M, O’Shaughnessy J A, Doren S, Carter C, Berenson R, Brown S, Moen R C, Greenblatt J, Stewart F M, et al. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 5.Hoogerbrugge P M, van Beusechem V W, Fischer A, Debree M, Le Deist F, Perignon J L, Morgan G, Gaspar B, Fairbanks L D, Skeoch C H, et al. Gene Ther. 1996;3:179–183. [PubMed] [Google Scholar]
- 6.Miller D G, Adam M A, Miller A D. Mol Cell Biol. 1990;10:4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones R J, Wagner J E, Celeno P, Zicha M S, Sharkis S J. Nature (London) 1990;347:188–189. doi: 10.1038/347188a0. [DOI] [PubMed] [Google Scholar]
- 8.Ploemacher R E, van der Sluijs J P, Voerman J S A, Brons N H C. Blood. 1989;74:2755–2783. [PubMed] [Google Scholar]
- 9.Ploemacher R E, van der Sluijs J P, van Beurden C A J, Baert M R M, Chan P L. Blood. 1991;78:2527–2533. [PubMed] [Google Scholar]
- 10.Traycoff C M, Kosak S T, Grigsby S, Srour E F. Blood. 1995;85:2059–2068. [PubMed] [Google Scholar]
- 11.Hao Q-L, Thiemann F T, Petersen D, Smogorzewska E M, Crooks G M. Blood. 1996;88:3306–3313. [PubMed] [Google Scholar]
- 12.Gothot A, van der Loo J C M, Clapp D W, Srour E F. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 13.Peters S O, Kittler L W, Ramshaw H S, Quesenberry P J. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 14.Tisdale J F, Hanazono Y, Sellers S E, Agricola B A, Metzger M E, Donahue R E, Dunbar C E. Blood. 1998;92:1131–1141. [PubMed] [Google Scholar]
- 15.Larochelle A, Vormoor J, Hanenberg H, Wang J C Y, Bhatia M, Lapidot T, Moritz T, Murdoch B, Xiao X L, Kato I, et al. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- 16.Harrison D E, Lerner C P, Spooncer E. Blood. 1987;69:1021–1025. [PubMed] [Google Scholar]
- 17.Bodine D M, Crosier P S, Clark S C. Blood. 1991;78:914–920. [PubMed] [Google Scholar]
- 18.Orlic D, Girard L J, Jordan C T, Anderson S M, Cline A P, Bodine D M. Proc Natl Acad Sci USA. 1996;93:11097–11102. doi: 10.1073/pnas.93.20.11097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crooks G M, Kohn D B. Blood. 1993;82:3290–3297. [PubMed] [Google Scholar]
- 20.Kiem H-P, Heyward S, Winkler A, Potter J, Allen J M, Miller A D, Andrews R G. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 21.Dunbar C E, Seidel N E, Doren S, Sellers S, Cline A P, Metzger M E, Agricola B A, Donahue R E, Bodine D M. Proc Natl Acad Sci USA. 1996;93:11871–11876. doi: 10.1073/pnas.93.21.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiem H-P, Andrews R G, Morris J, Peterson L, Heyward S, Allen J M, Rasko J E J, Potter J, Miller A D. Blood. 1998;92:1878–1886. [PubMed] [Google Scholar]
- 23.Dao M A, Nolta J A. Proc Natl Acad Sci USA. 1998;95:13006–13011. doi: 10.1073/pnas.95.22.13006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage F H, Verma I M, Trono D. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 25.Akkina R K, Walton R M, Chen M L, Li Q, Planelles V, Chen I S Y. J Virol. 1996;70:2581–2585. doi: 10.1128/jvi.70.4.2581-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naldini L, Blömer U, Gage F H, Trono D, Verma I M. Proc Natl Acad Sci USA. 1996;93:11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blömer U, Naldini L, Kafri T, Trono D, Verma I M. J Virol. 1997;71:6641–6649. doi: 10.1128/jvi.71.9.6641-6649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zufferey R, Nagy D, Mandel R J, Naldini L, Trono D. Nat Biotechnol. 1997;15:871–876. doi: 10.1038/nbt0997-871. [DOI] [PubMed] [Google Scholar]
- 29.Kafri T, Blömer U, Peterson D A, Gage F H, Verma I M. Nat Gen. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 30.Sutton R E, Wu H T M, Rigg R, Bohnlein E, Brown P O. J Virol. 1998;72:5781–5788. doi: 10.1128/jvi.72.7.5781-5788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uchida N, Sutton R E, Friera A M, He D, Reitsma M J, Chang W C, Veres G, Scollay R, Weissman I L. Proc Natl Acad Sci USA. 1998;95:11939–11944. doi: 10.1073/pnas.95.20.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dull T, Zufferey R, Kelly M, Mandel R J, Nguyen M, Trono D, Naldini L. J Virol. 1998;72:8463–8471. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gallay P, Swingler S, Song J, Busham F, Trono D. Cell. 1995;83:569–576. doi: 10.1016/0092-8674(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 34.Mastromarino P, Conti C, Goldoni P, Hauttecoeur B, Orsi N. J Gen Virol. 1987;68:2359–2369. doi: 10.1099/0022-1317-68-9-2359. [DOI] [PubMed] [Google Scholar]
- 35.Naviaux R K, Constanzi E, Haas M, Verma I M. J Virol. 1996;70:5701–5705. doi: 10.1128/jvi.70.8.5701-5705.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. Nucleic Acid Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu M, Winther B L, Kay M A. J Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins P B, Yu X J, Skelton D M, Pepper K A, Wasserman R M, Zhu L, Kohn D B. J Virol. 1997;71:9466–9474. doi: 10.1128/jvi.71.12.9466-9474.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hao Q-L, Shah A J, Thiemann F T, Smogorzewska E M, Crooks G M. Blood. 1995;86:3745–3753. [PubMed] [Google Scholar]
- 40.Jordan C T, Yamasaki G, Minamoto D. Exp Hematol. 1996;24:1347–1355. [PubMed] [Google Scholar]
- 41.Gerdes J, Schwab U, Lemke H, Stein H. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 42.Gerdes J, Lemke H, Baisch H, Wacker H H, Schwab U, Stein H. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 43.Nolta J A, Kohn D B. Hum Gene Ther. 1990;1:257–268. doi: 10.1089/hum.1990.1.3-257. [DOI] [PubMed] [Google Scholar]
- 44.Nolta J A, Crooks G M, Overell R W, Kohn D B. Exp Hematol. 1992;20:1065–1071. [PubMed] [Google Scholar]
- 45.Hao Q, Smogorzewska E M, Barsky L W, Crooks G M. Blood. 1998;91:4145–4151. [PubMed] [Google Scholar]
- 46.Kohn D B, Nolta J A, Crooks G M. In: Clinical Trials of Gene Therapy Using Hematopoietic Stem Cells. Hematopoietic Cell Transplantation. 2nd Ed. Forman S J, Blume K G, Thomas E D, editors. Boston: Blackwell Scientific; 1999. , in press. [Google Scholar]
- 47.Dick J E, Kamel-Reid S, Murdoch B, Doedens M. Blood. 1991;78:624–634. [PubMed] [Google Scholar]
- 48.Hajihosseini M, Iavachev L, Price J. EMBO J. 1993;12:4969–4974. doi: 10.1002/j.1460-2075.1993.tb06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roe T, Reynolds T C, Yu G, Brown P O. EMBO J. 1993;12:2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Springett G M, Moen R C, Anderson S, Blaese R M, Anderson W F. J Virol. 1989;63:3865–3869. doi: 10.1128/jvi.63.9.3865-3869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brummendorf T H, Dragowska W, Zijlmans J M J M, Thornbury G, Lansdorp P M. J Exp Med. 1998;188:1117–1124. doi: 10.1084/jem.188.6.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dao M A, Shah A J, Crooks G M, Nolta J A. Blood. 1998;91:1243–1255. [PubMed] [Google Scholar]