Abstract
Cancer cells maintain a high glycolytic rate even in the presence of oxygen, a phenomenon first described over 70 years ago and known historically as the Warburg effect. Fructose 2,6-bisphosphate is a powerful allosteric regulator of glycolysis that acts to stimulate the activity of 6-phosphofructo-1-kinase (PFK-1), the most important control point in mammalian glycolysis. The steady state concentration of fructose 2,6-bisphosphate in turn depends on the activity of the enzyme 6-phosphofructo-2-kinase (PFK-2)/fructose-2,6-bisphosphatase, which is expressed in several tissue-specific isoforms. We report herein the identification of a gene product for this enzyme that is induced by proinflammatory stimuli and which is distinguished by the presence of multiple copies of the AUUUA mRNA instability motif in its 3′-untranslated end. This inducible gene for PFK-2 is expressed constitutively in several human cancer cell lines and was found to be required for tumor cell growth in vitro and in vivo. Inhibition of inducible PFK-2 protein expression decreased the intracellular level of 5-phosphoribosyl-1-pyrophosphate, a product of the pentose phosphate pathway and an important precursor for nucleic acid biosynthesis. These studies identify a regulatory isoenzyme that may be essential for tumor growth and provide an explanation for long-standing observations concerning the apparent coupling of enhanced glycolysis and cell proliferation.
Keywords: phosphofructokinase; fructose 2,6-bisphosphate; cancer; oncogenesis
Cancer cells maintain a high glycolytic rate even in the presence of oxygen, a phenomenon first described over 70 years ago and known historically as the Warburg effect (1, 2). Despite many years of investigation, the specific regulatory mechanisms responsible for enhanced glycolysis and de novo nucleic acid synthesis in tumor cells have remained largely obscure. The discovery of fructose 2,6-bisphosphate (F2,6BP), a potent activator of the key glycolytic enzyme 6-phosphofructo-1-kinase (PFK-1), has led to the hypothesis that intracellular F2,6BP levels may “set” the glycolytic rate in proliferating cells by coupling hormonal signals with metabolic demand (3–5). F2,6BP synthesis can be induced by mitogens such as insulin and epidermal growth factor, and micromolar concentrations of F2,6BP function to relieve the tonic allosteric inhibition by ATP of PFK-1, the rate-limiting step of glycolysis (4).
The steady state concentration of F2,6BP depends on the activity of the homodimeric bifunctional enzyme 6-phosphofructo-2-kinase (PFK-2)/fructose-2,6-bisphosphatase, which is expressed in several tissue-specific isoforms (4). Multiple established cancer cell lines (i.e., Ehrlich ascites tumor cells, HeLa cells, HT29 colon adenocarcinoma cells, and Lewis lung carcinoma cells) have markedly elevated levels of F2,6BP when compared with normal tissue cells (6–9). Transformation of chick-embryo fibroblasts by retroviruses carrying either the v-src or v-fps oncogenes has been observed to induce F2,6BP synthesis and to cause increased glycolytic flux and cell proliferation (10). These data suggest that activation and/or over-expression of PFK-2-catalyzed F2,6BP synthesis may contribute to transformation and cancer cell proliferation (4, 5). A recent study also suggests that an important antineoplastic effect of 6-mercaptopurine may include the ability to form a mixed disulfide with a cysteine residue in PFK-2 and thus inhibit F2,6BP synthesis and enhanced glycolysis (11). Nevertheless, a direct examination of the role of F2,6BP in cancer cell metabolism has been limited by the unavailability of specific inhibitors of F2,6BP synthesis and the complex regulatory pathways governing tumor cell growth.
We report herein the identification of an isoform of PFK-2 that is induced by proinflammatory stimuli and that is distinguished by the presence of multiple copies of the AUUUA instability motif in its 3′ untranslated region (3′UTR). Inducible PFK-2 (iPFK-2) is expressed constitutively in several human cancer cell lines and is important for the increased synthesis of 5-phosphoribosyl-1-pyrophosphate (PRPP), a precursor for purine and pyrimidine biosynthesis. This regulatory isoenzyme of PFK-2 contributes to de novo nucleic acid synthesis in tumor cells and provides a potential mechanism to explain the apparent coupling of enhanced glycolysis and cell proliferation.
MATERIALS AND METHODS
Identification and Cloning of iPFK-2.
An expressed sequence tag (EST) containing several AUUUA elements was identified in the dbEST database at the National Center for Biotechnology Information by performing a tblastn search using multiple contiguous reiterations of the query sequence ATTTA. The selected EST (GenBank accession no. F00287) had been obtained from a Homo sapiens skeletal muscle cDNA library and was unrelated to previously identified sequences. 5′- and 3′- rapid amplification of cDNA ends was performed by using a Human Skeletal Muscle Marathon cDNA-ready rapid amplification of cDNA ends kit (CLONTECH). Gene-specific oligonucleotides used for the sequential 5′-directed rapid amplification of cDNA ends were 5′-ATTGGTCTGGCAACTGCAAA-3′, 5′-GATTGTACCATACCTGAAGCACAGCCTC-3′, 5′-TCTCCTGCCGCTCCAGCTCCATGATCAC-3′, and 5′-GTCAGCTTCTTGGAGATGTAGGTCTTGC-3′. Gene-specific oligonucleotides used for the 3′-directed rapid amplification of cDNA ends included 5′-TTGGTTTGGGAGCCTCCTATGTGTGACT-3′ and 5′-TTGGCGTCTACTGATTCCTCCAACTCTC-3′. DNA amplification products were purified with a QIAEX DNA gel extraction kit (Qiagen, Chatsworth, CA) and then were cloned into the pT7Blue T-vector (Novagen). For each amplification product, five recombinant clones were isolated, and each DNA insert was sequenced bidirectionally by using the Taq DyeDeoxy Terminator Cycle sequencing kit and an ABI Model 373A DNA sequencer (Applied Biosystems). The entire predicted amino acid sequences of human iPFK-2, placental PFK-2, and liver PFK-2 were aligned with the Lipman-Pearson method by using the DNAstar (Madison, WI) megalign application.
Lipopolysaccharide (LPS) Induction of Monocytes.
Peripheral blood mononuclear cells were isolated by density gradient centrifugation of whole blood through Ficoll-Paque (Pharmacia) and then were cultured in 6-well plates (3 × 106 cells/well in RPMI medium 1640 with 10% heat-inactivated human serum). Nonadherent cells were removed by aspiration after 24 hr of culture, and the remaining adherent monocytes were incubated alone as control or in the presence of 1 μg/ml LPS (Escherichia coli 0111:B4; Sigma). The monocyte cultures were >90% pure by FACS analysis for CD14 expression. After incubation for 1.5, 3, 6, 12, or 24 hr, the cells were lifted with ice-cold EDTA and were collected by centrifugation at 300 × g for 10 min, and the total cellular RNA was isolated immediately by a modified guanidinium isothiocyanate method (RNAzol, Cinna Biotecx Laboratories, Friendswood, TX).
Northern Blot Analysis.
Equivalent amounts of total cellular RNA (7.5 μg, measured by OD260) were electrophoresed through 1.5% agarose-formaldehyde gels, were transferred onto nylon membranes (Schleicher & Schuell), and were hybridized sequentially with the corresponding cDNA probes synthesized by PCR using the primers described below. 32P-labeling was by the random-priming method (Megaprime kit, Amersham Pharmacia), and the glyceraldehyde-3-phosphate dehydrogenase probe was obtained from CLONTECH. Autoradiography was performed at room temperature for 2–6 hr by using DuPont Reflection films and intensifying screens.
Reverse Transcription–PCR Analysis.
cDNA was prepared from 1.0 μg of total RNA by using 0.25 ng of oligo(dT) and Superscript II following the manufacturer’s protocol (GIBCO/BRL). Two-milliliter aliquots of cDNA then were amplified by PCR in a Perkin–Elmer model 9600 thermal cycler by using the primers listed below and the following cycling program: denaturation for 15 sec at 95°C, annealing for 20 sec at 55°C, and extension for 30 sec at 72°C for the indicated cycles with a final extension for 5 min at 72°C. The following human mRNA primers were custom synthesized: β-actin, 5′-TAAGGAGAAGCTGTGCTACG-3′, 5′-ATCTCCTTCTGCATCCTGTC-3′; interleukin (IL)-1β, 5′-CTGTACCTG-TCCTGCGTGTT-3′, 5′-AGCTCTCTTTAGGAAGACAC-3′; iPFK-2, 5′-ATTGGTCTGGCAACTG-CAAA-3′, 5′-GGAGCCTCCTATGTGTGACT-3′; and liver PFK-2, 5′-GAAGTCAAACTGAATGTGTC-3′, 5′-CCTCTTGTAGGCAGTAAGTC-3′ (and 5′-AGGCAGTAAGTCTTTATTCG-3′, 5′-AAGTCAAA-CTGCCTGTGTCC-3′). In control studies, the liver PFK-2 primers were found to produce a single DNA product of predicted size on amplification of cDNA from human liver (12). The amplification cycle number was varied initially so as to establish a linear amplification response, and the displayed cycle number illustrates representative differences in the amount of cDNA present.
Western Blot Analysis.
Cells were lysed in 2× Laemmli sample buffer for 5 min at 95°C, and the total cellular proteins were resolved by electrophoresis through a 7% SDS polyacrylamide gel under reducing conditions. After transfer to Immobilon P membranes (Millipore), the proteins were incubated with a polyclonal anti-PFK-2 antibody that was produced by immunization of rabbits with an iPFK-2 carboxy terminal peptide [(NH2)-GQPLLGQACLT-(COOH)] that was conjugated to keyhole limpet hemocyanin. This peptide comprises a unique region of iPFK-2 (amino acids 505–515) that differs from the corresponding portion of placental PFK-2 by a 5-aa deletion and eight amino acid mismatches. Immunoreactive iPFK-2 (Mr = 59,000) then was visualized by developing membranes with a donkey peroxidase-conjugated anti-rabbit IgG antibody (1:8,000) followed by reaction with enhanced chemiluminescence reagents (Amersham Pharmacia). For blocking studies, 100 μg of free peptide was incubated with 2 μg of anti-iPFK-2 antibody for 1 hr at room temperature before incubation with the membrane.
Inhibition of iPFK-2 Expression.
Human tumor cell lines were obtained from American Type Culture Collection and were cultured in triplicate in 96-well plates (5 × 103 cells/well) in RPMI medium 1640 (GIBCO/BRL) supplemented with 10% fetal calf serum. Cells in exponential growth phase were harvested, washed, and incubated with PBS as control or were transfected by the lipofectin method (GIBCO/BRL) for 20 hr with the 2 μM of the following phosphorothioate oligonucleotides: S-iPFK-2 (A) [sense, position 35–55], 5′-AGCCGCGAAGATGCCGTTGG-3′; AS-IPFK-2 (A) [anti-sense, position 35–55], 5′-CCAACGGCATCTTCGCGGCT-3′; S-IPFK-2 (B) [sense, position 42–62], 5′-AAGATGCCGTTGGAACTGAC-3′; AS-iPFK-2 (B) [anti-sense, position 42–62], 5′-GTCAGTTCCAACGGCATCTT-3′. Total intracellular F2,6BP and PRPP were measured by using described methods (13, 14). Cell proliferation was measured by the incorporation of [3H]thymidine (4 μCi/ml) (DuPont) into DNA over the last 4 hr of incubation/transfection.
In Vivo Studies.
K562 cells were collected from exponential growth phase culture in RPMI medium 1640 supplemented with 10% fetal calf serum and then were washed twice and were resuspended in PBS (1 × 107 cells/ml). Groups of five BALB/c nude female mice (20 gm) were injected s.c. with 0.10 ml of the K562 suspension (1 × 106 cells). The tumors were allowed to grow for 7 days to a mean weight of 0.4 g before treatment was begun. Alzet microosmotic pumps (Alza) loaded with 0.1 ml of pyrogen-free PBS or the phosphorothioate oligonucleotides S-iPFK-2 (B) or AS-iPFK-2 (B) (3.0 mM in pyrogen-free PBS) were implanted s.c. into the tumor-bearing mice. After the indicated period of treatment, tumor weight was determined in a blinded fashion with Vernier calipers according the following formula: weight (mg) = (width, mm)2 × (length, mm)/2 (15).
RESULTS
Identification of iPFK-2.
The mRNAs of several inflammatory cytokines (e.g., tumor necrosis factor α, IL-1, interferon γ, and granulocyte/macrophage colony-stimulating factor) and protooncogenes (e.g., c-fos, c-myc, and c-sis) that are members of the early response gene family have been noted to contain the sequence motif AUUUA in their 3′UTR (16). There is evidence that this AU-rich element confers both enhanced translation and instability to the mRNA molecule and that it plays an important role in regulating the half-life of the gene product under certain physiological circumstances (17, 18). To identify novel early response genes that could be involved in inflammation or oncogenesis, we searched the EST database for cDNA sequences containing the conserved AUUUA sequence motif(s). One AU-rich EST unrelated to previously described genes was identified and the complete cDNA was cloned and sequenced (Fig. 1). The amino acid sequence deduced from the ORF was found to share 98% identity with the sequence predicted from a shorter length cDNA clone obtained recently from human placenta (19) and 66% identity with human liver PFK-2 (12). The major difference between the regions encoded by the AU-containing PFK-2 cDNA and the placental PFK-2 sequence was in the length and composition of the carboxy terminus, suggesting that the two cDNA clones may represent alternatively spliced variants of one gene. Alternative splicing has been proposed to account for the generation of distinct PFK-2 isoforms in rat brain (20); however, there has been no previous association of sequences related to PFK-2 with AU-rich 3′UTRs.
Figure 1.
iPFK-2 nucleotide sequence and predicted amino acid sequence. (A) cDNA nucleotide sequence of an isoform of human PFK-2 cDNA (designated iPFK-2). The ATG initiation codon, TAA termination codon, and polyadenylation signal (AATAAA) are bold and underlined. The AT-rich mRNA destabilizing elements are bold and double-underlined (GenBank accession no. AF056320). (B) Predicted amino acid sequence and alignment of the PFK-2 cDNA with PFK-2 sequences deduced from a human placental (19) and a human liver (12) cDNA clone.
Expression and LPS Regulation of iPFK-2.
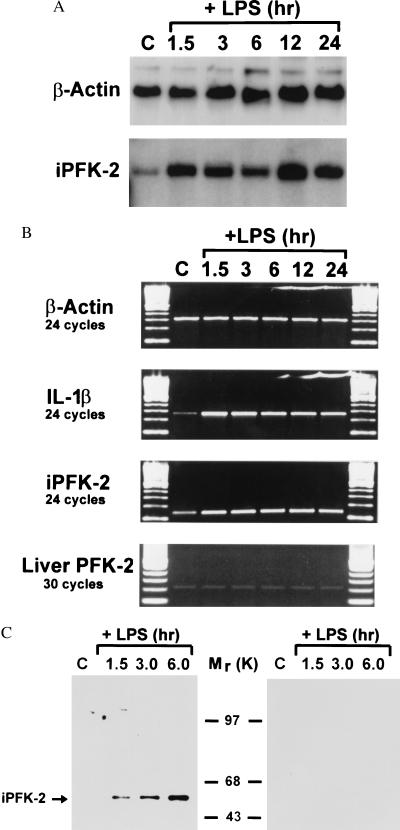
The expression of many protooncogenes and cytokines bearing the AUUUA motif increases in cells as a consequence of mitogenic or proinflammatory stimulation (16). Accordingly, only very low levels of the AU-containing PFK-2 sequence were detected by Northern blotting of normal human tissues (e.g., heart, brain, placenta, lung, liver, skeletal muscle, kidney, pancreas, spleen, and lymph node) (data not shown). By contrast, Northern blot analysis of human monocytes stimulated with LPS showed that this particular PFK-2 gene was rapidly induced (and therefore was designated iPFK-2) (Fig. 2A). The induction and increase in the level of iPFK-2 mRNA expression was similar to that observed for the cytokine IL-1β (which also contains AU-rich elements) whereas the expression level of the liver (constitutive) isoform of PFK-2 was unaffected by LPS stimulation (Fig. 2B). The induction of iPFK-2 mRNA was accompanied by a corresponding increase in immunoreactive iPFK-2 protein, as measured by Western blotting analysis using a specific anti-iPFK-2 peptide antibody (Fig. 2C). These data demonstrate that iPFK-2, like other genes with AU-rich motifs in their 3′UTR, is induced in primary human monocytes on proinflammatory activation in vitro.
Figure 2.
Time-dependent induction of iPFK-2 expression by LPS-stimulated human peripheral blood monocytes. (A) Northern blotting analysis of RNA obtained from control or LPS-stimulated monocytes using a 32P-labeled, iPFK-2 cDNA probe containing the AU-rich 3′UTR. (B) Reverse transcription–PCR analysis of monocyte mRNA by using gene-specific oligonucleotides for β-actin, IL-1β, iPFK-2, and liver PFK-2. (C) Western blot analysis of total monocyte protein by using an anti-iPFK-2 peptide specific antibody (Left) and after coincubation of the anti-iPFK-2 antibody with the iPFK-2-specific peptide (Right).
Expression of iPFK-2 in Tumor Cell Lines.
An increase in the level of stable expression of protooncogenes with AU-rich 3′UTRs is a characteristic feature of many transformed cells and can itself be directly oncogenic (21–23). We examined iPFK-2 mRNA in eight human tumor cell lines by Northern blotting and found this gene to be expressed at high levels (Fig. 3A). The intensities of the iPFK-2 hybridization signals were comparable to the iPFK-2 signals observed in the RNA obtained from the LPS-stimulated primary human monocytes (Fig. 2A). Closer examination of the K562 chronic myelogenous leukemia cell line showed that the expression of iPFK-2 was markedly higher than that of the previously described, hepatic PFK-2 isoform (Fig. 3B). Moreover, in each of four leukocyte-derived tumor cell lysates that were analyzed, there was a significant increase in the expression of iPFK-2 protein when compared with peripheral blood leukocytes (Fig. 3C). These data suggest that iPFK-2 expression may be important in regulating the glycolytic pathway during tumor cell growth.
Figure 3.
Expression of iPFK-2 in tumor cell lines. (A) Expression of iPFK-2 mRNA in human cancer cell lines. Shown is a Northern blot analysis of the cell lines HL-60 (promyelocytic leukemia), S3 (HeLa cell), K562 (chronic myelogenous leukemia), MOLT4 (lymphoblastic leukemia), Raji (Burkitt’s lymphoma), SW480 (colorectal adenocarcinoma), A549 (lung carcinoma), and G361 (melanoma). GADPH, glyceraldehyde-3-phosphate dehydrogenase RNA control. (B) Reverse transcription–PCR analysis of the expression of iPFK-2 and liver PFK-2 mRNA by K562 cells. (C) Western blot analysis of total cellular protein by using an anti-iPFK-2 antibody. PBL, human peripheral blood leukocytes, KG1a, human promyelocytic leukemia cells.
Biochemical Role of iPFK-2 in Tumor Cell Proliferation.
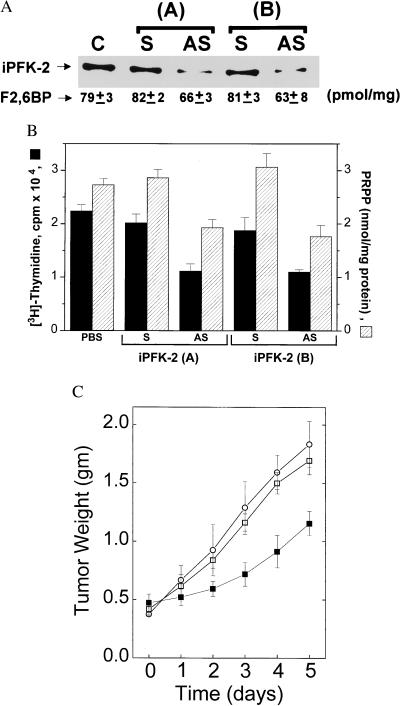
We next transfected K562 leukemia cells with iPFK-2-specific antisense oligonucleotides and found that both iPFK-2 protein and F2,6BP levels were significantly decreased when compared with cells transfected with sense oligonucleotide controls (Fig. 4A). These data indicate that the kinase activity of iPFK-2 contributes to intracellular F2,6BP levels. It has been hypothesized that the enhanced glycolytic flux in transformed cells provides substrates for the biosynthesis of PRPP, a critical precursor for purine and pyrimidine nucleotides (24). Inhibition of iPFK-2 was found to significantly decrease PRPP levels in K562 cells, and this decrease was associated with a corresponding decrease in K562 DNA synthesis and cell proliferation (Fig. 4B). A similar level of inhibition of DNA synthesis was observed after the transfection of iPFK-2 antisense oligonucleotides into the HL-60, MOLT4, SW480, G361, and KG1a cell lines (Table 1). These observations indicate that iPFK-2-catalyzed F2,6BP production may enhance glycolytic flux [through stimulation of PFK-1 (3, 4)] and thus permit the increased channeling of glucose metabolism in the direction of PRPP synthesis.
Figure 4.
Inhibition of iPFK-2 expression decreases intracellular F2,6BP and PRPP concentrations and decreases K562 tumor cell growth. (A) Western blotting and F2,6BP analysis of lysates obtained from untransfected, K562 control cells (C) and K562 cells transfected with two different pairs (A, B) of iPFK-2 sense (S) or iPFK-2 antisense (AS) oligonucleotides. The F2,6BP concentration of each lysate is indicated below the corresponding band of immunoreactive iPFK-2 protein (mean ± SD, oligonucleotide pair A, P = 0.0023 for AS vs. S; oligonucleotide pair B, P = 0.033 for AS vs. S, [t test statistic, independent variable (27)]. (B) Intracellular PRPP and [3H]thymidine incorporation measurements in untransfected, K562 control cells (PBS controls) and cells transfected with two pairs (A, B) of iPFK-2 sense (S) or iPFK-2 antisense (AS) oligonucleotides (mean ± SD) (25). Oligonucleotide pair A, P = 0.0017 for intracellular PRPP and P = 0.0018 for [3H]thymidine incorporation (AS vs. S). Oligonucleotide pair B, P = 0.0023 for intracellular PRPP and P = 0.0061 for [3H]thymidine incorporation (AS vs. S) (t test statistic, independent variable). (C) K562 tumor-bearing nude mice (n = 5 mice per group) were implanted with microosmotic pumps that administered PBS (○), iPFK-2 sense oligonucleotide B (□), or iPFK-2 antisense oligonucleotide B (■). After the indicated period of treatment, tumor weight was determined in a blinded fashion with Vernier calipers according the following formula: weight (mg) = (width, mm)2 × (length, mm)/2. AS vs. S, day 1, P = 0.176; day 2, P = 0.007; day 3, P = 0.0005; day 4, P < 0.00004; day 5, P = 0.0023 (t test statistic, independent variable).
Table 1.
Inhibition of iPFK-2 expression decreases human tumor cell proliferation in vitro
| Cell line | iPFK-2 AS | Percent inhibition | mean ± SD |
|---|---|---|---|
| HL60 | A | 32.0 ± 15.0 | P = 0.029 |
| B | 48.0 ± 22.0 | P = 0.006 | |
| MOLT4 | A | 32.6 ± 7.5 | P = 0.00004 |
| B | 49.5 ± 6.1 | P = 0.00002 | |
| SW480 | A | 28.7 ± 19.3 | P = 0.009 |
| B | 37.7 ± 12.6 | P = 0.0005 | |
| KG1a | A | 51.5 ± 19.5 | P = 0.022 |
| B | 58.6 ± 25 | P = 0.001 | |
| G361 | A | 19.6 ± 9.4 | P = 0.001 |
| B | 24.4 ± 11.1 | P = 0.002 |
Cells were cultured and transfected with two different iPFK-2 antisense oligonucleotides (AS: A or B). The G361 is a melanoma cell line. Proliferation was measured by the incorporation of [3H]thymidine (4 μCi/ml) (DuPont) into DNA over the last 16 hr of incubation. Percent inhibition was calculated relative to the corresponding sense control, which did not show any effect on baseline proliferation. All data are expressed as the mean ± SD of three experiments. Statistical significance was assessed by the two-sample t test (independent variable).
Finally, we examined the role of iPFK-2 expression in tumorigenesis in vivo by administering iPFK-2-specific anti-sense oligonucleotides to K562 tumor-bearing nude mice. BALB/c nude female mice were injected with K562 cells, and the resultant tumors were allowed to grow for 7 days to a mean weight of 0.4 g before treatment was begun. Microosmotic pumps loaded with antisense oligonucleotides then were implanted into the tumor-bearing mice. Within 2 days of treatment, tumors from the iPFK-2 anti-sense treated mice were significantly smaller than tumors from the iPFK-2 sense oligonucleotide- or PBS-treated mice (Fig. 4C). iPFK-2-catalyzed F2,6BP synthesis thus may serve a critical regulatory role for K562 tumor growth in vivo.
DISCUSSION
Early response genes encode a family of proteins that are rapidly induced on exposure to various extracellular stimuli, such as growth factors, cytokines, and neurotransmitters (16, 25). Several inflammatory cytokines (e.g., tumor necrosis factor α, IL-1, interferon γ, and granulocyte/macrophage colony-stimulating factor) and protooncogenes (e.g., c-fos, c-myc, and c-sis) that are members of the early response gene family contain the instability element AUUUA in their 3′UTR (16). iPFK-2 is the first metabolic enzyme to be identified whose mRNA molecule contains the AUUUA instability element. The expression of iPFK-2 is rapidly induced by proinflammatory activation of nontransformed cells, and expression levels are constitutively high in the transformed cells that were examined.
F2,6BP has been identified to be present in elevated amounts in many cancer cell lines (6, 8, 9, 11). F2,6BP is considered to be the most potent stimulator of glycolysis and acts by allosteric activation of PFK-1, which phosphorylates F6P to produce F1,6BP (3, 4). PFK-2 catalyzes the synthesis of F2,6BP from F6P, and, in chick-embryo fibroblasts, this catalytic activity has been shown to be increased by oncogenic retroviral transformation (10). Several tissue-specific isoenzymes of PFK-2 have been described, but the isoenzyme that is associated with the enhanced glycolysis of tumor cells has not been identified previously (4).
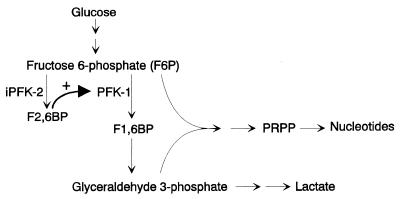
Glucose provides substrate both for energy production and for the pentose precursors required for nucleic acid synthesis by proliferating tumor cells. Elevated intracellular levels of pentose phosphate pathway metabolites, such as PRPP, are a characteristic feature of rapidly proliferating cells (26). The nonoxidative pentose phosphate pathway involves the transketolase–transaldolase transformation of 2 mol of F6P and 1 mol of glyceraldehyde 3-phosphate into 3 mol of ribose 5-phosphate. The observation that inhibition of iPFK-2 expression decreases PRPP levels is consistent with a pathway by which F2,6BP enhances flux through PFK-1 to provide increased substrate for the nonoxidative pentose phosphate pathway (Fig. 5) (24, 26).
Figure 5.
Scheme for the F2,6BP-mediated induction of glycolysis and nucleotide synthesis via iPFK-2.
The experiments described herein indicate that the iPFK-2-catalyzed production of F2,6BP plays an essential role in the glycolytic flux through PFK-1, leading to PRPP synthesis and DNA replication in proliferating cells. Enhanced glycolysis in the presence of oxygen, i.e., the Warburg effect, has been a poorly understood feature of fast growing cancer cells (26). Paradoxically, the high glycolytic flux of tumors represents an inefficient way of obtaining energy because the net ATP yield is much lower than that produced by the total oxidation of glucose in the mitochondria. The demand for nucleic acid precursors may require that transformed cells metabolize glucose “inefficiently” so as to maintain a high proliferation rate. We conclude that iPFK-2 is an important regulator of glycolysis that may be responsible for sustaining the high glycolytic flux of rapidly proliferating leukemia cells and may represent a molecular target for chemotherapeutic intervention.
Acknowledgments
These studies were supported by institutional funds from The Picower Institute for Medical Research and the Patterson Foundation.
ABBREVIATIONS
- EST
expressed sequence tag
- F2
6BP, fructose 2,6-bisphosphate
- LPS
lipopolysaccharide
- PFK-1
6-phoshofructokinase-1-kinase
- PFK-2
6-phoshofructokinase-2-kinase
- iPFK-2
inducible 6-phoshofructokinase-2-kinase
- PRPP
5-phosphoribosyl-1-pyrophosphate
- 3′UTR
3′ untranslated region
- IL
interleukin
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF056320).
References
- 1.Warburg O. Biochem Z. 1924;152:319–344. [Google Scholar]
- 2.Warburg O. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 3.Van Schaftingen E, Jett M F, Hue L, Hers H G. Proc Natl Acad Sci USA. 1981;78:3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hue L, Rousseau G G. Adv Enzyme Regul. 1993;33:97–110. doi: 10.1016/0065-2571(93)90011-2. [DOI] [PubMed] [Google Scholar]
- 5.Hue L, Rider M H. Biochem J. 1987;245:313–324. doi: 10.1042/bj2450313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nissler K, Petermann H, Wenz I, Brox D. J Cancer Res Clin Oncol. 1995;121:739–745. doi: 10.1007/BF01213320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mojena M, Bosca L, Hue L. Biochem J. 1985;232:521–527. doi: 10.1042/bj2320521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denis C, Paris H, Murat J C. Biochem J. 1986;239:531–536. doi: 10.1042/bj2390531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miralpeix J, Azcon-Bieto R, Bartrons R, Argiles J M. Cancer Lett. 1990;50:173–178. doi: 10.1016/0304-3835(90)90261-u. [DOI] [PubMed] [Google Scholar]
- 10.Bosca L, Mojena M, Ghysdael J, Rousseau R, Hue L. Biochem J. 1986;236:595–599. doi: 10.1042/bj2360595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mojena M, Bosca L, Rider M H, Rousseau G G, Hue L. Biochem Pharmacol. 1992;43:671–676. doi: 10.1016/0006-2952(92)90229-c. [DOI] [PubMed] [Google Scholar]
- 12.Lange A J, Pilkis S J. Nucleic Acids Res. 1990;18:3662. doi: 10.1093/nar/18.12.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sant M E, Lyons S D, Philips L, Christopherson R I. J Biol Biochem. 1992;267:11038–11045. [PubMed] [Google Scholar]
- 14.Van Schaftingen E. Methods Enzymol Anal. 1984;6:335–341. [Google Scholar]
- 15.Taetle R, Rosen F, Abramson I, Venditti J, Howell S. Cancer Treat Rep. 1987;71:297–304. [PubMed] [Google Scholar]
- 16.Greenberg M E, Belasco J G. In: Control of Messenger RNA Stability. Belasco J G, Brawerman G, editors. New York: Academic; 1993. pp. 199–218. [Google Scholar]
- 17.Caput D, Beutler B, Hartog K, Thayer R, Brown-Shimer S, Cerami A. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw G, Kamen R. Cell. 1986;46:659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- 19.Sakai A, Kato M, Fukusawa F, Ishiguro M, Furuya E, Sakakibara R. J. Biochem. 1996;119:506–511. doi: 10.1093/oxfordjournals.jbchem.a021270. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe F, Sakai A, Furuya E. J Neurochem. 1997;69:1–9. doi: 10.1046/j.1471-4159.1997.69010001.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee W M, Lin C, Curran T. Mol Cell Biol. 1988;8:5521–5527. doi: 10.1128/mcb.8.12.5521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabbitts P H, Forster A, Stinson M A, Rabbitts T H. EMBO J. 1985;4:659–667. doi: 10.1002/j.1460-2075.1985.tb04141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piecharczyk M, Yang J Q, Blanchard J M, Jeanteur P, Marcu K B. Cell. 1985;42:589–597. doi: 10.1016/0092-8674(85)90116-3. [DOI] [PubMed] [Google Scholar]
- 24.Eigenbrodt E, Glossmann H. Trends Pharmacol Sci. 1980;1:240–245. [Google Scholar]
- 25.Herschman H R. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- 26.Weber G. New Engl J Med. 1977;296:541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- 27.Zar J H. Biostatistical Analyses. Englewood Cliffs, NJ: Prentice–Hall; 1984. p. 718. [Google Scholar]