Abstract
Goblet-cell hyperplasia is a critical pathological feature in hypersecretory diseases of airways. However, the underlying mechanisms are unknown, and no effective therapy exists. Here we show that stimulation of epidermal growth factor receptors (EGF-R) by its ligands, EGF and transforming growth factor α (TGFα), causes MUC5AC expression in airway epithelial cells both in in vitro and in vivo. We found that a MUC5AC-inducing epithelial cell line, NCI-H292, expresses EGF-R constitutively; EGF-R gene expression was stimulated further by tumor necrosis factor α (TNFα). EGF-R ligands increased the expression of MUC5AC at both gene and protein levels, and this effect was potentiated by TNFα. Selective EGF-R tyrosine kinase inhibitors blocked MUC5AC expression induced by EGF-R ligands. Pathogen-free rats expressed little EGF-R protein in airway epithelial cells; intratracheal instillation of TNFα induced EGF-R in airway epithelial cells, and subsequent instillation of EGF-R ligands increased the number of goblet cells, Alcian blue–periodic acid–Schiff staining (reflecting mucous glycoconjugates), and MUC5AC gene expression, whereas TNFα, EGF, or TGFα alone was without effect. In sensitized rats, three intratracheal instillations of ovalbumin resulted in EGF-R expression and goblet-cell production in airway epithelium. Pretreatment with EGF-R tyrosine kinase inhibitor, BIBX1522, prevented goblet-cell production both in rats stimulated by TNFα-EGF-R ligands and in an asthma model. These findings suggest potential roles for inhibitors of the EGF-R cascade in hypersecretory diseases of airways.
Goblet-cell hyperplasia is an important feature in many chronic airway diseases including chronic bronchitis (1), cystic fibrosis (2), and bronchiectasis (3). Hypersecretion from hyperplastic goblet cells causes airway mucous plugging, especially in peripheral airways, where large, numerous secreting goblet cells can more easily cause obstruction (4, 5); this phenomenon is reported as a major cause of death in acute asthma (6). In spite of the importance of goblet-cell hyperplasia in airways, the analysis of mechanisms of goblet-cell production has been difficult because of the heterogeneity of these hypersecretory diseases. Hence, the mechanisms causing goblet-cell hyperplasia in hypersecretory diseases remain unknown.
Growth factors could be involved in goblet-cell production, because hypersecretory diseases are associated with abnormal epithelial-cell growth and proliferation. Among the growth factors, a possible candidate is epidermal growth factor (EGF) and its receptor (EGF-R). EGF-R, a 170-kDa membrane glycoprotein, is expressed on the surface of various cells, and may be related to mucin production in stomach (7), urothelium (8), and other epithelia (9). In airways, EGF-R is expressed in the fetus, where it is important in cell proliferation, branching morphogenesis, and epithelial-cell differentiation (10). In healthy adult human airways, expression of EGF-R is sparse. However, EGF-R and its ligands are expressed in malignant lung tumors (11), in airway epithelium of bleomycin-induced pulmonary fibrosis (12), and in asthma (13), suggesting a potential role not only in tumor pathogenesis but also in epithelial inflammatory diseases. Moreover, EGF-R is known to be up-regulated by the proinflammatory cytokine tumor necrosis factor α (TNFα) (14–16), which is increased in lungs in hypersecretory diseases (17). Therefore, we hypothesize that the EGF-R system plays a role in goblet-cell production that may be regulated, at least in part, by TNFα.
Here we report that stimulation of airway epithelial cells with TNFα induces EGF-R in epithelial cell cultures and in rats in vivo. We show that stimulation of EGF-R by its ligands results in mucin-producing goblet cells and that ovalbumin sensitization in rats causes induction of EGF-R and goblet-cell production in airways. Most importantly, EGF-R tyrosine kinase inhibitors prevent mucin production in each of these systems. We suggest that inhibitors of EGF-R may be useful in inhibiting goblet-cell production and hypersecretion in disease.
METHODS
In Vitro Studies.
Cell culture. A human pulmonary mucoepidermoid carcinoma cell line, NCI-H292, were grown in RPMI 1640 medium containing 10% fetal bovine serum, penicillin (100 units/ml), streptomycin (100 μg/ml), and Hepes (25 mM) at 37°C in a humidified 5% CO2 water-jacketed incubator. When confluent, cells were incubated with EGF (recombinant human EGF, 25 ng/ml, Genzyme), TGFα (recombinant human TGFα, 25 ng/ml, Genzyme), TNFα (recombinant human TNFα, 20 ng/ml, Genzyme), or EGF plus TNFα or TGFα plus TNFα for 12 h, 24 h, or 48 h. In inhibition studies, cells were pretreated with the selective tyrosine kinase inhibitors, BIBX1522 (10 μg/ml, generously provided by Boehringer Ingelheim), tyrphostin AG1478 (10 μM, Calbiochem) or Compound 56 (10 μM, Calbiochem) 30 min before adding growth factors. The effects of a selective tyrosine kinase inhibitor of platelet-derived growth factor (tyrphostin AG1295, 100 μM, Calbiochem) and a negative control for tyrphostins (tyrphostin A1, 100 μM, Calbiochem) were also examined.
Immunoblotting for EGF-R.
Cells grown in T-75 flasks were lysed and scraped with PBS containing 1% Triton X-100, 1% sodium deoxycholate and 10 mg/ml phenylmethylsulfonyl fluoride. Total protein was estimated by using BCA protein assay reagent (Pierce). Proteins were separated by using SDS/PAGE in 8% acrylamide gels. The resulting gels were equilibrated in the transfer buffer: 25 mM Tris⋅HCl, 192 mM glycine, and 20% (vol/vol) methanol (pH 8.3). The proteins were then transferred electrophoretically to nitrocellulose membranes. The membranes were incubated with 5% fat-free skim milk in PBS containing 0.05% Tween 20 for 1 h and incubated with monoclonal mouse anti-EGF-R antibody (1:100, Calbiochem) at 4°C overnight. Bound antibody was visualized according to standard protocols for the avidin–biotin–alkaline phosphatase complex method (ABC kit, Vector Laboratories). As a positive control for EGF-R, cell lysates from A431 cells were used (18).
Immunocytochemical localization of EGF-R and MUC5AC in NCI-H292 cells.
Cells grown on 8-chamber slides were fixed with 4% paraformaldehyde for 1 h. PBS containing 0.05% Tween 20, 2% normal goat serum, and Levamisol (2 mM) was used as diluent for the antibody. Cells were incubated with mouse mAb to EGF-R (1:250) or to MUC5AC (clone 45 M1, 1:100, New Markers, Fremont, CA) overnight at 4°C, and then washed 3 times with PBS to remove excess primary antibody. Cells were then incubated with biotinylated horse anti-mouse IgG (Vector Laboratories) at 1:200 dilution for 1 h at room temperature. Bound antibody was visualized according to standard protocols for avidin–biotin–alkaline phosphatase complex method.
Mucin analysis.
Cells were fixed similarly to the immunocytochemical technique and stained with Alcian blue/periodic acid–Schiff (AB–PAS). MUC5AC protein was measured by using ELISA. Cell lysates were prepared with PBS at multiple dilutions, and 50 μl of each sample was incubated with bicarbonate–carbonate buffer (50 μl) at 40°C in a 96-well plate (Nunc), until dry. Plates were washed three times with PBS and blocked with 2% BSA, fraction V (Sigma) for 1 h at room temperature. Plates were again washed three times with PBS and then incubated with 50 μl of mouse monoclonal MUC5AC antibody (1:100), which was diluted with PBS containing 0.05% Tween 20, and dispensed into each well. After 1 h, the wells were washed three times with PBS, and 100 μl of horseradish peroxidase–goat anti-mouse lgG conjugate (1:10,000) was dispensed into each well. After 1 h, plates were washed three times with PBS. Color reaction was developed with 3,3′,5,5′-tetramethylbenzidine (TMB) peroxidase solution (Kirkegaard & Perry Laboratories) and stopped with 1 M H2SO4. Absorbance was read at 450 nm.
Northern blotting.
Total RNA was extracted from NCI-H292 cells grown in a T-75 tissue culture flask in each condition, using Trizol (GIBCO). Total RNA (10 μg) was electrophoresed on 1% agarose/formaldehyde gel and transferred to a nylon membrane (Amersham Pharmacia) by capillary blotting. The probes (pTRI-EGF-R-human probe template, Ambion, Austin, TX; and human MUC5AC, generously provided by Carol Basbaum, University of California, San Francisco) were labeled with 32P by using the Random Primed DNA labeling kit (Boehringer Mannheim). Hybridization was performed as described (19).
In Vivo Studies.
The experimental animal protocol was approved by the Committee on Animal Research, University of California, San Francisco. Specific pathogen-free male F344 Fisher rats, weighing 230–250 g (Simonsen Laboratories, Gilroy, CA), were maintained in a temperature-controlled (21°C) room with standard laboratory food and water freely available.
Healthy rats.
Rats were anesthetized with methohexital sodium (Brevital sodium, 50 mg/kg, i.p.; Eli Lilly) and allowed to breathe spontaneously. TNFα (200 ng, 100 μl) was instilled into the trachea, and the animals were euthanized 24 h later. EGF (600 ng, 100 μl) or TGFα (rat synthetic TGFα, 250 ng, 100 μl; Sigma) was instilled into the trachea either alone or 24 h after the instillation of TNFα (200 ng, 100 μl), and the animals were euthanized 48 h later. In each study, sterile PBS (100 μl) was instilled into the trachea as control. In inhibition studies, rats were pretreated with BIBX1522 (3, 10, or 30 mg/kg, i.p., dose estimated from studies using the inhibitor to prevent cancer growth), 1 h before and 24 h after instillation of TGFα. The trachea and lungs were removed for examination 48 h after the instillation of TGFα.
Sensitized rats.
Rats were sensitized on days 0 and 10 with intraperitoneal injections (i.p.) of ovalbumin (10 mg, grade V; Sigma), complexed with 100 mg of aluminum hydroxide in 0.5 ml of sterile saline. On days 20, 22, and 24, ovalbumin (0.1%, 100 μl) was delivered by intratracheal (i.t.) instillation. Rats were euthanized either without i.t. instillation (day 20) or 48 h after the third i.t. instillation (day 26). To study the effect of BIBX1522 on goblet-cell production in sensitized rats, BIBX1522 was given i.p. (10 mg/kg) 1 h before the i.t. instillation of ovalbumin and instilled into the trachea (10−5M, 100 μl) on days 20, 22 and 24. BIBX1522 was also injected i.p. (10 mg/kg) every 24 h until the day before the rats were euthanized. Forty-eight hours after the third i.t. instillation, the animals were euthanized, and the trachea and lungs were removed.
Tissue preparation.
At preselected times during anesthesia, the systemic circulation was perfused with 1% paraformaldehyde in DEPC-treated PBS at a pressure of 120 mmHg (1 mmHg = 133 Pa). For frozen sections, tissues were removed and placed in 4% paraformaldehyde for 1 h and placed in 30% sucrose for cryoprotection overnight. The tissues were embedded in optimal cutting temperature (OCT) compound. For plastic sections, the tissues were placed in 4% paraformaldehyde for 24 h then dehydrated and embedded in JB-4 plus monomer solution A. The embedded tissues were cut as cross sections (4 μm thick) and placed on glass slides.
Cell analysis.
The total number of epithelial cells was determined by counting epithelial cell nuclei over 2 mm of the basal lamina with an oil immersion objective lens (×1,000 magnification). The linear length of the basal lamina under each analyzed region of epithelium was determined by tracing the contour of the digitized image of the basal lamina. The epithelial cells were identified as described (20, 21). In brief, goblet cells are goblet to low columnar in shape, with abundant AB–PAS-stained granules filling most of the cytoplasm. Pre-goblet cells contain smaller mucus-stained areas (<1/3 height in epithelium from basement membrane to luminal surface) or sparse granules stained with AB–PAS. Ciliated cells are recognized by their ciliated borders, lightly stained cytoplasm, and large, round nuclei. Nongranulated secretory cells are columnar in shape and extend from the lumen to the basal lamina. The cytoplasm stains light pink, and a few small, PAS-positive and AB-negative granules are observed in the cytoplasm. Basal cells are small, flattened cells with large nuclei located just above the basal lamina but not reaching the airway lumen.
Quantification of goblet-cell production.
Goblet-cell production was determined by the volume density of AB–PAS-stained mucous glycoconjugates on the epithelial mucosal surface, by using a semiautomatic imaging system described elsewhere (22). We measured the AB–PAS-positive stained area and the total epithelial area and expressed the data as the percentage of the total area stained by AB–PAS. The analysis was performed with the public domain NIH image program (developed at the U.S. National Institutes of Health and available by anonymous FTP from zippy.nimh.gov or on floppy disk from the National Technical Information Service, Springfield, VA, part number PB95–500195GEI).
Immunohistochemical localization of EGF-R in rat epithelium.
The localization of EGF-R was examined by using immunohistochemical staining with an antibody to EGF-R (Calbiochem) in frozen sections of rat trachea. Immunostaining was performed similarly to the in vitro studies.
In situ hybridization.
The cDNA for rat MUC5AC was generously provided by Carol Basbaum. A 320-bp cDNA fragment of rat MUC5AC was subcloned into the XbaI/HindIII site of the transcription vector, pBluescript-SK(−) (Stratagene). The preparation of RNA probes and in situ hybridization were performed as described (20).
Statistics.
All data are expressed as mean ± SEM. One-way ANOVA was used to determine statistically significant differences between groups. Scheffe’s F test was used to correct for multiple comparisons when statistical significances were identified in the ANOVA. A probability of <0.05 for the null hypothesis was accepted as indicating a statistically significant difference.
RESULTS
TNFα Stimulates Production of EGF-R in NCI-H292 Cells.
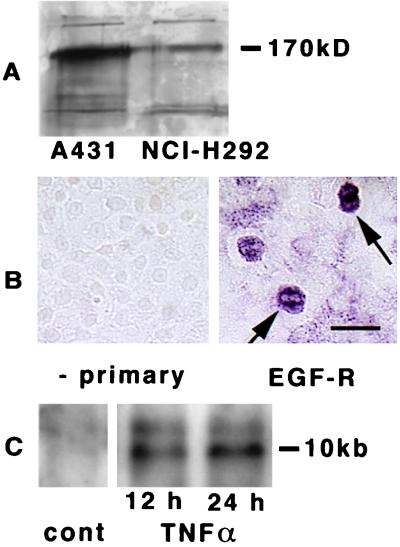
First, we determined whether NCI-H292 cells express EGF-R constitutively. Immunoblot analysis of cell lysates identified the presence of EGF-R protein in confluent cultures of NCI-H292 cells (Fig. 1A). Protein from A431 cells provided a positive control for EGF-R (Fig. 1A); these cells express EGF-R constitutively (18). Immunocytochemical analysis with an anti-EGF-R antibody revealed that most cells were positive, although some cells had more intense staining than the others (Fig. 1B). Northern blotting in NCI-H292 cells showed little expression of EGF-R mRNA constitutively, but TNFα up-regulated EGF-R mRNA at 12 h, accentuated at 24 h (Fig. 1C).
Figure 1.
Expression of EGF-R in cultured cells. (A) Immunoblotting of EGF-R in A431 and in NCI-H292 cells. Cells were examined after becoming confluent. Lysates were electrophoresed in 8% acrylamide gels and blotted with anti-EGF-R antibody. Molecular mass of marker protein is reported on the right. (B) Immunocytochemical analysis with anti-EGF-R antibody in cultures of NCI-H292 cells. At confluence, positive staining was seen in most of cells, and certain cells had more intense staining (arrows, right side). In the absence of the primary antibody, no staining was seen (left side). (Bar = 25 μm.) (C) Northern analysis of EGF-R in NCI-H292 cells was performed on total RNA extracted from confluent cultures incubated with TNFα (20 ng/ml) for 12 or 24 h. The RNA (10 μg) was electrophoresed on a formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized with the 32P-labeled EGF-R cDNA probe. After hybridization, the membrane was washed and autoradiographed.
EGF-R Ligands Stimulate MUC5AC Protein and Gene Expression in NCI-H292 Cells.
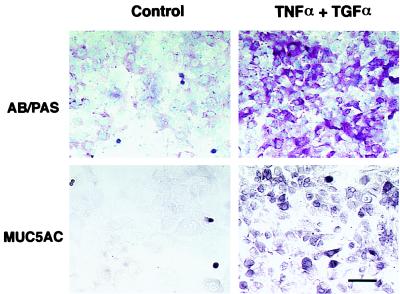
EGF-R are expressed constitutively in NCI-H292 cells; we assessed the ability of EGF-R ligands (EGF and TGFα) to induce mucous glycoconjugate production (assessed by AB–PAS staining; Fig. 2, upper column): Some control cells showed AB–PAS staining; incubation with either EGF or with TGFα increased PAS-positive staining; incubation with TNFα alone did not affect staining (data not shown). However, TNFα potentiated the stimulatory effect of EGF-R ligands on mucous glycoconjugate production (Fig. 2). Immunocytochemical analysis with an anti-MUC5AC antibody showed that the cytoplasm of a few control cells stained positive, similar to AB–PAS staining. Incubation with TNFα plus TGFα increased the staining for MUC5AC protein in a pattern similar to AB–PAS staining (Fig. 2, Lower). To quantify the MUC5AC production, an ELISA was performed: EGF alone or TGFα alone caused an ≈2-fold increase in MUC5AC production. Thus, EGF-R ligands induce the production of MUC5AC protein in NCI-H292 cells. TNFα alone caused little increase in MUC5AC, but TNFα potentiated the effect of EGF-R ligands (Fig. 3). To examine MUC5AC gene expression, Northern blotting was performed. MUC5AC gene was expressed at low levels in NCI-H292 cells treated with control medium. Incubation with EGF or TGFα increased MUC5AC gene expression beginning at 12 h and reaching a maximum at 24 h. TNFα alone had no effect on MUC5AC gene expression but greatly potentiated the stimulatory effect of EGF-R ligands; TGFα showed a greater effect than EGF (Fig. 4).
Figure 2.
AB–PAS staining of NCI-H292 cells for identification of mucous glycoconjugates (Upper) and immunocytochemical analysis for MUC5AC protein (Lower). Immunocytochemical analysis with an anti-MUC5AC antibody showed positive staining in only a few cells in the control state in a similar pattern to that of AB–PAS staining. However, incubation with TNFα (20 ng/ml) plus TGFα (25 ng/ml) increased both the PAS-positive staining and MUC5AC protein markedly. (Bar = 50 μm.)
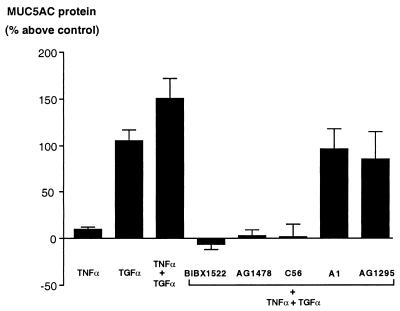
Figure 3.
ELISA for MUC5AC in NCI-H292 cells. MUC5AC protein was measured as described in Methods. TNFα caused little expression of MUC5AC protein (8.7 ± 3.8%, n = 3); TGFα alone caused ≈100% increase in MUC5AC protein, an effect that was potentiated by coincubation with TNFα (149.5 ± 21.7%, n = 6). The stimulatory effect of TNFα plus TGFα was inhibited by each of the three selective EGF-R tyrosine kinase inhibitors (BIBX1522, −6.0 ± 5.5%; tyrphostin AG1478, 2.1 ± 6.7%; Compound 56, 1.0 ± 14.5%; n = 3), whereas a negative control for tyrphostins (tyrphostin A1) and a selective inhibitor of platelet-derived growth factor (tyrphostin AG1295) were without effect.
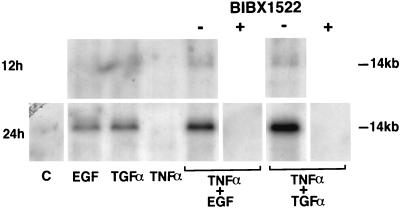
Figure 4.
Northern analysis for MUC5AC gene expression in NCI-H292 cells. Total RNA was extracted from the cells, and 10 μg of RNA was electrophoresed on a formaldehyde-agarose gel, transferred to a nylon membrane, and hybridized with the 32P-labeled MUC5AC cDNA probe. After hybridization, the membrane was washed and autoradiographed. Cultures were obtained with medium alone (C), EGF or TGFα (25 ng/ml), TNFα (20 ng/ml), or the combination of TNFα plus either EGF or TGFα for 12 h (Upper) or 24 h (Lower) on MUC5AC gene expression. Cultures were also obtained with TNFα plus either EGF or TGFα after preincubation with EGF-R tyrosine kinase inhibitor (BIBX1522; 10 μg/ml; Lower); the inhibitor prevented MUC5AC gene expression. Three different experiments showed similar results.
EGF-R Tyrosine Kinase Inhibitors Prevent MUC5AC Gene and Protein Expression in NCI-H292 Cells.
To test the hypothesis that activation of EGF-R induces MUC5AC gene and protein expression, cells were incubated with various tyrosine kinase inhibitors. Pretreatment of NCI-H292 cells with selective kinase inhibitors for EGF-R (BIBX1522, AG1478, Compound 56) prevented the increased staining for MUC5AC protein that usually occurred with EGF-R ligands (Fig. 3). A selective tyrosine kinase inhibitor of platelet-derived growth factor (AG1295) and a negative control for tyrphostins (A1) were without effect (Fig. 3). On Northern analysis, BIBX1522 completely inhibited MUC5AC gene expression caused by the combination of TNFα plus EGF-R ligands (Fig. 4). These results implicate activation of EGF-R tyrosine kinase in the induction of MUC5AC gene and protein expression in NCI-H292 cells.
TNFα Stimulates EGF-R Production in Rat Airways.
In the control state, tracheal epithelium contained few EGF-R-positive cells (Fig. 5A, Left). However, intratracheal instillation of TNFα induced EGF-R protein in the tracheal epithelium (Fig. 5A, Right).
Figure 5.
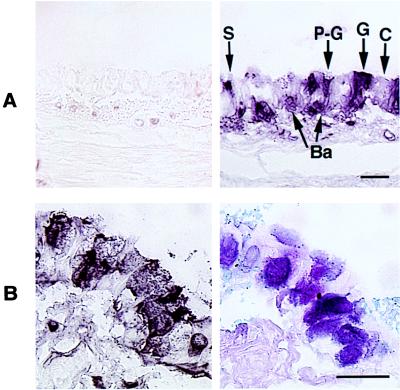
Immunohistochemical analysis of EGF-R with an anti-EGF-R antibody in pathogen-free rats. (A) TNFα-treated rats. Control animals showed little EGF-R staining (Left); 24 h after intratracheal instillation of TNFα (200 ng, 100 μl), EGF-R-positive staining was present in goblet cells (G), pre-goblet cells (P-G), nongranulated secretory cells (S), and basal cells (Ba), but not in ciliated cells. (Bar = 50 μm.) (B) Ovalbumin sensitization. After three intratracheal instillations of ovalbumin (0.1%, 100 μl), EGF-R immunoreactivity was strongly expressed in goblet and pre-goblet cells (Left), the same cells that stained positively with AB–PAS (Right). (Bar = 50 μm.)
Role of EGF-R Ligands in Mucous Glycoconjugate Production and MUC5AC Gene Expression in Rats.
In the control state, tracheal epithelium contained few goblet and pre-goblet cells. Intratracheal instillation of EGF-R ligands (EGF or TGFα) alone had no effect on goblet cell production (Table 1). However, when TNFα was given first, followed 24 h later by TGFα or by EGF (data not shown), the numbers of goblet and pre-goblet cells were increased markedly; the numbers of nongranulated secretory cells and basal cells decreased significantly, whereas there was no change in the total number of cells or in the number of ciliated cells (Table 1). In situ hybridization for MUC5AC gene showed no expression in control animals. When TNFα was instilled intratracheally, followed by EGF or TGFα, expression of MUC5AC was visible in the epithelium (data not shown). Thus, induction of EGF-R alone or stimulation by EGF-R ligands alone was insufficient to induce goblet-cell production. However, after the induction of EGF-R by TNFα, instillation of EGF-R ligands stimulated goblet-cell production markedly.
Table 1.
Cell analysis in tracheal epithelium
| Cell type | Control | TGFα | TNFα/TGFα | OVA sensitization
|
|
|---|---|---|---|---|---|
| i.p. only | i.p. + i.t. | ||||
| Goblet | 2.8 ± 0.7 | 5.8 ± 1.2 | 28.8 ± 3.4* | 5.4 ± 1.5 | 38.2 ± 6.3* |
| Pre-goblet | 7.8 ± 1.3 | 12.8 ± 1.6 | 44.8 ± 3.6* | 13.8 ± 1.4 | 36.0 ± 6.3* |
| Secretory | 82.0 ± 2.0 | 72.2 ± 4.0 | 40.8 ± 2.4* | 67.6 ± 7.0 | 49.8 ± 4.2* |
| Ciliated | 49.6 ± 2.0 | 54.6 ± 2.3 | 53.2 ± 1.8 | 56.4 ± 3.8 | 52.4 ± 7.1 |
| Basal | 57.8 ± 2.6 | 56.8 ± 2.3 | 43.0 ± 3.5* | 60.2 ± 3.4 | 59.8 ± 2.9 |
| Indeterminate | 1.4 ± 0.5 | 2.0 ± 0.4 | 0.8 ± 0.4 | 1.4 ± 0.2 | 2.6 ± 0.5 |
| Total | 201.4 ± 2.2 | 204.2 ± 3.3 | 211.4 ± 4.8 | 204.8 ± 6.6 | 238.8 ± 4.4* |
Effects of mediators and ovalbumin sensitization on tracheal epithelial cells in rats. Cells were analyzed as described in Methods; five rats per group. Control airways and airways stimulated by TGFα (250 ng) alone contained few goblet and pre-goblet cells; TNFα (200 ng) followed by TGFα (250 ng) resulted in increased numbers of goblet (P < 0.001) and pre-goblet (P < 0.0001) cells, accompanied by a decrease of nongranulated secretory cells (P < 0.0001) and basal cells (P < 0.05). Sensitization of rats with ovalbumin (OVA) intraperitoneally (i.p.) had no effect on cell distribution, but when OVA was given i.p. followed by intratracheal (i.t.) instillation, the total number of epithelial cells increased significantly (P < 0.001), and there were increased numbers of goblet (P < 0.001) and pre-goblet (P < 0.001) cells. Nongranulated secretory cells decreased significantly (P < 0.01). In all conditions, the number of ciliated cells was unchanged.
Ovalbumin Sensitization in Rats Induces EGF-R and Goblet-Cell Production.
Injections of ovalbumin i.p. on days 0 and 10 did not alter the total number of epithelial cells and did not increase the number of goblet cells or pre-goblet cells (Table 1). However, when this was followed by three i.t. instillations of ovalbumin on days 20, 22, and 24, the total number of epithelial cells increased significantly compared with control state. The numbers of goblet and pre-goblet cells were increased markedly, but the numbers of ciliated and basal cells were unchanged (Table 1). Thus, ovalbumin i.p. followed by ovalbumin i.t. caused goblet cell hyperplasia. Immunohistochemical studies with an anti-EGF-R antibody showed no staining in control tracheas (data not shown). Animals sensitized to both i.p. and i.t. showed EGF-R staining (Fig. 5B, Left) selectively in cells that stained positively with AB–PAS (Fig. 5B, Right).
EGF-R Tyrosine Kinase Inhibitor, BIBX1522 Prevents Goblet-Cell Production Induced by Instillation of TNFα Plus EGF-R Ligands and by Ovalbumin Sensitization in Rats.
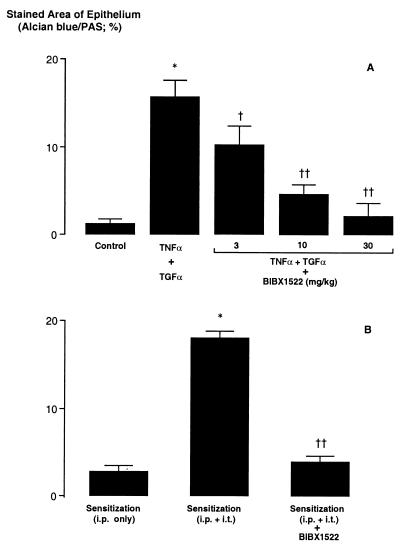
Because BIBX1522 prevented mucin production in cultured cells, the effect of this inhibitor was examined in pathogen-free rats. Alcian blue–PAS staining, which was increased by tracheal instillation of TNFα followed by EGF-R ligand TGFα, was inhibited dose-dependently by pretreatment with BIBX1522 (Fig. 6A). Similarly, three i.t. instillations of ovalbumin caused a significant increase in goblet-cell production, which was inhibited by pretreatment with BIBX1522 (Fig. 6B).
Figure 6.
Effect of EGF-R tyrosine kinase inhibitor (BIBX1522) on production of goblet cells (expressed as % stained area of airway epithelium occupied by AB–PAS-positive stained cells). (A) Stimulation with TNFα (200 ng, 100 μl). Tracheal instillation of TNFα, followed by the EGF-R ligand TGFα, increased goblet-cell production significantly (n = 5; ∗, P < 0.0001), an effect that was inhibited by pretreatment with BIBX1522 (3–30 mg/kg, i.p.) dose-dependently (n = 5; P compared with TNFα followed by TGFα: †, P = 0.003; ††, P < 0.0001). (B) Ovalbumin sensitization. Animals given ovalbumin intraperitoneally (i.p.) only showed little AB–PAS-positive staining in bronchial epithelium. Animals first sensitized with ovalbumin (OVA) i.p., followed by three intratracheal (i.t.) instillations of OVA, showed a marked increase in AB–PAS-positive staining (n = 5; ∗, P < 0.0001). Pretreatment with BIBX1522 (10 mg/kg, i.p.) inhibited OVA-induced production of goblet cells (n = 5; ††, P < 0.0001).
DISCUSSION
We investigated the role of EGF-R and its ligands in mucin production in airway epithelium by using NCI-H292 cells (23) and by using pathogen-free rats whose airways contain few goblet cells. TNFα induced expression of EGF-R in both NCI-H292 cells and rat-airway epithelial cells. After induction of EGF-R by TNFα, subsequent stimulation of EGF-R by its ligands resulted in MUC5AC production at both gene expression and protein levels. Selective inhibitors of EGF-R tyrosine kinase blocked MUC5AC gene and protein expression caused by TNFα plus EGF-R ligands, suggesting that EGF-R signaling is involved in MUC5AC production.
The mechanism by which TNFα acted on MUC5AC gene and protein expression in NCI-H292 cells requires discussion. In our studies, TNFα alone had little effect on MUC5AC production, although one study reported that TNFα induces mucin MUC2 gene expression (24). Our results show that TGFα had a large effect on MUC5AC gene expression, and that the effect of TGFα was markedly potentiated by coincubation with TNFα. The effect of TNFα plus TGFα was greater than additive. A possible explanation for the potentiating effect of TNFα is the up-regulation of EGF-R. In fact, TNFα caused EGF-R gene expression in NCI-H292 cells; similar findings are reported in A431 cells (15), human pancreatic cancer cells (14), and epidermal keratinocytes (16). The fact that the inhibition of EGF-R tyrosine kinase completely blocked MUC5AC gene and protein expression caused by TNFα plus EGF-R ligands implicates the EGFR pathway in the TNFα–TGFα responses. Two other selective EGF-R tyrosine kinase inhibitors (AG1478 and C56) also inhibited MUC5AC protein production, similar to BIBX1522. However, a selective platelet-derived growth factor tyrosine kinase inhibitor (AG1295) and a negative control for tyrphostins (A1) were without effect, implicating EGF-R tyrosine phosphorylation as the signaling pathway inducing MUC5AC expression.
Present results also suggest a possible sequence for the evolution of goblet-cell production based on the expression of EGF-R: Stimulation with TNFα induced intense EGF-R staining of nongranulated secretory cells; their subsequent activation by EGF-R ligands caused progressive staining for mucous glycoconjugates, and the cells became “pre-goblet” and then “goblet” cells. Instillation of TNFα followed by EGF-R ligands induced goblet-cell production without altering the total number of epithelial cells, suggesting that EGF-R activation promoted selective cell differentiation (not proliferation). These findings suggest that goblet cells are derived from nongranulated secretory cells that express EGF-R and are stimulated by EGF-R ligands to produce mucins. In our study, sensitization caused goblet cell production associated with an increase of the total number of cells in airway epithelium, whereas instillation of TNFα and TGFα caused goblet cell production but not epithelial hyperplasia. One reason for the difference may be the duration after the stimulus. A second reason for the difference may be because of the presence of another mediator that causes epithelial cell proliferation in the sensitized animal, such as cysteinyl leukotrienes, which are increased in asthmatic airways (25). We show that EGF-R are not expressed in control airway epithelium but are expressed in sensitized airways. Cells that stained were pre-goblet and goblet cells, suggesting that EGF-R is involved in goblet cell production. Pretreatment with BIBX1522 prevented airway goblet cell production, confirming the role of EGF-R activation in goblet cell production in experimental asthma. However, two issues remain. (i) The mechanism of expression of EGF-R: TNFα is a likely candidate; it is produced by mast cells (26), neutrophils (27), and macrophages (28) and is elevated in bronchoalveolar lavage fluid obtained in subjects with symptomatic asthma (29); and (ii) The source of EGF-R ligands: in asthmatics, epithelial cells are reported to express EGF (13); another possible source is leukocytes, because eosinophils (30), neutrophils, and monocytes (31) produce EGF-R ligands. In our study, i.p. sensitization with ovalbumin did not cause goblet-cell hyperplasia, but airway instillation of ovalbumin in sensitized rats, which results in leukocyte recruitment (32), caused goblet-cell production. This result suggests a possible role in leukocytes as a source of EGF-R ligands.
Previous studies showed that various stimuli such as ozone (33), sulfur dioxide (34), viruses (34), lipopolysaccharide (33, 35), and platelet activating factor (20) up-regulate mucin expression and secretion. Recently, downstream signaling causing mucin MUC2 expression induced by Pseudomonas aeruginosa was shown to go through a c-Src-Ras-MEK1/2-MAPK-pp90rsk pathway (36); we suggest that EGF-R could provide the upstream signaling for those responses. Additional studies will be required to evaluate the relationship of various inflammatory stimuli to the EGF-R system.
Hypersecretion is a major manifestation in many chronic inflammatory diseases of airways. Presently, there is no effective therapy to relieve the symptoms and to halt the progression of these diseases. Our findings provide a mechanism and a strategy for therapy: by inhibiting EGF-R activation, goblet-cell production is prevented. Inhibition of EGF-R activation is proposed as therapy in hypersecretory airway diseases. Proof of concept in humans will require testing in patients with hypersecretory diseases.
Acknowledgments
The work was supported in part by National Heart, Lung, and Blood Institute Program Project Grant HL-24136.
ABBREVIATIONS
- EGF
epidermal growth factor
- EGF-R
EGF receptor(s)
- TFGα
transforming growth fator α
- TNFα
tumor necrosis factor α
- AB
Alcian blue
- PAS
periodic acid–Schiff
References
- 1.Snider G L, Faling L J, Rennard S I. In: Textbook of Respiratory Medicine. Murray J F, Nadel J A, editors. New York: Saunders; 1994. , Chap. 41. [Google Scholar]
- 2.Boat T F, Boucher R C. In: Textbook of Respiratory Medicine. Murray J F, Nadel J A, editors. New York: Saunders; 1994. , Chapter 43. [Google Scholar]
- 3.Fahy J V, Schuster A, Ueki I, Boushey H A, Nadel J A. Am Rev Respir Dis. 1992;146:1430–1433. doi: 10.1164/ajrccm/146.6.1430. [DOI] [PubMed] [Google Scholar]
- 4.Cardell B S, Pearson R S B. Thorax. 1959;14:341–352. [Google Scholar]
- 5.Dunnill M S. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Chest. 1992;101:916–921. doi: 10.1378/chest.101.4.916. [DOI] [PubMed] [Google Scholar]
- 7.Kelly S M, Hunter J O. Clin Sci. 1990;79:425–427. doi: 10.1042/cs0790425. [DOI] [PubMed] [Google Scholar]
- 8.Vinter-Jensen L, Julh C O, Djurhuus J C, Poulsen S S, Dajani E Z, Brown K D, Ørntoft T F, Teglbjærg P S, Nexø E. Am J Pathol. 1995;147:1330–1338. [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman K, Randell S R, Nettesheim P. Biochem Biophys Res Commun. 1995;217:412–418. doi: 10.1006/bbrc.1995.2792. [DOI] [PubMed] [Google Scholar]
- 10.Ruocco S, Lallemand A, Tournier J M, Gaillard D. Pediatr Res. 1996;39:448–455. doi: 10.1203/00006450-199603000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Hendler F J, Ozanne B W. J Clin Invest. 1984;74:647–651. doi: 10.1172/JCI111463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madtes D K, Busby H K, Strandjord T P, Clark J G. Am J Respir Cell Mol Biol. 1994;11:540–551. doi: 10.1165/ajrcmb.11.5.7524566. [DOI] [PubMed] [Google Scholar]
- 13.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Am J Crit Care Med. 1998;157:1907–1912. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 14.Schmiegel W, Roeder C, Schmielau J, Rodeck U, Kalthoff H. Proc Natl Acad Sci USA. 1993;90:863–867. doi: 10.1073/pnas.90.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donato N J, Rosenblum M G, Steck P A. Cell Growth Differ. 1992;3:259–268. [PubMed] [Google Scholar]
- 16.Valyi-Nagy I, Jensen P J, Albelda S M, Rodeck U. J Invest Dermatol. 1992;99:350–356. doi: 10.1111/1523-1747.ep12616672. [DOI] [PubMed] [Google Scholar]
- 17.Ulich T R. In: Cytokines of the Lung. Kelley J, editor. New York: Dekker; 1993. pp. 307–332. [Google Scholar]
- 18.Weber W, Gill G N, Spiess J. Science. 1984;224:294–297. doi: 10.1126/science.6324343. [DOI] [PubMed] [Google Scholar]
- 19.Massion P P, Inoue H, Richman-Eisenstat J, Grunberger D, Jorens P G, Housset B, Pittet J-F, Wiener-Kronish J P, Nadel J A. J Clin Invest. 1994;93:26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lou Y-P, Takeyama K, Grattan K M, Lausier J A, Ueki I F, Agustí C, Nadel J A. Am J Respir Crit Care Med. 1998;157:1927–1934. doi: 10.1164/ajrccm.157.6.9709113. [DOI] [PubMed] [Google Scholar]
- 21.Mercer R R, Russell M L, Roggli V L, Crapo J D. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 22.Takeyama K, Agustí C, Ueki I, Lausier J, Cardell L O, Nadel J A. Am J Physiol. 1998;275:L294–L302. doi: 10.1152/ajplung.1998.275.2.L294. [DOI] [PubMed] [Google Scholar]
- 23.Kai H, Yoshitake K, Hisatsune A, Kido T, Isohama Y, Takahama K, Miyata T. Am J Physiol. 1996;271:L484–L488. doi: 10.1152/ajplung.1996.271.3.L484. [DOI] [PubMed] [Google Scholar]
- 24.Levine S J, Larivée P, Logun C, Angus C W, Ognibene F P, Shelhamer J H. Am J Respir Cell Mol Biol. 1995;12:196–204. doi: 10.1165/ajrcmb.12.2.7865217. [DOI] [PubMed] [Google Scholar]
- 25.Leikauf G D, Claesson H E, Doupnik C A, Hybbinette S, Grafstrom R C. Am J Physiol. 1990;259:L255–L261. doi: 10.1152/ajplung.1990.259.4.L255. [DOI] [PubMed] [Google Scholar]
- 26.Gordon J R, Galli S J. Nature (London) 1990;346:274–276. doi: 10.1038/346274a0. [DOI] [PubMed] [Google Scholar]
- 27.Xing Z, Kirpalani H, Torry D, Jordana M, Gauldie J. Am J Pathol. 1993;143:1009–1015. [PMC free article] [PubMed] [Google Scholar]
- 28.Ohkawara Y, Yamauchi K, Tanno Y, Tamura G, Ohtani H, Nagura H, Ohkuda K, Takishima T. Am J Respir Cell Mol Biol. 1992;7:385–392. doi: 10.1165/ajrcmb/7.4.385. [DOI] [PubMed] [Google Scholar]
- 29.Broide D H, Lotz M, Cuomo A J, Coburn D A, Federman E C, Wasserman S I. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 30.Wong D T W, Weller P F, Galli S J, Elovic A, Rand T H, Gallagher G T, Chiang T, Chou M Y, Matossian K, McBride J, Todd R. J Exp Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calafat J, Janssen H, Ståhle-Bäckdahl M, Zuurbier A E M, Knol E F, Egesten A. Blood. 1997;90:1255–1266. [PubMed] [Google Scholar]
- 32.Agustí C, Takeyama K, Cardell L O, Ueki I, Lausier J, Lou Y-P, Nadel J A. Am J Respir Crit Care Med. 1998;158:1253–1258. doi: 10.1164/ajrccm.158.4.9801041. [DOI] [PubMed] [Google Scholar]
- 33.Harkema J R, Hotchkiss J A. Toxicol Lett. 1993;68:251–263. doi: 10.1016/0378-4274(93)90136-l. [DOI] [PubMed] [Google Scholar]
- 34.Jany B, Gallup M, Tsuda T, Basbaum C. Biochem Biophys Res Commun. 1991;181:1–8. doi: 10.1016/S0006-291X(05)81373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu T, Takahashi Y, Kawaguchi S, Sakakura Y. Am J Respir Crit Care Med. 1996;153:1412–1418. doi: 10.1164/ajrccm.153.4.8616574. [DOI] [PubMed] [Google Scholar]
- 36.Li J-D, Feng W, Gallup M, Kim J-H, Gum J, Kim Y, Basbaum C. Proc Natl Acad Sci USA. 1998;95:5718–5723. doi: 10.1073/pnas.95.10.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]