Abstract
The sodium–calcium exchanger (NCX) is a critical mediator of calcium homeostasis. In the heart, NCX1 predominantly operates in forward mode to extrude Ca2+; however, reverse-mode NCX1 activity during ischemia/reperfusion (IR) contributes to Ca2+ loading and electrical and contractile dysfunction. IR injury has also been associated with altered fat metabolism and accumulation of long-chain acyl CoA esters. Here, we show that acyl CoAs are novel, endogenous activators of reverse-mode NCX1 activity, exhibiting chain length and saturation dependence, with longer chain saturated acyl moieties being the most effective NCX1 activators. These results implicate dietary fat composition as a plausible determinant of IR injury. We further show that acyl CoAs may interact directly with the XIP (exchanger inhibitory peptide) sequence, a known region of anionic lipid modulation, to dynamically regulate NCX1 activity and Ca2+ homeostasis. Additionally, our findings have broad implications for the coupling of Ca2+ homeostasis to fat metabolism in a variety of tissues.
Keywords: acyl CoA, fat metabolism, ischemia/reperfusion injury, NCX1, sodium–calcium exchange
Introduction
Sodium–calcium exchangers (NCX) are a class of membrane proteins intimately involved in the regulation of intracellular Ca2+ homeostasis, playing key roles in diverse signaling pathways in a variety of cell types. In the heart, the NCX1 isoform is a critical modulator of cardiomyocyte Ca2+ cycling, typically operating in forward mode to extrude one Ca2+ ion for 3–4 Na+ ions (Dong et al, 2002; Hinata and Kimura, 2004) and generating an inward Na+ current that contributes to the plateau phase of the action potential (Weber et al, 2002). In addition to regulating physiological Ca2+ levels, alterations in the ionic, electrical and metabolic milieu that accompany cardiac pathologies such as ischemia/reperfusion (IR) injury (Schafer et al, 2001) and heart failure (Piacentino et al, 2002; Schillinger et al, 2003) promote reverse-mode NCX1 activity, leading to calcium overload and electrical dysfunction (Tani, 1990). In this regard, there is much interest in developing pharmacological inhibitors of reverse-mode NCX1 (Hobai and O'Rourke, 2004).
While much is known about the molecular biology and biophysics of NCX1, the intrinsic regulation of both forward- and reverse-mode NCX1 activity by endogenous signaling and metabolic pathways that may be altered in physiological and pathophysiological conditions has not been fully characterized. Identification of these regulatory processes may therefore provide valuable insights into the cellular mechanisms by which NCX1 is modulated in health and disease in a variety of tissues. One key area of interest is the role of lipids and lipid metabolism in regulating ionic homeostasis via modulation of ion channels and exchangers. Lipid-containing moieties such as the anionic phosphatidylinositol 4,5-bisphosphate (PIP2) are capable of increasing reverse-mode NCX1 activity by reducing Na+-dependent (I1) inactivation, leading to a net increase in Ca2+ influx (Hilgemann and Ball, 1996; He et al, 2000). Interestingly, intracellular levels of anionic long-chain fatty acyl CoA esters (acyl CoAs) are increased in exercise (Goodwin and Taegtmeyer, 2000), ischemia (Whitmer et al, 1978), hypertrophy (Finck et al, 2003) and the failing heart (Sharma et al, 2004) owing to changes in metabolic enzyme activity, substrate preference and oxygen supply. Like PIP2, acyl CoAs also possess a negatively charged head group (CoA) and a hydrophobic tail. Our group and others have shown that acyl CoAs are potent activators of ATP-sensitive potassium (KATP) channels (Larsson et al, 1996; Riedel et al, 2003; Branstrom et al, 2004) and that acyl CoAs and PIP2 may share a common mechanism of action (Schulze et al, 2003) via interaction with common positively charged residues in the KATP channel (Manning Fox et al, 2004). Furthermore, we have shown that alterations in the side-chain length and degree of saturation of the acyl tail greatly affect the efficacy and persistence of KATP channel activation by acyl CoAs (Riedel and Light, 2005).
Therefore, we sought to determine (1) the effects of physiological levels of common acyl CoAs on recombinant and native NCX1 activity and (2) the effects of intracellular acyl CoA elevation on NCX1-mediated Ca2+ overload in an intact cardiomyocyte model. Our findings provide the first direct evidence linking altered fat metabolism and lipotoxicity to NCX1 activity and have broad implications for our current understanding of NCX1 regulation and function in a variety of tissues.
Results
Reverse-mode NCX1 activity is increased by acyl CoAs
Previous measurements of electrogenic NCX1 currents have relied on either whole-cell (O'Rourke et al, 1999; Maack et al, 2005) or giant excised patch clamp techniques (Hilgemann, 1990; Matsuoka et al, 1997). Here we show that NCX1 current can be routinely measured using the conventional excised inside-out patch technique with minor modifications. Adenoviral infection of tsA201 cells with AdVNCX1 resulted in a >75% infection efficiency and excised inside-out membrane patches yielded average peak currents of 12.9±1.1 pA. Reverse-mode NCX1 activity displayed a characteristic rapidly developed peak current followed by a slow Na+-dependent (I1) inactivation to a steady-state level that stabilized at 25±2.2% of the peak current (Figure 1Ai; Hilgemann, 1990). NCX1 currents were sensitive to the NCX inhibitors NiCl2 (5 mM) and KB-R7943 (5 μM), which resulted in a 91.1±2.6 and 84.3±4.9% inhibition of total current, respectively, but were not inhibited by the selective L-type Ca2+ channel blocker nifedipine (Figure 1B).
Figure 1.
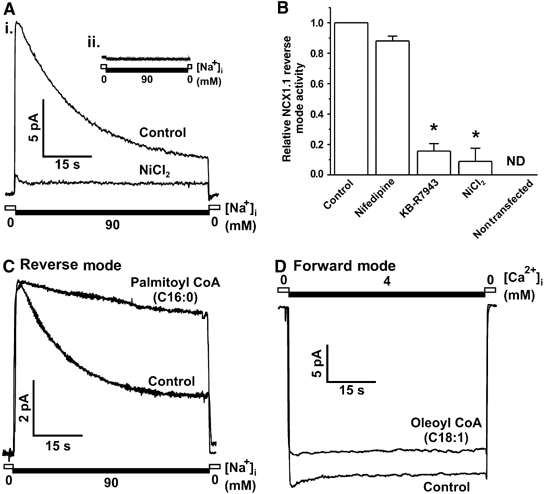
Forward- and reverse-mode NCX1 activity is modulated by acyl CoAs. (A) (i) Representative macroscopic current recording of reverse-mode NCX1 activity from tsA201 cells infected with AdVNCX1 virus showing activation by high intracellular Na+ and inhibition by NiCl2 (5 mM). (ii) Uninfected tsA201 cells are devoid of endogenous NCX activity. (B) Grouped data showing inhibition of reverse-mode NCX1 activity by the specific inhibitors KB-R7943 (5 μM) and NiCl2 (5 mM) but not by the L-type Ca2+ blocker nifedipine (10 μM). n=3–6 patches per group, *P<0.05 versus control, ND=not detected. (C) Representative trace showing that palmitoyl CoA (1 μM) inhibits I1 inactivation. (D) Representative current recording indicating that oleoyl CoA (1 μM) inhibits forward-mode NCX1 activity.
The process of intracellular Na+-induced I1 inactivation (Hilgemann, 1990) is critically regulated by the 20-amino-acid exchanger inhibitory peptide (XIP) sequence in the cytosolic portion of NCX1, with mutations throughout the XIP region dramatically altering inactivation properties (Matsuoka et al, 1997). In addition, PIP2 has been shown to inhibit the transition of the active exchanger into its I1 inactive state (He et al, 2000) thereby increasing reverse-mode activity. PIP2 also influences the activity of a number of ion channels (Suh and Hille, 2005) and in some cases shares a common mechanism of action with acyl CoAs (Schulze et al, 2003; Manning Fox et al, 2004). In this study, we investigated the effects of acyl CoAs on NCX1 activity (Figure 1C and D). Application of 1 μM palmitoyl CoA to the cytosolic surface of the membrane patch significantly inhibited reverse-mode I1 inactivation (Figure 1C) resulting in a 214±74% increase in the late-to-peak current ratio and a 65.2±31.1% increase in total reverse-mode activity, as measured by integrating the area under the curve (AUC; Figure 2B and C). Conversely, we found that oleoyl CoA caused a small yet significant decrease in total forward-mode NCX1 activity (12.7±4.3%; Figure 1D). As Ca2+ loading via increased reverse-mode NCX1 is considered to be a major contributor to cardiac damage sustained during IR injury (Piper et al, 2003), we further examined the modulation of I1 inactivation by acyl CoAs.
Figure 2.
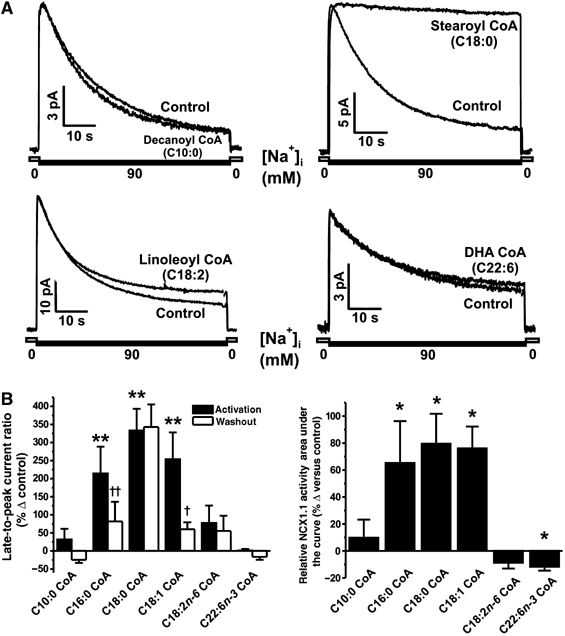
Activation of reverse-mode NCX1 activity by acyl CoAs exhibits saturation and chain length dependence. (A) Representative macroscopic NCX1 current recordings showing that short-chain (decanoyl CoA, C10:0) and polyunsaturated acyl CoAs (linoleoyl CoA, C18:2; DHA CoA, C22:6) do not inhibit I1 inactivation, unlike stearoyl CoA (C18:0). (B) Grouped data showing the maximum effect (black bars) and reversibility (white bars) of each acyl CoA on the late-to-peak current ratio. n=3–11 patches per group. **P<0.01 versus control in the respective group, †P<0.05 and ††P<0.01 versus maximum activation in the respective group. (C) Grouped data indicating that total NCX1 reverse-mode activity was increased by palmitoyl, stearoyl and oleoyl CoA only. n=4–6 patches per group. *P<0.05 versus control activity measured in the same patch before acyl CoA application.
Regulation of I1 inactivation by acyl CoAs demonstrates side-chain length and saturation dependence
Our group and others have previously shown that the regulation of KATP channels by acyl CoAs is dependent on both chain length and saturation (Branstrom et al, 2004; Riedel and Light, 2005). Here we tested a number of acyl CoAs on NCX1 activity and found similar trends to those observed for activation of the β-cell KATP channel (Figure 2; Riedel and Light, 2005). Specifically, we found that at a concentration of 1 μM, the shorter chain decanoyl CoA (C10:0) had no significant effect on either the late-to-peak current ratio or relative NCX1 activity (Figure 2A). Increasing the chain length to 16 carbons (palmitoyl CoA, C16:0) and further to 18 carbons (stearoyl CoA, C18:0) significantly increased both the late-to-peak current ratio (Figure 2B) and relative exchanger activity (Figure 2C). Addition of one double bond (oleoyl CoA, C18:1) still caused an increase in NCX1 activity similar to that of palmitoyl and stearoyl CoA, whereas addition of a second double bond (linoleoyl CoA, C18:2) eliminated the effect of acyl CoAs on I1 inactivation (Figure 2B and C). Application of the n-6 polyunsaturated fish oil docosahexaenoyl (DHA) CoA resulted in a small yet significant decrease in total NCX1 reverse-mode activity resulting from a decrease in peak exchanger activity (Figure 2C).
Regulation of I1 inactivation by acyl CoAs does not require ATP
Several studies have demonstrated a stimulatory effect of Mg-ATP on NCX1 activity (Hilgemann, 1990; Condrescu et al, 1995; Hilgemann and Ball, 1996; Berberian et al, 1998), a mechanism that may occur via phosphorylation of PIP and the maintenance of membrane PIP2 levels. To determine that the observed activation of NCX1 by acyl CoAs in our study was not contaminated by membrane-bound PIP2 or by associated ATP-mediated events, we repeated our experiments in the absence of Mg-ATP (Figure 3). Under these conditions, I1 inactivation occurred more rapidly (τ=7.8±1.1 versus 17.5±1.7 s (P<0.01)) and to a greater extent than in the presence of 2 mM MgATP (Figure 3C, compare white bars). Perfusion of PIP2-depleted membrane patches with acyl CoAs resulted in a much larger activation of NCX1 current (∼3.8- and ∼19-fold for palmitoyl and oleoyl CoA, respectively) than in the presence of Mg-ATP (∼2.4-fold for both palmitoyl and oleoyl CoA; Figure 3C).
Figure 3.

Activation of reverse-mode NCX1 activity by acyl CoAs occurs in the absence of Mg-ATP. Representative macroscopic NCX1 current recordings in the presence (A) and absence (B) of 2 mM Mg-ATP. Under both conditions, acyl CoAs (oleoyl CoA, 1 μM shown here) inhibited I1 inactivation. (C) Grouped data indicating that the increase in late-to-peak current ratio as a result of acyl CoA exposure (black bars) was greater in the absence of Mg-ATP. n=7–11 patches per group. *P<0.05 versus corresponding control patches in the absence of acyl CoAs (white bars).
Acyl CoAs interact with XIP, but at a site distinct from that of PIP2
PIP2 regulates NCX1 activity via interaction with the XIP region located on the intracellular loop between transmembrane segments 5 and 6 (Li et al, 1991). Residue F255 seems especially important in governing this PIP2-mediated modulation (He et al, 2000). To assess whether acyl CoAs interact with NCX1 via a similar mechanism, we applied an antibody directed against XIP (AbXIP) directly to membrane patches. In the absence of additional modulators, AbXIP reduced peak current by ∼80% while eliminating the steady-state current. However, pre-exposing the membrane patch to 1 μM oleoyl CoA resulted in a significantly impaired AbXIP-mediated inhibition of both peak and late currents (22.6±8.2 and 32.5±8.4% inhibition, respectively; Figure 4A). To elucidate further similarities between binding of acyl CoAs and PIP2, we created the previously described F255E mutant (Matsuoka et al, 1997). NCX1(F255E) mutant exchangers are inactivated more rapidly and PIP2 is incapable of inducing NCX1(F255E) exchanger activity following I1 inactivation (He et al, 2000) as a result of a weakened PIP2–XIP interaction. Although we have successfully reproduced this finding using a similar protocol and found that acyl CoAs are likewise unable to stimulate significant activity in inactivated mutant NCX1 (Figure 4B), we report two additional observations. Firstly, unlike PIP2, oleoyl CoA can reduce inactivation when bound before NCX1(F255E) activation (Figure 4C). Under these conditions, total NCX1(F255E) activity was significantly increased 10.80±3.34-fold (P<0.05, n=11) compared to a nonsignificant 3.09±1.21-fold (P=0.11, n=11) when the exchanger was exposed to 30 μM PIP2. This suggests that the NCX1(F255E) mutant exchanger retains the ability to be significantly modified by acyl CoAs but not by PIP2. Secondly, we found that the inability of acyl CoAs to stimulate NCX1 activity in a population of inactivated transporters was a property limited to the F255E mutant. Figure 4D shows that oleoyl CoA readily stimulated wild-type NCX1 activity, increasing current levels by 91.6±4.5% (n=5) over that measured at the base of the inactivation, whereas PIP2 failed to do so. Conversely, even the wild-type exchanger was not significantly re-activated by PIP2 (32.4±14% increase in current following PIP2 perfusion versus base inactive current, n=6, P=0.08; Figure 4D).
Figure 4.

The activation of reverse-mode NCX1 activity by acyl CoAs may occur via their interaction with the XIP region, but at a site distinct from that of PIP2. (A) Grouped data indicating that pre-exposure of membrane patches to oleoyl CoA (1 μM) abolishes the inhibitory action of an anti-XIP antibody. n=3–4 patches per group. *P<0.05 versus respective peak (black bars) and late (white bars) current in patches exposed to the anti-XIP antibody alone. (B) Representative current recording indicating that neither acyl CoAs (1 μM oleoyl CoA shown here) or PIP2 (30 μM) is able to re-activate NCX1(F255E) mutant exchangers following I1 inactivation. (C) Representative current recordings indicating that acyl CoAs (1 μM oleoyl CoA shown here) but not PIP2 (30 μM) are able to prevent the entry of NCX1(F255E) mutant exchangers into the I1 inactive state. (D) Representative macroscopic current recording indicating the ability of acyl CoAs (oleoyl CoA, 1 μM shown here) but not PIP2 (30 μM) to re-activate wild-type NCX1 exchanger activity following a period of I1 inactivation.
Acyl CoA accumulation increases NCX1 reverse-mode activity and intracellular Ca2+ loading
Accumulation of acyl CoAs within transgenic mouse cardiomyocytes is associated with cardiomyopathy (Chiu et al, 2001); however, the mechanisms underlying this association are not clearly delineated. In light of our own findings and those of Chiu and colleagues, we investigated the cellular consequences of acyl CoA accumulation and subsequent increases in NCX1 activity by examining whole-cell NCX1 currents in intact adult rat ventricular myocytes and Ca2+ loading via reverse-mode NCX1 activity in an intact model cellular system in which acyl CoA levels were manipulated.
Reverse-mode NCX1 activity was elicited in adult rat cardiomyocytes using a previously described external Na+ removal protocol (Su et al, 1999) in an attempt to visualize a change in I1 inactivation in response to pathophysiological increases in intracellular Na+ and acyl CoA levels as are seen during IR (Figure 5). The observed reverse-mode NCX1 activity was again sensitive to nickel and KB-R7943 (Figure 5B and C). Dialysis of myocytes with 20 mM Na+ mimicked ischemic intracellular Na+ concentrations, and activation of reverse-mode activity produced the characteristic I1 inactivation response (Figure 5A; Hilgemann et al, 1992). The addition of 1 μM oleoyl CoA to the intracellular milieu, via dialysis of the pipette solution, resulted in a significant increase in the late-to-peak current ratio (0.673±0.03 versus 0.512±0.06, P<0.05).
Figure 5.
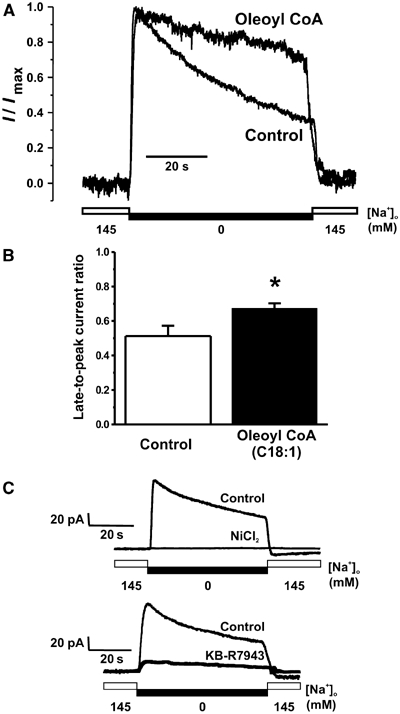
Activation of rat ventricular myocyte reverse-mode whole-cell NCX1 current by oleoyl CoA. (A) Representative whole-cell NCX1 current recordings from myocytes dialyzed with either control pipette solution or oleoyl CoA-containing pipette solution. (B) Grouped data demonstrating a significant increase in late-to-peak current ratio in myocytes dialyzed with oleoyl CoA (1 μM, black bars, n=6) when compared to control myocytes (white bars, n=7). (C) Example whole-cell recordings demonstrating inhibition of the current changes recorded in our system by the NCX blocker NiCl2 (5 mM) and the reverse-mode specific blocker KB-R7943 (5 μM). *P<0.05.
Neonatal rat cardiomyocytes were used as a model system for measuring Ca2+ loading because of their robust expression of NCX1 (Boerth et al, 1994) and the ability to maintain their phenotype under standard culture conditions. We used an established protocol for inducing reverse-mode NCX activity in intact spontaneously beating primary myocyte cultures that involves an initial loading of the cells with Na+ via inhibition of the Na/K-ATPase pump using a K+-free solution. Subsequent removal of extracellular Na+ promotes Na+ extrusion and concomitant Ca2+ loading via reverse-mode NCX1 activity (Figure 6A; Eigel and Hadley, 2001). Changes in cytosolic Ca2+ were monitored using the Ca2+-sensitive fluorophore calcium green. The observed Ca2+ loading was inhibited by nickel and external Ca2+ removal, but not by nifedipine (Figure 6E)—observations that are consistent with the measurement of reverse-mode NCX1 activity. Acyl CoA levels within neonatal rat cardiomyocytes were elevated by adenoviral-mediated overexpression of fatty acyl CoA synthetase-1 (FACS-1 or ACSL1), the primary enzyme involved in free fatty acid (FFA) esterification to acyl CoAs (Figure 6C, inset; Suzuki et al, 1990; Coleman et al, 2002). Incubation of cardiomyocytes overexpressing FACS-1 in the presence of FFAs led to a significant increase in maximum and total Ca2+ loading (Figure 6B–D). Total cytosolic acyl CoA content was subsequently measured in each experimental group using HPLC to quantify the extent of acyl CoA loading (Figure 6F). Whereas the expression of FACS-1 alone was insufficient to significantly increase total acyl CoA content (0.171±0.053 pmol/μg total protein, P=0.14, NS), the combination of FACS-1 overexpression and treatment of myocytes with elevated FFAs produced a significant ∼7-fold increase in total acyl CoA content (0.174±0.043 pmol/μg total protein). It was in this treatment group that we observed the greatest activation of reverse-mode NCX1 activity and Ca2+ loading (Figure 6D).
Figure 6.

Reverse-mode NCX1 activity is increased in neonatal rat cardiomyocytes following elevation of intracellular acyl CoA levels. (A) Representative Ca2+ transient recording from a group of neonatal myocytes subjected to a protocol designed to induce reverse-mode NCX1 activity. (B) Representative recordings showing typical reverse-mode NCX1-mediated Ca2+ loading in neonatal myocytes. Panel shows only the reverse-mode NCX1 activity-related portion of the recording obtained during Na+-free solution exposure. (C) Grouped data showing significant increase in maximum Ca2+ loading in FACS-1-overexpressing neonatal myocytes incubated with 0.2 mM palmitate and 0.1 mM oleate. n=5–7 experiments per group with 6–8 cells per experiment. **P<0.01 versus control group, #P<0.05 versus FFA group, @P<0.05 versus FACS-1 group. Inset: Representative Western blot showing overexpression of FACS-1 protein after treatment with AdVFACS-1 virus. (D) Total reverse-mode NCX1 activity in control myocytes and those treated with FFA or FACS-1 alone or in combination. n=5–7 experiments per group with 6–8 cells per experiment. *P<0.05 versus control group. †P<0.05 versus FFA+FACS-1 group. (E) Total reverse-mode NCX1 activity was inhibited by a Ca2+-free extracellular perfusate and by the specific inhibitor NiCl2 (5 mM) but not by the L-type Ca2+ channel blocker nifedipine (10 μM). n=3–4 experiments per group with 6–8 cells per experiment. **P<0.01 versus control group. (F) Total cytosolic acyl CoA levels were increased significantly by palmitate and oleate incubation in FACS-1-overexpressing cells. n=3 experiments per group. *P<0.05 versus control group. #P<0.05 versus FFA group.
Discussion
Accumulation of Ca2+ within the cardiomyocyte contributes to hallmark electrical and contractile dysfunction of IR injury (Carmeliet, 1999; Eigel and Hadley, 2001). The contribution of reverse-mode NCX activity to Ca2+ overload in this setting has been the focus of a number of important studies. Of particular note is the observation that cardiac-specific overexpression of the NCX1 gene resulted in increased susceptibility to IR-mediated cardiac dysfunction (Cross et al, 1998), whereas antisense inhibition or complete cardiac-specific ablation of NCX1 provided significant protection (Eigel and Hadley, 2001; Imahashi et al, 2005). These studies highlight the importance of NCX1 activity in contributing to IR injury. Increased NCX1 reverse-mode activity by whatever mechanism therefore represents a major burden to contractile recovery following IR injury. Results from our study provide the first direct evidence that NCX1 activity is under the influence of acyl CoAs, and importantly, by direct intracellular application of acyl CoAs, we demonstrate that this regulation occurs in cardiac myocytes. These common fatty acid intermediates represent a novel class of NCX1 modulators that may be involved in the observed increase in NCX1 reverse-mode activity in IR injury as well as the physiological coupling of Ca2+ homeostasis to lipid metabolism in a variety of tissues.
Mechanistic insights
Previous studies have demonstrated that transition of active reverse-mode NCX1 to the I1 inactive state is governed by intracellular sodium ions and the endogenous regulatory XIP sequence (Hilgemann et al, 1992; Matsuoka et al, 1997). Our results provide evidence that acyl CoAs may interact with NCX1 at or near this XIP sequence, as these anionic lipids can prevent an XIP antibody from reaching its epitope. We also show that acyl CoAs may disrupt the XIP-mediated conformational change required for inactivation of NCX1, thereby increasing Ca2+ loading under conditions used to stimulate reverse-mode NCX1 activity.
The differences observed between the effects of acyl CoAs on the wild-type and F255E mutant exchanger provide insights into the molecular mechanisms mediating XIP-induced inactivation. While wild-type NCX1 can be prevented from entering the I1 inactive state in the presence of both PIP2 (He et al, 2000) and acyl CoAs (this study), wild-type NCX1 can be re-activated only by acyl CoAs. This suggests that the ability of the endogenous XIP region to mediate inactivation is hindered by acyl CoAs. The addition of a negative charge in the XIP region of the NCX1(F255E) mutant may act to repel the negatively charged PIP2 and acyl CoA molecules, resulting in the observed enhancement of inactivation (Figure 4B and C). Differences in either net charge or charge location may explain why PIP2 is less capable of altering NCX1(F255E) activity than acyl CoAs (Figure 4C). The inability of either PIP2 or acyl CoAs to re-activate mutant NCX1(F255E) currents (Figure 4B) suggests that the interaction of XIP with these anionic lipids is weakened by the addition of a negative charge at position 255 of the XIP region facilitating a faster rate of inactivation.
Physiological significance
Acyl CoAs are dynamically regulated within the cardiomyocyte and serve as a major substrate for β-oxidation and lipogenesis. Acyl CoAs are increased in both physiological (during exercise; Goodwin and Taegtmeyer, 2000) and pathophysiological (during IR; Whitmer et al, 1978) situations. Acyl CoAs are also known regulators of a number of proteins including ion channels (Faergeman and Knudsen, 1997; Haber et al, 2003). The total acyl CoA pool in the heart is large but free cytosolic acyl CoA levels are highly buffered to levels near 1 μM by several acyl CoA and fatty acid binding proteins (Faergeman and Knudsen, 1997). Although the actual levels of free acyl CoA at distinct subcellular locations are not known, they have been shown to increase during cardiac ischemia (Whitmer et al, 1978) as plasma fatty acid levels increase. Accordingly, we show that a rebalancing of NCX1 activity towards increased outward current occurs in the presence of acyl CoAs (at 1 μM), with forward-mode activity being slightly reduced and reverse-mode activity being dramatically increased. During exercise, when the exchanger is operating primarily in forward mode to extrude Ca2+, a small decrease in NCX1 activity may have a positive inotropic effect by maintaining higher or prolonged intracellular Ca2+ levels and may decrease the likelihood of arrhythmogenic delayed afterdepolarizations (Bers et al, 2002). Conversely, under pathophysiological conditions such as IR injury where reverse-mode activity is favored, a large increase in reverse-mode NCX1 activity by acyl CoAs may result in increased Ca2+ loading, thus further impairing contractile function and increasing the likelihood of developing Ca2+-dependent pro-arrhythmic events. In this regard, reverse-mode NCX1 inhibitors have been shown to reduce the occurrence of ischemia-induced arrhythmias (Takahashi et al, 2003).
The contribution of elevated plasma FFAs to the severity of IR injury is not clearly established in humans; however it has been shown that obese adult Zucker fatty rats exhibit greater contractile dysfunction following a period of global ischemia than their lean control counterparts (Zhou et al, 2000; Sidell et al, 2002). This contractile dysfunction was completely reversed with the use of peroxisome proliferator-activated receptor-gamma agonists that among other effects decrease plasma FFA levels (Zhou et al, 2000; Sidell et al, 2002), suggesting that elevated FFAs may increase the risk for contractile dysfunction in the ischemic heart. These results support our suggestion that increased reverse-mode NCX activity under conditions of elevated intracellular saturated acyl CoAs represents an important novel regulatory pathway contributing to cardiac dysfunction in multiple pathological settings including ischemia, hypertrophy, diabetic cardiomyopathies, heart failure and other conditions associated with lipotoxic heart disease.
Acyl CoA saturation and implications for IR injury
Altering the composition of dietary fatty acids can change cellular membrane constituents and alter lipid profiles within cells (Hulbert et al, 2005). Modulation of both the level and composition of intracellular acyl CoAs that fluctuate as a result of changing metabolism may serve as an important modulatory mechanism for controlling signaling processes including NCX1 activity. We demonstrate that modulation of NCX1 by acyl CoAs is dependent on the number of carbons and double bonds present in the hydrophobic tail, with longer chain more saturated acyl CoAs causing the greatest increases in reverse-mode NCX1 activity. This suggests that the severity of contractile dysfunction sustained during IR injury may be linked to plasma FFA composition and hence to the type of fat consumed in the diet. In particular, we have shown that saturated and monounsaturated acyl CoAs of chain length 16 or greater are more effective NCX1 activators than shorter chain or polyunsaturated acyl CoAs. We have established a similar dependence on side-chain length and degree of saturation for the activation of KATP channels by acyl CoAs including a lack of effect by the fish oil ester DHA CoA (Riedel and Light, 2005). Interestingly, Xiao et al (2004) have shown that the polyunsaturated fats DHA and eicosapentaenioc acid inhibit both forward- and reverse-mode NCX activity, whereas the saturated stearic acid does not. In agreement with these results, we show that the polyunsaturated fish oil ester DHA CoA slightly inhibits reverse-mode NCX1 activity, a mechanism that may contribute to the well-documented cardioprotective effects of dietary fish oils (Kris-Etherton et al, 2002; Marchioli et al, 2002). Conversely, diets higher in saturated fats such as stearic or palmitic acid may increase susceptibility to IR injury via enhanced reverse-mode NCX1 activation. This hypothesis is supported by the previous finding that diabetic mice fed diets high in long-chain fatty acids exhibited increased susceptibility to cardiomyopathies, whereas those fed diets high in medium-chain fatty acids did not (Finck et al, 2003). Our results show that unlike the long-chain acyl CoAs, the medium-chain decanoyl CoA was incapable of significantly increasing reverse-mode NCX1 activity and may therefore provide a contributory mechanism for the observed benefits of strategies designed to lower serum lipids and/or specifically decrease long-chain fatty acyl triglycerides in the treatment of diabetic cardiomyopathies (Finck et al, 2003).
Implications for other tissues
NCX is expressed in a number of tissues including the heart, endocrine pancreas and brain. Three separate genes have been identified, each encoding several splice variants in these and other tissues (Quednau et al, 1997), suggesting that NCX activity is an important physiological process for the maintenance of appropriate ion homeostasis. NCX1 is the most ubiquitously expressed of the three genes and alternatively spliced variants are highly homologous, with differences from one splice variant to another existing only within a small alternative splicing region in the large intracellular loop (Quednau et al, 1997). The XIP region, which we have identified as a putative acyl CoA interaction site, is conserved among all NCX1 isoforms (Quednau et al, 1997) and is similar in NCX2 and NCX3 (Li et al, 1994; Nicoll et al, 1996). The presence of the XIP region in all NCX gene products suggests that regulation of NCX by acyl CoAs may regulate Ca2+ homeostasis in many tissues. This may be especially important in disease states such as obesity and diabetes, where fat metabolism can be altered by high levels of plasma FFAs (Golay et al, 1986; Reaven et al, 1988) leading to elevated intracellular acyl CoA levels.
In conclusion, our results provide evidence for a novel link between cellular metabolism and calcium homeostasis. Given that free acyl CoA levels within cardiomyocytes are dynamically regulated according to metabolic demand, as well as during several pathologies, it is tempting to speculate that acyl CoAs may be a key endogenous regulator of NCX1 activity in the heart. Further studies are therefore warranted to determine the contributions of acyl CoA modulation of NCX activity to cellular function both under physiological and pathophysiological conditions in a variety of tissues expressing this important class of ion exchanger.
Materials and methods
Molecular biology
The rat heart NCX1 (Low et al, 1993) clone in pcDNA3 was obtained from Dr Jonathan Lytton and the NCX1 adenovirus was obtained from Dr Joseph Cheung. Labeling of amino acids beginning with the first in-frame methionine results in the highly conserved XIP region spanning amino acids 251–270. Therefore, the equivalent mutation of F223 from previous studies (Matsuoka et al, 1997; He et al, 2000) corresponded to F255 in our clone. The discrepancy occurs owing to the potential existence of an NH2-terminal 32-amino-acid signaling sequence that may be cleaved before insertion into the plasma membrane (Nicoll et al, 1990). The F255E XIP region mutant was created from wild-type template using standard PCR protocols. The presence of the mutation was verified by diagnostic digest and sequence analysis.
Cell culture
tsA201 cells, which are devoid of endogenous NCX activity (Figure 1Aii), were maintained in DMEM supplemented with 25 mM glucose, 2 mM L-glutamine, 10% FCS and 0.1% penicillin/streptomycin in a humidified incubator at 37°C with 5% CO2. Cells were passaged and plated at 60–80% confluency in 35 mm culture dishes ∼8 h before infection or transfection.
Isolation of rat cardiac myocytes
All animal protocols were performed in accordance with the University of Alberta Animal Policy and Welfare Committee and the Canadian Council on Animal Care (CCAC) Guidelines. Adult right ventricular myocytes were obtained from male Sprague–Dawley rats as described previously (Bouchard et al, 1993; Light et al, 1998).
Neonatal cardiac myocytes were isolated from the hearts of 1- to 3-day-old neonatal rat pups as described previously (Kovacic et al, 2003). The cells were plated on fibronectin-coated coverslips at ∼100% confluency.
Cellular infection/transfection
Wild-type NCX1 was expressed in tsA201 cells via adenovirus delivery of the NCX1 and green fluorescent protein clones (GFP; pGreenLantern, Life Technologies, Gaithersburg, MD) driven under separate CMV promoters. Cells were exposed to ∼30 PFU/cell AdVNCX1 for 2–4 h and macroscopic NCX1 current recordings were performed 24–48 h later.
The NCX1(F255E) mutant exchanger was inserted into pcDNA3.1, transfected using the Ca2+ phosphate precipitation technique and visualized by coexpression of GFP. Macroscopic NCX1(F255E) recordings were performed after 24–48 h.
After 18 h of culture, neonatal rat cardiomyocytes were allocated to three different groups. Group 1 cells were continued in culture with no additional treatment. Group 2 and 3 cells were infected with 10 PFU/cell AdVFACS-1. At 24 h post-infection, group 3 cells were incubated with 0.2 mM palmitate and 0.1 mM oleate complexed to BSA in DMEM/F12 media for 18 h.
Electrophysiological measurements
The excised inside-out patch clamp technique was used to measure macroscopic outward (reverse-mode) and inward (forward-mode) NCX currents from infected or transfected tsA201 cells. Large tip diameter patch pipettes were pulled from borosilicate glass (G85150T-3; Warner Instruments Inc., Hamden, CT) to yield resistances between 400 and 700 kΩ when backfilled with buffer solution.
For outward reverse-mode NCX current measurements, the pipette (extracellular) solution contained the following (in mM): CsCl 140, TEA 20, HEPES 5, glucose 10, MgCl2 1.4 and CaCl2 4. pH was adjusted to 7.4 with CsOH. Outward currents were elicited by rapidly switching from an intracellular cesium-based solution containing (in mM) CsCl 120, TEA 20, HEPES 5, glucose 10, MgCl2 1.4 and CaCl2 4.28 to an intracellular sodium-based solution containing (in mM) CsCl 30, NaCl 90, TEA 20, HEPES 5, glucose 10, MgCl2 1.4 and CaCl2 4.28. Free calcium concentrations were buffered to ∼800 nM with 5 mM EGTA and pH was adjusted to 7.2 with CsOH.
For inward forward-mode NCX current measurements, the pipette (extracellular) solution contained (in mM) CsCl 30, NaCl 90, TEA 20, HEPES 5, glucose 10, MgCl2 1.4 and CaCl2 4.28. Free calcium concentrations were buffered to ∼800 nM with 5 mM EGTA and pH was adjusted to 7.4 with CsOH. Inward currents were elicited by rapidly changing the intracellular solution from a cesium-based low-calcium solution containing (in mM) CsCl 120, TEA 20, HEPES 5, glucose 10, MgCl2 1.4, CaCl2 4.28 and EGTA 5 to a cesium-based high-calcium solution containing (in mM) CsCl 140, TEA 20, HEPES 5, glucose 10, MgCl2 1.4 and CaCl2 4. pH of the intracellular solutions was adjusted to 7.2 with CsOH. In some experiments, 2 mM MgATP was added to both extracellular and intracellular solutions where indicated in the text. All solution changes were achieved in <2 s.
Membrane patches were held at 0 mV and NCX currents were measured and analyzed using an Axopatch 200B amplifier and Clampex 8.1 software (Axon Instruments, Foster City, CA). All experiments were performed at room temperature (22±1°C).
Whole-cell myocyte NCX1 currents were measured as previously described (Su et al, 1999) with the exception that pipettes were pulled from borosilicate glass (G85150T-3) to yield resistances between 600 and 850 MΩ and the pipette solution contained 20 mM NaCl and no ATP.
Measurement of Ca2+ transients
Neonatal rat cardiomyocytes from three different groups outlined above were rinsed and loaded for 30 min at room temperature and for 30 min at 37°C with the Ca2+-sensitive fluorescent probe Calcium Green-1AM (4 μM, in a 1:1 v/v dimethyl sulfoxide:pluronic acid mixture; Molecular Probes, Eugene, OR, USA). A Photomultiplier Detection System (PTI, Lawrenceville, NJ, USA) with Clampex 8.1 was used for data acquisition and analysis. Calcium Green-1AM was excited with 480 nm light and the emitted light intensity at 520 nm was digitized and stored. Cells were subjected to a superfusion protocol designed to evoke reverse-mode NCX activity as described previously (Eigel and Hadley, 2001). Briefly, cells were superfused for 2 min with a solution containing (in mM) NaCl 140, KCl 4, HEPES 10, CaCl2 2.5, MgCl2 1 and glucose 10 to evoke control Ca2+ transients. Cells were then superfused for 5 min with a K+-free solution containing (in mM) NaCl 144, HEPES 10, CaCl2 2.5, MgCl2 1 and glucose 10, which resulted in intracellular Na+ loading. Finally, a Na+-free solution containing (in mM) LiCl 140, KCl 4, HEPES 10, CaCl2 2.5, MgCl2 1 and glucose 10 was superfused for 5 min to evoke reverse-mode NCX activity. pH was adjusted to 7.4 in all solutions. TTX (20 μM) was added to the K+-free and Na+-free solutions to block TTX-sensitive INa, thus preventing Na+ loading via voltage-gated Na+ channel activity. NiCl2 (5 mM) was added to the Na+-free solution in some experiments to inhibit NCX activity (Hinde et al, 1999). The increase in diastolic Ca2+ indicator fluorescence and AUC during reverse-mode NCX activity were normalized to the amplitude of the Ca2+ transient under control conditions. The Ca2+ transient experiments were performed at room temperature.
Experimental compounds
Nifedipine was dissolved in DMSO as a 10 mM stock solution and diluted to concentrations indicated in text before use. The final DMSO concentration of 0.1% did not affect NCX currents in the absence of nifedipine (data not shown). NiCl2 was dissolved directly into solutions to concentrations indicated in the text. All acyl CoAs were purchased from Sigma (Sigma-Aldrich, Oakville, ON) as Li+ salts and dissolved in ddH2O as 10 mM stock solutions. PIP2 was purchased from Avanti Polar Lipids Inc. (Alabaster, AL) as a tri-ammonium salt and dissolved in ddH2O as a 5 mM stock solution. Before use, stock solutions were sonicated for 5–10 min and diluted in intracellular solution to concentrations indicated in the text. The α-XIP antibody (Alpha Diagnostic Intl, San Antonio, TX) was reconstituted in PBS at 1 mg/ml, and subsequently diluted 1:100 in intracellular solution before use.
Determination of free acyl CoA content
The free intracellular acyl CoA content of neonatal myocytes was determined using HPLC as described previously (Larson and Graham, 2001). Cells were plated at 100% confluency on 60 mm culture dishes and treated as described in text with fatty acids and/or AdVFACS-1 virus. Values obtained were normalized to total protein content.
Statistical analysis
Recombinant macroscopic and whole-cell NCX1 currents were normalized to the stable peak current obtained under control conditions. Inactivation was calculated as the ratio of the steady-state current obtained after 1 min of activation to the peak current obtained during activation (late-to-peak current ratio). Total NCX1 current was measured as the area under the activation curve during the 1 min activation period. Statistical significance was assessed using the paired or unpaired Student's t-test where required. P<0.05 was considered to be significantly different. Data are expressed as means±s.e.
Acknowledgments
We thank Suzanne Kovacic for the isolation of neonatal rat cardiomyocytes and Ken Strynadka for help with the acyl CoA HPLC measurements. This work was supported by funding from the Alberta Heritage Foundation for Medical Research (AHFMR) and the Canadian Institutes of Health Research (CIHR, MOP 39745). MJR was supported by AHFMR and Canadian Diabetes Association Scholarships. IB and GJS received salary supported by the CIHR Strategic Training Initiative. PEL and JRBD received salary support as AHFMR Scholars and CIHR New Investigators.
References
- Berberian G, Hidalgo C, DiPolo R, Beauge L (1998) ATP stimulation of Na+/Ca2+ exchange in cardiac sarcolemmal vesicles. Am J Physiol 274: C724–C733 [DOI] [PubMed] [Google Scholar]
- Bers DM, Pogwizd SM, Schlotthauer K (2002) Upregulated Na/Ca exchange is involved in both contractile dysfunction and arrhythmogenesis in heart failure. Basic Res Cardiol 97 (Suppl 1): I36–I42 [DOI] [PubMed] [Google Scholar]
- Boerth SR, Zimmer DB, Artman M (1994) Steady-state mRNA levels of the sarcolemmal Na(+)–Ca2+ exchanger peak near birth in developing rabbit and rat hearts. Circ Res 74: 354–359 [DOI] [PubMed] [Google Scholar]
- Bouchard RA, Clark RB, Giles WR (1993) Role of sodium–calcium exchange in activation of contraction in rat ventricle. J Physiol 472: 391–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branstrom R, Aspinwall CA, Valimaki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, Larsson O (2004) Long-chain CoA esters activate human pancreatic beta-cell K(ATP) channels: potential role in Type 2 diabetes. Diabetologia 47: 277–283 [DOI] [PubMed] [Google Scholar]
- Carmeliet E (1999) Cardiac ionic currents and acute ischemia: from channels to arrhythmias. Physiol Rev 79: 917–1017 [DOI] [PubMed] [Google Scholar]
- Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE (2001) A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest 107: 813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Lewin TM, Van Horn CG, Gonzalez-Baro MR (2002) Do long-chain acyl-CoA synthetases regulate fatty acid entry into synthetic versus degradative pathways? J Nutr 132: 2123–2126 [DOI] [PubMed] [Google Scholar]
- Condrescu M, Gardner JP, Chernaya G, Aceto JF, Kroupis C, Reeves JP (1995) ATP-dependent regulation of sodium–calcium exchange in Chinese hamster ovary cells transfected with the bovine cardiac sodium–calcium exchanger. J Biol Chem 270: 9137–9146 [DOI] [PubMed] [Google Scholar]
- Cross HR, Lu L, Steenbergen C, Philipson KD, Murphy E (1998) Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ Res 83: 1215–1223 [DOI] [PubMed] [Google Scholar]
- Dong H, Dunn J, Lytton J (2002) Stoichiometry of the Cardiac Na+/Ca2+ exchanger NCX1.1 measured in transfected HEK cells. Biophys J 82: 1943–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigel BN, Hadley RW (2001) Antisense inhibition of Na+/Ca2+ exchange during anoxia/reoxygenation in ventricular myocytes. Am J Physiol Heart Circ Physiol 281: H2184–H2190 [DOI] [PubMed] [Google Scholar]
- Faergeman NJ, Knudsen J (1997) Role of long-chain fatty acyl-CoA esters in the regulation of metabolism and in cell signalling. Biochem J 323 (Part 1): 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP (2003) A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci USA 100: 1226–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golay A, Swislocki AL, Chen YD, Jaspan JB, Reaven GM (1986) Effect of obesity on ambient plasma glucose, free fatty acid, insulin, growth hormone, and glucagon concentrations. J Clin Endocrinol Metab 63: 481–484 [DOI] [PubMed] [Google Scholar]
- Goodwin GW, Taegtmeyer H (2000) Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol 279: H1490–H1501 [DOI] [PubMed] [Google Scholar]
- Haber EP, Ximenes HM, Procopio J, Carvalho CR, Curi R, Carpinelli AR (2003) Pleiotropic effects of fatty acids on pancreatic beta-cells. J Cell Physiol 194: 1–12 [DOI] [PubMed] [Google Scholar]
- He Z, Feng S, Tong Q, Hilgemann DW, Philipson KD (2000) Interaction of PIP(2) with the XIP region of the cardiac Na/Ca exchanger. Am J Physiol Cell Physiol 278: C661–C666 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW (1990) Regulation and deregulation of cardiac Na(+)–Ca2+ exchange in giant excised sarcolemmal membrane patches. Nature 344: 242–245 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Ball R (1996) Regulation of cardiac Na+,Ca2+ exchange and KATP potassium channels by PIP2. Science 273: 956–959 [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Matsuoka S, Nagel GA, Collins A (1992) Steady-state and dynamic properties of cardiac sodium–calcium exchange. Sodium-dependent inactivation. J Gen Physiol 100: 905–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinata M, Kimura J (2004) Forefront of Na+/Ca2+ exchanger studies: stoichiometry of cardiac Na+/Ca2+ exchanger; 3:1 or 4:1? J Pharmacol Sci 96: 15–18 [DOI] [PubMed] [Google Scholar]
- Hinde AK, Perchenet L, Hobai IA, Levi AJ, Hancox JC (1999) Inhibition of Na/Ca exchange by external Ni in guinea-pig ventricular myocytes at 37 degrees C, dialysed internally with cAMP-free and cAMP-containing solutions. Cell Calcium 25: 321–331 [DOI] [PubMed] [Google Scholar]
- Hobai IA, O'Rourke B (2004) The potential of Na+/Ca2+ exchange blockers in the treatment of cardiac disease. Expert Opin Investig Drugs 13: 653–664 [DOI] [PubMed] [Google Scholar]
- Hulbert AJ, Turner N, Storlien LH, Else PL (2005) Dietary fats and membrane function: implications for metabolism and disease. Biol Rev Camb Philos Soc 80: 155–169 [DOI] [PubMed] [Google Scholar]
- Imahashi K, Pott C, Goldhaber JI, Steenbergen C, Philipson KD, Murphy E (2005) Cardiac-specific ablation of the Na+/Ca2+ exchanger confers protection against ischemia/reperfusion injury. Circ Res 97: 916–921 [DOI] [PubMed] [Google Scholar]
- Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR (2003) Akt activity negatively regulates phosphorylation of AMP-activated protein kinase in the heart. J Biol Chem 278: 39422–39427 [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Harris WS, Appel LJ (2002) Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 106: 2747–2757 [DOI] [PubMed] [Google Scholar]
- Larson TR, Graham IA (2001) Technical advance: a novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25: 115–125 [DOI] [PubMed] [Google Scholar]
- Larsson O, Deeney JT, Branstrom R, Berggren PO, Corkey BE (1996) Activation of the ATP-sensitive K+ channel by long chain acyl-CoA. A role in modulation of pancreatic beta-cell glucose sensitivity. J Biol Chem 271: 10623–10626 [DOI] [PubMed] [Google Scholar]
- Li Z, Matsuoka S, Hryshko LV, Nicoll DA, Bersohn MM, Burke EP, Lifton RP, Philipson KD (1994) Cloning of the NCX2 isoform of the plasma membrane Na(+)–Ca2+ exchanger. J Biol Chem 269: 17434–17439 [PubMed] [Google Scholar]
- Li Z, Nicoll DA, Collins A, Hilgemann DW, Filoteo AG, Penniston JT, Weiss JN, Tomich JM, Philipson KD (1991) Identification of a peptide inhibitor of the cardiac sarcolemmal Na(+)–Ca2+ exchanger. J Biol Chem 266: 1014–1020 [PubMed] [Google Scholar]
- Light P, Shimoni Y, Harbison S, Giles W, French RJ (1998) Hypothyroidism decreases the ATP sensitivity of KATP channels from rat heart. J Membr Biol 162: 217–223 [DOI] [PubMed] [Google Scholar]
- Low W, Kasir J, Rahamimoff H (1993) Cloning of the rat heart Na(+)–Ca2+ exchanger and its functional expression in HeLa cells. FEBS Lett 316: 63–67 [DOI] [PubMed] [Google Scholar]
- Maack C, Ganesan A, Sidor A, O'Rourke B (2005) Cardiac sodium–calcium exchanger is regulated by allosteric calcium and exchanger inhibitory peptide at distinct sites. Circ Res 96: 91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning Fox JE, Nichols CG, Light PE (2004) Activation of adenosine triphosphate-sensitive potassium channels by acyl coenzyme A esters involves multiple phosphatidylinositol 4,5-bisphosphate-interacting residues. Mol Endocrinol 18: 679–686 [DOI] [PubMed] [Google Scholar]
- Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F (2002) Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)-Prevenzione. Circulation 105: 1897–1903 [DOI] [PubMed] [Google Scholar]
- Matsuoka S, Nicoll DA, He Z, Philipson KD (1997) Regulation of cardiac Na(+)–Ca2+ exchanger by the endogenous XIP region. J Gen Physiol 109: 273–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll DA, Longoni S, Philipson KD (1990) Molecular cloning and functional expression of the cardiac sarcolemmal Na(+)–Ca2+ exchanger. Science 250: 562–565 [DOI] [PubMed] [Google Scholar]
- Nicoll DA, Quednau BD, Qui Z, Xia YR, Lusis AJ, Philipson KD (1996) Cloning of a third mammalian Na+–Ca2+ exchanger, NCX3. J Biol Chem 271: 24914–24921 [DOI] [PubMed] [Google Scholar]
- O'Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R, Marban E (1999) Mechanisms of altered excitation–contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84: 562–570 [DOI] [PubMed] [Google Scholar]
- Piacentino V III, Weber CR, Gaughan JP, Margulies KB, Bers DM, Houser SR (2002) Modulation of contractility in failing human myocytes by reverse-mode Na/Ca exchange. Ann NY Acad Sci 976: 466–471 [DOI] [PubMed] [Google Scholar]
- Piper HM, Meuter K, Schafer C (2003) Cellular mechanisms of ischemia–reperfusion injury. Ann Thorac Surg 75: S644–S648 [DOI] [PubMed] [Google Scholar]
- Quednau BD, Nicoll DA, Philipson KD (1997) Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am J Physiol 272: C1250–C1261 [DOI] [PubMed] [Google Scholar]
- Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD (1988) Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37: 1020–1024 [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Boora P, Steckley D, de Vries G, Light PE (2003) Kir6.2 polymorphisms sensitize beta-cell ATP-sensitive potassium channels to activation by acyl CoAs: a possible cellular mechanism for increased susceptibility to type 2 diabetes? Diabetes 52: 2630–2635 [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Light PE (2005) Saturated and cis/trans unsaturated acyl CoA esters differentially regulate wild-type and polymorphic beta-cell ATP-sensitive K+ channels. Diabetes 54: 2070–2079 [DOI] [PubMed] [Google Scholar]
- Schafer C, Ladilov Y, Inserte J, Schafer M, Haffner S, Garcia-Dorado D, Piper HM (2001) Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc Res 51: 241–250 [DOI] [PubMed] [Google Scholar]
- Schillinger W, Fiolet JW, Schlotthauer K, Hasenfuss G (2003) Relevance of Na+–Ca2+ exchange in heart failure. Cardiovasc Res 57: 921–933 [DOI] [PubMed] [Google Scholar]
- Schulze D, Rapedius M, Krauter T, Baukrowitz T (2003) Long-chain acyl-CoA esters and phosphatidylinositol phosphates modulate ATP inhibition of KATP channels by the same mechanism. J Physiol 552: 357–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H (2004) Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J 18: 1692–1700 [DOI] [PubMed] [Google Scholar]
- Sidell RJ, Cole MA, Draper NJ, Desrois M, Buckingham RE, Clarke K (2002) Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the zucker Fatty rat heart. Diabetes 51: 1110–1117 [DOI] [PubMed] [Google Scholar]
- Su Z, Bridge JH, Philipson KD, Spitzer KW, Barry WH (1999) Quantitation of Na/Ca exchanger function in single ventricular myocytes. J Mol Cell Cardiol 31: 1125–1135 [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B (2005) Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol 15: 370–378 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Kawarabayasi Y, Kondo J, Abe T, Nishikawa K, Kimura S, Hashimoto T, Yamamoto T (1990) Structure and regulation of rat long-chain acyl-CoA synthetase. J Biol Chem 265: 8681–8685 [PubMed] [Google Scholar]
- Takahashi K, Takahashi T, Suzuki T, Onishi M, Tanaka Y, Hamano-Takahashi A, Ota T, Kameo K, Matsuda T, Baba A (2003) Protective effects of SEA0400, a novel and selective inhibitor of the Na+/Ca2+ exchanger, on myocardial ischemia–reperfusion injuries. Eur J Pharmacol 458: 155–162 [DOI] [PubMed] [Google Scholar]
- Tani M (1990) Mechanisms of Ca2+ overload in reperfused ischemic myocardium. Annu Rev Physiol 52: 543–559 [DOI] [PubMed] [Google Scholar]
- Weber CR, Piacentino V III, Ginsburg KS, Houser SR, Bers DM (2002) Na(+)–Ca(2+) exchange current and submembrane [Ca(2+)] during the cardiac action potential. Circ Res 90: 182–189 [DOI] [PubMed] [Google Scholar]
- Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR (1978) Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem 253: 4305–4309 [PubMed] [Google Scholar]
- Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A (2004) Inhibitory effect of n-3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun 321: 116–123 [DOI] [PubMed] [Google Scholar]
- Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci USA 97: 1784–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]


