Abstract
Plasma membrane transport of single amino-acid methionine in yeast is shown to be mediated by at least seven different permeases whose activities are transcriptionaly and post-transcriptionaly regulated by different ubiquitin-dependent mechanisms. Upon high extracellular methionine exposure, three methionine-permease genes are repressed while four others are induced. SCFMet30, SCFGrr1 and Rsp5 ubiquitin ligases are the key actors of the ubiquitin-dependent remodeling of methionine transport. In addition to regulating the activity of Met4, the SCFMet30 ubiquitin ligase is shown to convey an intracellular signal to a membrane initiated signaling pathway by controlling the nuclear concentration of the Stp1 transcription factor. By coupling intra- and extracellular metabolite sensing, SCFMet30 thus allows yeast cells to accurately adjust the intermediary sulfur metabolism to the growth conditions. The multiple ubiquitin-dependent mechanisms that function in methionine transport regulation further exemplify the pervasive role of ubiquitin in the adaptation of single-cell organisms to environmental modifications.
Keywords: ligase, methionine, permease, ubiquitin
Introduction
All the cells have the capacity to respond to changes in their environment and their adaptation mostly relies on modifications of gene expression programs. The mechanisms whereby differential environmental cues, such as modifications of ions, minerals or nutrient concentrations, stimulate transcription changes are of particular importance for unicellular organisms whose growth and development are often limited by the availability of the elemental nutrients in the environment. The ability of single-cell microorganisms to rapidly adapt to changing environmental growth conditions often depend on membrane receptors that respond to the binding of specific ligands. Signals initiated from these membrane sensing systems are then transduced to specific transcription factors that are either activated or repressed (Brivanlou and Darnell, 2002). The accuracy of adaptation also relies on the integration of intracellular metabolite-sensing systems in order to dynamically adjust the cell metabolic status to the prevailing environmental conditions.
The regulation of amino-acid biosynthesis in the yeast Saccharomyces cerevisiae furnishes a powerful example of a large gene network which is primarily controlled at the level of transcription by both highly specific and general regulation mechanisms that are activated in response to either intra- or extracellular signals (Hinnebusch, 2005). Yeast amino-acid biosynthesis is mainly controlled by a cross pathway regulatory system, which is known as the general amino-acid control and is mediated by the Gcn4 transcription activator (Hinnebusch, 2005). The primary event that leads to the activation of Gcn4 is the intracellular accumulation of uncharged tRNA (Hinnebusch, 1997). In addition, several amino-acid biosynthetic genes are regulated by pathway-specific mechanisms that can be activated in response to the presence of a specific extracellular amino acid and can override the Gcn4-mediated derepression regulation (Hinnebusch, 2005). More recently, it became evident that most of these regulations are in addition associated with the modification of the expression of several permeases that mediate the uptake of amino acids into yeast cells (Forsberg and Ljungdahl, 2001).
S. cerevisiae cells express about 20 distinct amino-acid transporters which are structurally related and belong to the APC transporter superfamily (André, 1995). These permeases display either sharp substrate specificity, transporting only one amino acid such as the high-affinity lysine permease Lyp1, or recognize larger sets of amino acids, even comprising ones which are not found in proteins (Horak, 1997). Regulation of the expression of amino-acid permease-encoding genes was shown to be dependent on different signaling pathways, including one initiated at the plasma membrane by a multimeric sensing system call SPS and which appears to transduce signal information regarding the presence of extracellular amino acids (Jorgensen et al, 1998; Iraqui et al, 1999; Klasson et al, 1999; Boles and Andre, 2004). A central component of the SPS system is the membrane protein Ssy1, which, while structurally related to the amino-acid permease, is apparently devoid of transport capacity and rather functions as a sensor of extracellular amino acids (Didion et al, 1998; Bernard and Andre, 2001a).
Among amino acids, methionine displays inherent features that distinguish it from all other amino acids. Indeed, aside from its role as a primary building block of proteins, methionine is an important determinant of one-carbon metabolism. Under its activated form, S-adenosylmethionine (AdoMet), it serves as a methyl donor in hundreds of transmethylation reactions of nucleic acids, proteins and lipids. AdoMet is, moreover, used as precursor for the biosynthesis of polyamines, vitamins and modified nucleotides (Cantoni, 1977). Appropriate control of intracellular concentrations of methionine and its derivatives is thus expected to be crucial for proper cell growth and development.
In yeast, the methionine metabolism includes the sulfate assimilation pathway, the trans-sulfuration pathways that enable interconversion of cysteine and homocysteine and the methionine cycles. Upon exposure to high extracellular methionine, the expression of most of the methionine (MET) genes is turned off (Thomas and Surdin-Kerjan, 1997). More recently, the MET gene network was demonstrated to be regulated by additional environmental changes such as the growth in complex media comprising a mixture of sulfur-containing compounds, the presence of heavy metals such as cadmium or the exposure to acetaldehyde (Fauchon et al, 2002; Kuras et al, 2002; Aranda and del Olmo, 2004; Barbey et al, 2005). Most of these regulations involve ubiquitylation processes that function by either degradation-dependent or -independent mechanisms (Kaiser et al, 2000; Kuras et al, 2002). To get further insights on the mechanisms that sustain the regulation of the MET gene network, we asked whether and how the specific regulation of methionine transport helps the cells to cope with the required maintenance of methionine homeostasis. We uncovered a yet unexpected complexity in methionine transport regulation with ubiquitylation standing at the core of each unraveled mechanism.
Results
Dual regulation of the methionine permease genes upon methionine exposure
In S. cerevisae cells, transport of methionine is achieved through seven membrane permeases Mup1, Mup3, Agp1, Agp3, Bap2, Bap3 and Gnp1 (see Supplementary data). To analyze how these permeases are regulated, we first measured the level of expression of the seven corresponding genes in cells that were grown in minimal B medium and exposed to 1 mM extracellular methionine, a concentration known to trigger repression of the MET gene network. The results showed that the methionine permease genes could be classified into two distinctive families, according to their transcriptional response to methionine exposure (Figure 1A). The first class comprised the AGP3, MUP1 and MUP3 genes whose expression was ∼20-fold repressed upon high methionine exposure. The second class comprised the AGP1, BAP2, BAP3 and GNP1 genes whose expression was, in a strong contrast, 3–10-fold induced after methionine exposure. The four latter permeases are all classified as broad substrate specificity permeases and all display an intermediate affinity for methionine (Isnard et al, 1996; Regenberg et al, 1999, data not shown). The results thus indicated that upon high extracellular methionine exposure, yeast cells profoundly remodel its methionine transport capacities.
Figure 1.

Met4 and the SPS sensor control methionine permease gene expression. Methionine permease gene expression was assessed by quantitative real-time RT–PCR in (A) wild-type (CD269), (B) met4Δ (CC849-8A) and (C) ssy1Δ (CD317) cells grown in the absence and presence of 1 mM L-methionine. Values represent the average of two independent experiments and error bars indicate standard deviations. (D) Wild-type (W303-1A), ssy5Δ (CD319), met3Δ (CY14-5D), met4Δ (CC849-8A), ssy5Δ met3Δ (CY343-1C) and ssy5Δ met4Δ (CY33-2B) cells were plated on minimal medium supplemented with 0.1 mM L-methionine or 0.5 mM AdoMet.
Roles of Met4 and the SPS sensor in methionine permeases regulation
To decipher the mechanisms underlying such a modification of membrane composition, we next asked whether the methionine permease genes were targets of Met4, the prominent transcription activator of the MET gene network (Thomas and Surdin-Kerjan, 1997). We found that the three methionine-repressed genes, AGP3, MUP1 and MUP3, were not expressed in the absence of a functional Met4 (Figure 1B) as well as in the absence of Met31p and Met32p (data not shown) that furnish the prominent DNA-binding platform for Met4 (Blaiseau and Thomas, 1998). In contrast, the four other methionine permease genes, AGP1, BAP2, BAP3 and GNP1, were activated in met4Δ mutant cells, strongly suggesting that these genes are not Met4-target genes.
Several yeast amino-acid permease genes are known to respond to particular extracellular amino acids, a regulation that requires the Ssy1, Ssy5 and Ptr3 proteins that function together and form the SPS sensing system (Bernard and Andre, 2001a; Forsberg et al, 2001). We thus used ssy1Δ, ssy5Δ and ptr3Δ mutant cells to examine the role of the SPS sensor in the methionine-mediated regulation of methionine transport. It is worth to note that, due to the auxotrophic requirements of the tested strains, the minimal growth medium used in this study did contain several amino acids (leucine, tryptophan and histidine, see Materials and methods). As shown in Figure 1C, deletion of ssy1 (or ssy5 or ptr3, data not shown) abolished the methionine-triggered activation of the AGP1, BAP2, BAP3 and GNP1 genes, while transcription of the methionine-repressed AGP3, MUP1 and MUP3 genes was not affected by either mutation.
Phenotypic assays next confirmed the additive effects of Met4 and the SPS sensor on methionine transport. On the contrary to single met4Δ mutant cells, the organic sulfur requirement of which could be fulfilled by either methionine or AdoMet, double met4Δ ssy5Δ mutant cells were able to grow in the presence of AdoMet only. This phenotype did result from the impairment of methionine transport and not from the abolishment of the assimilatory sulfur metabolism since, in contrast, double met3Δ ssy5Δ mutant cells grew in the presence of either methionine or AdoMet as single met3Δ cells which lack ATP-sulfurylase and are thus unable to assimilate sulfate (Figure 1D).
Substrate-triggered control of the methionine permease genes is mediated by the Stp1, Stp2 and Met4 transcription activators
Two related zinc-finger-containing transcription factors, Stp1 and Stp2, have been shown to be essential for the SPS-dependent induction of the BAP2 and BAP3 genes in response to high concentrations of extracellular leucine or phenylalanine, while the induction of the AGP1 was shown to require the Uga35 and Stp1 factors (Abdel-Sater et al, 2004a). As shown in Figure 2A–C, the transcription of the AGP3, MUP1 and MUP3 genes was shown to be independent of functional Stp1, Stp2 and Uga35 factors. In contrast, both methionine-activated and basal transcription levels of the AGP1, BAP2, BAP3 and GNP1 genes were abrogated in cells which did not express functional Stp1 and Stp2 proteins (Figure 2B). In addition, deletion of the uga35 gene only led to 2–5-fold decrease of the expression of the four genes in the presence of high methionine (Figure 2C). The prominent role of Met4, Stp1 and Stp2 in transcription activation of the seven yeast methionine permeases was further illustrated by showing that unlike met4Δ or uga35Δ met4Δ mutant cells that grew in the presence of either methionine or AdoMet, the sulfur auxotrophy of the triple stp1Δ stp2Δ met4Δ mutant was fulfilled by AdoMet only (Figure 2D).
Figure 2.

Methionine permeases are controlled by the Stp1, Stp2 and Met4 transcription activators. Methionine permease gene expression was assessed in (A) wild-type (CD269), (B) stp1Δ stp2Δ (CD354) and (C) in uga35Δ (CD357) as in Figure 1. (D) Serial dilutions of wild-type (W303-1A), met4Δ (CC849-8A), stp1Δ stp2Δ (CD354), stp1Δ stp2Δ met4Δ (CY350-1C), uga35Δ (CD357) and met4Δ uga35Δ (CY353-16C) cells were plated on minimal medium in the absence and presence of either 0.1 mM L-methionine or 0.2 mM AdoMet.
Chromatin immunoprecipitation (ChIP) was next used to assess the abundance of Stp1 and Met4 at individual promoters. Association of the general transcription factor TFIIB was analyzed in parallel to determine the correlation between the presence of either Stp1 or Met4 and transcriptional activity. Crosslinked chromatin was prepared from wild-type and ssy1Δ cells grown in the absence and presence of methionine. In both strains, Met4 and TFIIB were found to be heavily associated with the MUP1, MUP3 and AGP3 gene promoters in the absence, but not in the presence, of methionine (Figure 3A and C). In contrast, Met4 was not recruited to the AGP1, BAP2, BAP3 and GNP1 gene promoters in either growth conditions.
Figure 3.

Met4, Stp1 and TFIIB occupancy at methionine permease promoters. CD362 (SSY1, stp1∷STP1HA, sua7∷SUA7MYC9) and CD364 (ssy1Δ, stp1∷STP1HA, sua7∷SUA7MYC9) cells were grown in minimal B medium and exposed to 1 mM L-methionine. After crosslinking, the chromatin was immunoprecipitated with either anti-Met4 (A), anti-HA (B) or anti-Myc (C) antibody. Total DNA was analyzed by quantitative PCR with primer-pairs-specific promoters. IME2 ORF was used as a control. The indicated IP/total ratio corresponds to the concentration of target DNA in the immunoprecipitated sample relative to that in the corresponding input sample. The reported values represent the average of two independent experiments, and error bars indicate standard deviations.
The identical chromatin preparations further revealed that Stp1 is only weakly associated with most of the promoter regions in the absence of methionine. Exposure of wild-type cells to high methionine led to a 5–12-fold increase in Stp1 and TFIIB occupancies at the AGP1, BAP2, BAP3 and GNP1 promoters (Figure 3B and C). ssy1 inactivation abrogated methionine-triggered recruitments of both Stp1 and TFIIB at the same promoters. All told, our results suggest that the methionine-triggered activation of the SPS-dependent methionine transporters is mainly mediated by the Stp1 and Stp2 factors.
Methionine transport regulation involves two SCF ubiquitin ligases, SCFMet30 and SCFGrr1
Ubiquitylation and members of the SCF ubiquitin ligase family control both the Met4- and the SPS-dependent signaling pathways. When yeast cells are grown in minimal medium and exposed to a high concentration of methionine, SCFMet30-mediated ubiquitylation targets Met4 for degradation by the 26S proteasome (Rouillon et al, 2000; Kuras et al, 2002). The role of the SCFGrr1 ubiquitin ligase in the SPS transduction pathway is less well understood, but the receptor subunit Grr1 was shown to be essential for proper expression of several amino-acid permease genes (Iraqui et al, 1999; Bernard and Andre, 2001b; Andreasson and Ljungdahl, 2002; Abdel-Sater et al, 2004b). To address the role of each SCF ubiquitin ligases in methionine transport regulation, the expression of the seven methionine permease genes was measured in mutant cells bearing either a met30-10 mutation that causes a constitutive MET gene expression (Thomas et al, 1995) or a grr1Δ null mutation. As shown in Figure 4A, high methionine exposure did not trigger repression of the three Met4-dependent methionine permeases in met30-10 cells. In contrast, methionine-triggered repression of the AGP3, MUP1 and MUP3 genes was not affected by the presence of a grr1Δ gene disruption. Consistent with the critical role of the SCFGrr1 ubiquitin ligase in the SPS pathway, the methionine-triggered activation of the four AGP1, BAP2, BAP3 and GNP1 genes was severely impeded in the presence of the grr1Δ null mutation (Figure 4B).
Figure 4.

Regulation of the methionine permease genes depends on both SCFMet30 and SCFGrr1 ubiquitin ligases. (A) Met4-dependent methionine permease gene expression was analyzed by quantitative RT–PCR in met30-10 (CY213-8A) and grr1Δ (CD344) cells exposed or not to 1 mM L-methionine. Total RNA was extracted before and 40 min after methionine addition. MET25 gene expression was used as a control of methionine repression. (B) SPS-dependent methionine permease gene expression was analyzed by quantitative RT–PCR in wild-type (W303-1A), met30-10 (CY213-8A) and grr1Δ (CD344) cells exposed or not to high extracellular methionine (1 mM). Total RNA was extracted before and 40 min after methionine addition. (C) Met30 regulates the binding of Stp1 on the AGP1, BAP2, BAP3 and GNP1 promoters. CD384 (stp1∷STP1HA) and CD383 (met30-10, stp1∷STP1HA) cells were grown in minimal B medium and exposed to 1 mM L-methionine for 40 min. ChIPs were performed as in Figure 3. (D) Addition of Cd2+ to the medium compromised the methionine-mediated regulation of both classes of methionine permeases. Total RNA was extracted before and 40 min after the addition of 1 mM L-methionine, in the absence and presence (+Cd2+) of 100 μM Cd2+, and analyzed by quantitative RT–PCR.
Quite unexpectedly, we found that the three AGP1, BAP3 and GNP1 genes were significantly activated in met30-10 mutant cells grown in the absence of high extracellular methionine, suggesting that the SCFMet30 ubiquitin ligase was involved in the methionine-triggered activation of the SPS dependent methionine permeases (Figure 4B). Accordingly, ChIP analyses showed that the met30-10 mutation caused a marked increase (5–10-fold) in Stp1 occupancy at the AGP1, BAP2, BAP3 and GNP1 promoters in cells grown in the absence of methionine (Figure 4C). In contrast to what was observed in wild-type cells, in met30-10 cells, Stp1 is thus recruited to the SPS-dependent methionine permease promoters independently of the presence of extracellular methionine, and with the exception of the BAP2 gene, activates the transcription.
To further ascertain the function of the SCFMet30 ligase, we characterized the transcription of the methionine permease genes in cells simultaneously exposed to cadmium (Cd2+) and high methionine. Indeed, Cd2+ was demonstrated to inhibit the activity of the SCFMet30 ligase by disrupting the interaction between the substrate-binding subunit Met30 and the core catalytic apparatus (Barbey et al, 2005; Yen et al, 2005). As shown in Figure 4D, the presence of Cd2+ compromised both methionine-dependent regulations, repression of the AGP3, MUP1 and MUP3 genes and activation of the AGP1, BAP2, BAP3 and GNP1 genes.
The SCFMet30 ubiquitin ligase controls the methionine-induced nuclear accumulation of Stp1
Activation of Stp1 and Stp2 by extracellular tryptophan or leucine was found to require an SPS-dependent endoproteolytic cleavage that removes a ∼10 kDa N-terminal fragment and allows both factors to accumulate in the nucleus (Andreasson and Ljungdahl, 2002). The processing of Stp1 and Stp2 is believed to be the major regulatory event that transduces extracellular amino-acid signals from the SPS sensor to permease promoters (Andreasson and Ljungdahl, 2002). To further address the mechanism of methionine signaling, we analyzed the status of Stp1 in a wild-type and ssy1Δ strains, both grown in the absence and presence of 1 mM methionine. As expected, Stp1 was revealed as high molecular weight form of 68 kDa in ssy1Δ cells. In contrast, Stp1 was revealed as a low molecular form in wild-type cells in either growth conditions, suggesting that it was actively processed in the absence and presence of high methionine (Figure 5A). It thus appeared that in the growth conditions used throughout this study (minimal medium supplemented with amino acids required to satisfy the auxotrophic requirement of the strains, see Material and methods), Stp1 is processed by an SPS-dependent mechanism, but is unable to bind the methionine permease promoters in the absence of high extracellular methionine.
Figure 5.
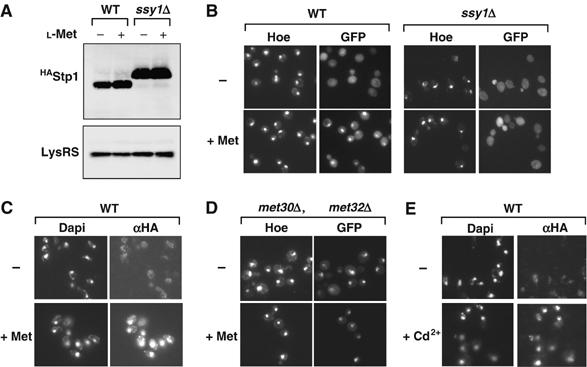
High methionine targets Stp1 to the nucleus. (A) CD362 (SSY1, stp1∷STP1HA) and CD364 (ssy1Δ, stp1∷STP1HA) cells were grown in minimal B medium and exposed to 1 mM L-methionine. Total proteins were extracted and processed for immunoblotting with monoclonal antibody to Ha and polyclonal antibody to the yeast lysyl-tRNA synthetase. (B) Methionine induces Stp1 nuclear localization. The Stp1-GFPVenus fusion protein was expressed in the SSY1 (CD386) and ssy1Δ (CD387) cells from the GAL1 promoter for 1 h. To limit the expression level of the Stp1-GFPVenus fusion protein, the cells were transferred to a glucose-containing medium to repress the GAL1 promoter. The culture was then split into two cultures, and the cells grown for 40 min, either in the absence of methionine (−) or in the presence of 1 mM L-methionine (+Met). Fluorescence images were then acquired. The fluorescence dye Hoesch labels the nucleus. (C) Stp1 is localized in the nucleus upon methionine exposure. Localization of the tagged Stp13HA protein expressed from the endogenous promoter was analyzed by immunofluorescence in CY352 cells exposed or not to 1 mM L-methionine for 40 min. (D) Constitutive nuclear localization of Stp1 in met30Δ met32Δ mutant cells. The Stp1-GFPVenus protein was expressed in the met30Δ met32Δ mutant cells (CD396) from the GAL1 promoter. Cells were grown as in (B). Images were acquired in the absence of methionine (−) and in the presence of 1 mM methionine (+Met). (E) Cd2+ exposure leads to Stp1 nuclear accumulation. Localization of the tagged Stp13HA protein was analyzed by immunofluorescence in CD362 cells exposed or not to Cd2+.
Taken together, the results, therefore, suggested that high methionine exposure triggers an additional activation step of Stp1 that follows its SPS-dependent processing and permits the activation of the AGP1, BAP2, BAP3 and GNP1 genes. To address the corresponding mechanism, we analyzed the cellular localization of the Stp1 factor in cells exposed or not to high extracellular methionine. We first used an Stp1-GFPVenus fusion protein, which was expressed from the chromosomes of stp1Δ stp2Δ (CD386) and ssy1Δ (CD387) cells and was shown to be functional (Menant et al, manuscript in preparation). In the absence of high extracellular methionine, the Stp1-GFPVenus fusion protein was found to be predominantly localized within the cytoplasm of both strains (Figure 5B). Upon high methionine exposure, the Stp1-GFPVenus fusion protein was shown to be concentrated within the nucleus of CD386 cells, while the fusion protein remained mainly cytoplasmic in ssy1Δ cells. This result thus suggested that high methionine exposure triggers the nuclear accumulation of Stp1 through a mechanism that requires its previous processing by the SPS system. To further assess this hypothesis, we performed immunofluorescence assays with cells that expressed a tagged Stp13Ha derivative from the endogenous promoter. As shown in Figure 5C, immunofluorescence assays confirmed that Stp13Ha concentrated within the nucleus upon high extracellular methionine exposure. Finally, we examined whether the SCFMet30 ubiquitin ligase might play a role in the nuclear accumulation of Stp1 by analysing the cellular localization of an Stp1-GFPVenus fusion protein in met30Δ met32Δ cells that do not express a functional Met30 protein and are methionine prototroph (Patton et al, 2000). As shown in Figure 5D, the Stp1-GFPVenus fusion protein was found to be constitutively concentrated within the nucleus of met30Δ mutant cells, independently of the presence of high extracellular methionine. Accordingly, exposure of cells to Cd2+, which impairs SCFMet30 function, led to the nuclear localization of the Stp13Ha protein (Figure 5E).
Nuclear accumulation of Stp1 depends upon an intracellular sulfur compound
The nuclear accumulation of Stp1 thus appears to be controlled by at least two different systems, the SPS sensor that triggers the removing of the N-terminal moiety and the SCFMet30 ubiquitin ligase. While the former mechanism clearly depends upon extracellular cues (Klasson et al, 1999; Bernard and Andre, 2001a; Forsberg and Ljungdahl, 2001), the proximal effector of the latter mechanism remained to be established as the SCFMet30 ligase is now recognized to be regulated by different signaling pathways (Rouillon et al, 2000; Aranda and del Olmo, 2004; Barbey et al, 2005; Yen et al, 2005). To gain insight on a plausible effector, we first analyzed methionine permease transcription in cells exposed to high concentrations of AdoMet, a sulfur comprising nucleotide which is actively transported into yeast cells (Rouillon et al, 1999). As shown in Figure 6A, addition of either 0.2 or 0.5 mM AdoMet into the growth medium promoted the same transcriptional response than high methionine exposure: repression of the AGP3, MUP1 and MUP3 permease genes and activation of the AGP1, BAP2, BAP3 and GNP1 genes. As expected, Stp1 is revealed as a low molecular weight form in wild-type cells grown in the presence and absence of AdoMet (Figure 6B). Importantly, the Stp1-GFPVenus fusion protein was shown to be concentrated within the nucleus of wild-type cells exposed to high AdoMet (Figure 6C).
Figure 6.
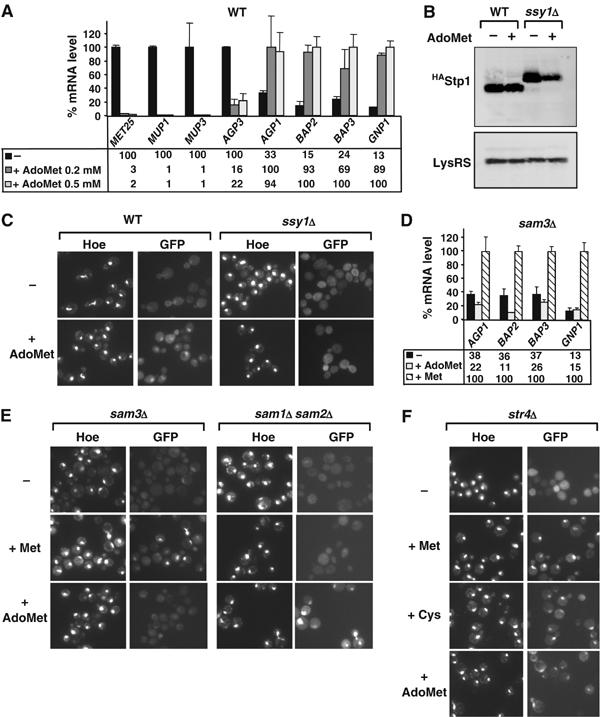
Stp1 nuclear targeting depends upon an intracellular sulfur-containing compound. (A) Methionine permease gene expression was analyzed by quantitative real time RT–PCR in wild-type cells (CD269) grown in minimal medium before and after addition of either 0.2 or 0.5 mM AdoMet. (B) CD362 (SSY1, stp1∷STP1HA) and CD364 (ssy1Δ, stp1∷STP1HA) cells were grown in minimal B medium and exposed to 0.5 mM AdoMet. Total proteins were extracted and processed as in Figure 5. (C) High extracellular AdoMet induces Stp1 nuclear localization. The Stp1-GFPVenus fusion protein was expressed as in Figure 5B except that 0.5 mM AdoMet, instead of 1 mM methionine, was added into the medium. (D) SPS-dependent methionine permease genes expression was assessed by quantitative real-time RT–PCR analysis in sam3Δ cells (CD192) exposed or not to either1 mM methionine or 0.5 mM AdoMet. (E) High intracellular AdoMet induces Stp1 nuclear accumulation. The Stp1-GFPVenus fusion protein was expressed in sam3Δ (CY356-3B) and sam1Δ sam2Δ (CD398) cells exposed to either 1 mM methionine or 0.5 mM AdoMet. (F) Intracellular conversion of AdoMet into cysteine is not required for Stp1 nuclear accumulation. The Stp1-GFPVenus fusion protein was expressed in str4Δ (CD400) cells exposed to either 1 mM methionine (+Met), 1 mM cysteine (+Cys) or 0.5 mM AdoMet (+AdoMet).
We next took advantage that AdoMet is transported by Sam3, an SPS-independent permease (Rouillon et al, 1999), to decipher whether the SCFMet30-mediated activation of Stp1 is triggered by an extra- or intracellular signal. As shown in Figure 6D, exposure to high extracellular methionine but not to high extracellular AdoMet, triggered the induction of the Stp1 target genes (AGP1, BAP2, BAP3 and GNP1) in sam3Δ cells that lack an active AdoMet transport. Accordingly, the Stp1-GFPVenus is concentrated within the nucleus upon methionine exposure only in such cells (Figure 6E). From these results, we concluded that the nuclear accumulation of processed Stp1 that allows its binding to the promoters of the methionine permease genes is controlled by the increase of an intracellular sulphur-containing compound. However, experiments performed with double sam1Δ sam2Δ and single str4Δ mutant cells showed that, in contrast to Met4 degradation (Menant et al, 2006), Stp1 movement to the nucleus, while requiring methionine transformation into AdoMet (Figure 6E), does not depend upon AdoMet transformation into cysteine (Figure 6F).
Rsp5-mediated destabilization of the high-affinity methionine permease Mup1
High methionine exposure triggers two opposite transcription responses: a rapid repression of the Met4-dependent AGP3, MUP1 and MUP3 genes and the activation of the SPS-dependent AGP1, BAP2, BAP3 and GNP1 genes. To further appreciate the physiological importance of this gene expression switch, we asked whether the repression of the Met4-dependent methionine permease genes might be accompanied by a downregulation of the existing permeases. For this purpose, we examined the stability of a Mup1-Gfp fusion protein expressed from the endogenous promoter in cells grown in minimal medium and exposed to high methionine. In the absence of methionine, Mup1-Gfp was detected as a punctuate staining at the periphery of the cells (Figure 7A). This plasma membrane signal gradually disappeared after methionine exposure, with intracellular dots transiently formed, and most of the fluorescent signal disappeared within 90 min. This result was reminiscent to the internalization and vacuolar targeting of several membrane transporters which are mediated by the Rsp5 ubiquitin ligase (for a review, see Horak, 2003). We thus analyzed the behavior of the Mup1-Gfp fusion protein in rps5 mutant cells shifted to restrictive temperature and exposed to high methionine. As shown in Figure 7B, rsp5 inactivation abolished the methionine-triggered endocytosis and vacuolar degradation of Mup1-Gfp. The use of doa4Δ mutant cells that have a reduced internal pool of monomeric ubiquitin (Amerik et al, 2000) further confirmed that Mup1 destabilization is an ubiquitin-dependent process. Indeed, methionine-triggered degradation of Mup1-Gfp was not observed in doa4Δ mutant cells (Figure 7C). Therefore, high methionine exposure results into a rapid elimination of the high-affinity methionine permease Mup1 through two additive ubiquitin-based mechanisms: repression of transcription and protein destabilization.
Figure 7.

High extracellular methionine induces the ubiquitin-dependent destabilization of Mup1-Gfp fusion protein. (A) Destabilization of the Mup1-Gfp fusion protein in living cells exposed to high methionine levels. CD378 (mup1∷MUP1-GFP) cells were grown in the absence (−Met) or in the presence of 1 mM L-methionine (+Met). Cells were imaged at the indicated times after methionine addition. (B) Stabilization of the Mup1-Gfp fusion protein in a rsp5 mutant. CD379 (mup1∷MUP1-GFP, rsp5-1) cells were grown in minimal B medium, shifted to the nonpermissive temperature 37°C for 2 h and then exposed or not to 1 mM methionine. Cells were imaged at the indicated times after the addition of methionine (+Met) or not (−Met). (C) Stabilization of the Mup1-Gfp fusion protein in a doa4 mutant. CD380 (mup1∷MUP1-GFP, doa4∷LEU2) cells were grown in the absence (−Met) or in the presence of 1 mM methionine (+Met).
Discussion
The adaptation of a microbial organism derives from its ability to adjust its intermediary metabolism to the environmental conditions and from its capacity to appropriately control the amounts and the forms of the nutrients taken up from the extracellular medium. Recent studies have illuminated the diversity and the functional versatility of the transporters that are expressed at the surface of the microbial cells (Van Belle and Andre, 2001). Moreover, redundancy appears to be a widely found scheme, a single nutrient being often transported by several different plasma membrane systems that, however, may display different kinetic and specificity properties (Regenberg et al, 1999).
We report here a striking example of such a functional redundancy: S. cerevisiae cells are indeed shown to express no less than seven different plasma membrane transporters that take up extracellular methionine. Since these permeases differ from each other by their affinity, transport capacity and specificity, such a redundancy likely allows yeast cells to withdraw methionine from a great variety of environments and thereby to limit their consumption of the expensive reducing equivalents which are needed for the synthesis of methionine from sulfate ions. However, a puzzling observation is that these seven membrane proteins fall into two distinctive families according to the regulation of their expression. We indeed show that upon high methionine exposure, yeast cells profoundly remodel their methionine transport capacities by turning off the expression of three out seven methionine permeases while inducing the expression of the four other transporters. The physiological reasons for such a two-classes partition are not obviously apparent as the three repressed transporters correspond to two highly specific permeases, Mup1 and Mup3, and one transporter of broad specificity, Agp3 (Schreve and Garrett, 2004). The four methionine-induced permeases are all classified as broad substrate specificity permeases. Each of the three repressed permeases appears to be strictly coregulated with MET biosynthesis genes and to be a target of Met4, the main transcription activator of the MET gene network. Accordingly to previous studies, repression of the AGP3, MUP1 and MUP3 genes is shown to result from the degradation of Met4, which is triggered by the SCFMet30 ubiquitin ligase.
Interestingly, we show here that, at least for one Met4-dependent methionine permease, Mup1, the repression of transcription is associated with the methionine-dependent elimination of the pre-exisiting Mup1 transporters present within the plasma membrane. Mup1 removal depends upon a second ubiquitin ligase, the Hect domain-containing ligase Rsp5. As described for several other yeast transporters, such as the tryptophan permease Tat2 and the uracil permease Fur4 (Horak, 2003), the Rsp5-dependent trafficking and degradation of Mup1 is impaired in cells lacking the ubiquitin hydrolase Doa4. Therefore, in yeast cells grown in minimal medium and exposed to high methionine, the rapid elimination of the high-affinity methionine permease results from two concomitant ubiquitin-dependent mechanisms that target two different cellular components, the proteasome that mediates the degradation of ubiquitylated Met4 and the vacuolar lumen where ubiquitylated Mup1 is proteolysed. This double substrate-induced downregulation mechanism might be classically interpreted as helping cells to protect themselves from excess methionine, an issue of importance regarding the various essential processes in which methionine is involved. However, it must be noticed that, while Mup1 is the permease that displays the highest affinity for methionine, its transport capacity is limited (Isnard et al, 1996). Future studies aimed to establish whether the two other Met4-dependent permeases Agp3 and Mup3 are also actively removed from cell membranes, will be required to ensure the physiological causes of this concerted repressive action of two ubiquitin-dependent pathways.
Concomitantly to the repression of the three Met4 target permeases, the four other methionine permeases, Agp1, Bap2, Bap3 and Gnp1 are induced under high methionine exposure. The methionine-mediated induction of the corresponding genes requires the integrity of the amino-acid sensing system SPS, a multimeric complex which resides at the cell membranes: inactivation of either component, Ssy1, Ssy5 or Ptr3, of the SPS sensor impairs methionine activation. Accordingly to previous works on the SPS signaling pathway, we found that the methionine-mediated activation of the four Agp1, Bap2, Bap3 and Gnp1 permeases in addition requires the integrity of the SCFGrr1 ubiquitin ligase. The SCFGrr1 ligase is hypothesized to be involved in the ubiquitin-dependent, proteasome-independent processing of two effectors of the SPS pathway, the transcription factors Stp1 and Stp2 (Abdel-Sater et al, 2004b; Boles and Andre, 2004). In response to SPS activation, Stp1 and Stp2, which exist as latent forms in the cytoplasm, where shown to be processed through a proteolytic event that removes an amino-terminal peptide of ∼10 kDa from each factor. This processing was hypothesized to drive the translocation of Stp1 and Stp2 into the nucleus (Andreasson and Ljungdahl, 2002, 2004). Measures of mRNA levels, phenotypic assays and ChIP experiments confirmed the primordial roles of the transcription factors Stp1 and Stp2 in the methionine-mediated activation of the four SPS-dependent methionine permeases.
An additional layer of complexity in Stp1/Stp2 regulation, however, emerged from our studies which were performed with cells grown in minimal medium in the presence of the amino acids needed to meet their auxotrophic requirements. In the absence of high extracellular methionine, Stp1 is efficiently processed into a low molecular weight form, as expected from the presence of various amino acids in the medium, but ChIP experiments revealed that Stp1 is unable to bind the promoters of the four SPS-dependent methionine permease genes, which consequently are not expressed. In contrast, upon exposure of high extracellular methionine, Stp1 bind to the promoter regions of the AGP1, BAP2, BAP3 and GNP1 genes, which accordingly are highly transcribed. Several lines of evidences strongly suggest that in the absence of extracellular methionine, the activity of Stp1/Stp2 is restricted by the SCFMet30 ubiquitin ligase. First, activation of the SPS-dependent methionine permeases is observed in response to either high methionine or AdoMet exposure and is triggered by the increase of an intracellular sulfur-containing compound. Second, a constitutive derepression of the SPS-dependent methionine permease genes as well as high Stp1 occupancy levels at the corresponding promoters are measured in cells bearing a met30-10 mutation. Both Gfp-based and immunofluorescence assays reveal that in wild-type cells, processed Stp1 is not accumulated within the nucleus until either high methionine or AdoMet are added to the growth medium. In contrast, in met30Δ cells or in the presence of Cd2+, a divalent metal known to specifically inactivate the SCFMet30 ligase (Barbey et al, 2005; Yen et al, 2005), Stp1 accumulates within the nucleus in the absence of high extracellular methionine. These findings indicate that the SCFMet30 ubiquitin ligase negatively regulates the nuclear accumulation of Stp1 and thereby its capacity of activating the SPS-dependent methionine permeases. How the SCFMet30 ligase is achieving its function is not deciphered. However, it is worth to note that the signaling pathway that controls Stp1 nuclear accumulation differs from the one triggering Met4 degradation (Menant et al, 2006). Indeed, Stp1 movement to the nucleus requires the synthesis of AdoMet from extracellular methionine but not the subsequent transformation of AdoMet into cysteine. In addition, as shown in Supplementary Figure 2, extracellular methionine-triggered accumulation of Stp1 appears to be independent of Asi1, an integral component of the inner nuclear membrane which inhibits the unprocessed Stp1 and Stp2 proteins to bind the SPS-regulated promoters (Boban et al, 2006). In any cases, the SCFMet30 ligase acts downstream the SPS sensor after the processing of Stp1, as we have shown that the methionine-induced nuclear concentration of Stp1 and its subsequent binding to the SPS-dependent methionine permease genes require both the Ssy1 and SCFGrr1 activities.
The SCFMet30 ubiquitin ligase illustrates the strategy that is used to dynamically coordinate the transcription responses in response to both extra- and intracellular signals. SCFMet30 first orchestrates the reduction of inorganic sulfur and its incorporation into various organic compounds by regulating the activity of Met4, the main transcription activator of the MET gene network. By conveying intracellular signals to the Stp1/Stp2 factors, which are the downstream effectors of a membrane initiated signaling pathway, the intervention of the SCFMet30 ligase further provides yeast cells with an efficient means to assess the availability of both extra- and intracellular sulfur-containing compounds. The here reported involvement of three different ubiquitin ligases in the regulation of the membrane transport of a single amino acid further underlines the pervasive role played by ubiquitin in the adaptation of single-cell organisms to the various changes of their habitats.
Materials and methods
Yeast culture
S. cerevisiae strains used in this study are listed in Table I. Standard yeast media and minimal B medium were prepared as previously described (Kuras et al, 2002). Amino acids needed to complement the auxotrophic requirements for each strain were added (0.8 mM L-leucine, 0.25 mM L-histidine, 0.1 mM L-tryptophan). For high methionine exposure assays, cells were grown in B medium supplemented with 0.2 mM DL-homocysteine to early log phase, transferred to a B medium without sulfur source for 1 h before the addition of 1 mM L-methionine. Transformation was by the lithium acetate method (Gietz et al, 1992).
Table 1.
Yeast strains
| Strain | Genotype | Source |
|---|---|---|
| CA113-2B | Matα, ade2, his3, leu2, ura3, trp1, mup3∷LEU2 | Isnard et al (1996) |
| CC849-8A | Matα, his3, leu2, ura3, trp1, met4∷TRP1 | Rouillon et al (2000) |
| CD192 | Matα, ade2, his3, leu2, ura3, trp1, sam3∷URA3 | Rouillon et al (1999) |
| CD269 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9MYC-TRP1 | This study |
| CD317 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9MYC-TRP1, ssy1∷HIS3 | This study |
| CD319 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9MYC-TRP1, ssy5∷HIS3 | This study |
| CD344 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9-MYC-TRP1, grr1∷HIS3 | This study |
| CD354 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9 MYC-TRP1, stp2∷HPH, stp1∷LEU2 | This study |
| CD357 | Mat-α, his3, leu2, trp1, ura3, met4∷FLU-MET4, sua7∷SUA7-9 MYC-TRP1, uga35∷LEU2 | This study |
| CD362 | Matα, ade2, his3, leu2, ura3, trp1, sua7∷SUA7-9MYC-TRP1, stp1∷STP1-HA-HIS3 | This study |
| CD364 | Matα, ade2, his3, leu2, ura3, trp1, sua7∷SUA7-9MYC-TRP1, ssy1∷HIS3, stp1∷STP1-HA-Kan | This study |
| CD368 | Matα, ade2, his3, leu2, ura3, trp1, mup1∷HIS3, mup3∷LEU2, agp1∷URA3, bap3∷TRP1,bap2∷HPH | |
| CD369 | Matα, ade2, his3, leu2, ura3, trp1, mup1∷HIS3, mup3∷LEU2, agp1∷URA3, bap3∷TRP1, | This study |
| bap2∷Hph, gnp1∷Kan | ||
| CD371 | Matα, ade2, his3, leu2, ura3, trp1, mup1∷HIS3, mup3∷LEU2, agp1∷URA3, bap3∷TRP1, | This study |
| bap2∷HPH, gnp1∷Kan, agp3∷Ble | ||
| CD375 | Matα, ade2, his3, leu2, ura3, trp1, mup1∷HIS3, mup3∷LEU2, agp3∷URA3 | This study |
| CD378 | Matα, his3, leu2, ura3, trp1, mup1∷MUP1-GFP-Kan | This study |
| CD379 | Matα, his4-912δR5, lys2-1285, ura3-S2, rsp5-1, mup1∷MUP1-GFP-Kan | This study |
| CD380 | Matα, his3, leu2, ura3, trp1, lys2, gal2, doa4∷LEU2, mup1∷MUP1-GFP-Kan | This study |
| CD383 | Mata, ade2, his3, trp1, leu2∷proMET16-XylE∷LEU2, met30-10, stp1∷STP1-HA-HIS3 | This study |
| CD384 | Mata, ade2, his3, trp1, leu2∷proMET16-XylE∷LEU2, stp1∷STP1-HA-HIS3 | This study |
| CD386 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9 MYC-TRP1, stp2∷HPH, stp1∷LEU2, | This study |
| ura3∷pGal-STP1-GFPVenus∷URA3 | ||
| CD387 | Matα, his3, leu2, ura3, trp1, met4∷HA3-MET4, sua7∷SUA7-9MYC-TRP1, ssy1∷HIS3, | This study |
| ura3∷pGal-STP1-GFPVenus∷URA3 | ||
| CD396 | Mat-α, his3, leu2, ura3, trp1, met30∷LEU2, met32∷TRP1, ura3∷pGal-STP1-GFPVenus∷URA3 | This study |
| CD398 | Mata,his3, leu2, ura3, ade2, trp1, can1, sam1∷LEU2, sam2∷HIS3, ura3∷pGal-STP1-GFPVenus∷URA3 | This study |
| CD399 | Mata, ura3, asi1∷Kan, ura3∷pGal-STP1-GFPVenus∷URA3 | This study |
| CD400 | Mat-a, ura3, str4∷KanMX4, , ura3∷pGal-STP1-GFPVenus∷URA3 | This study |
| CY352 | Mata, ade2, his3, trp1, leu2∷proMET16-XylE∷LEU2, stp1∷STP1-HA-HIS3/ | This study |
| Matα, ade2, his3, leu2, ura3, trp1, sua7∷SUA7-9MYC-TRP1, stp1∷STP1-HA-HIS3 | ||
| CY14-5D | Mata, his3, leu2, ura3, trp1, met3∷URA3 | Y Surdin-Kerjan |
| CY33-2B | Mata, his3, ura3, trp1, met4∷TRP1, ssy5∷HIS3 | This study |
| CY213-8A | Mata, ade2, his3, trp1, leu2∷proMET16-XylE∷LEU2, met30-10 | P Baudouin-Cornu |
| CY343-1C | Mata, his3, leu2, ura3, trp1, ssy5∷HIS3, met3∷URA3 | This study |
| CY347-6C | Matα, ade2, his3, leu2, ura3, trp1, mup1∷HIS3, mup3∷LEU2, agp1∷URA3, bap3∷TRP1 | This study |
| CY350-1C | Mata, his3, leu2, ura3, trp1, met4∷TRP1, stp2∷HPH, stp1∷LEU2, sua7∷SUA7-9MYC-TRP1 | This study |
| CY353-16C | Mata, his3, leu2, ura3, trp1, uga35∷LEU2, met4∷TRP1, sua7∷SUA7-9MYC-TRP1 | This study |
| CY356-3B | Matα, his3, leu2, ura3, trp1, sam3∷URA3, ura3∷pGal-STP1-GFPVenus∷URA3 | This study |
| W303-1A | Mata, ade2, his3, leu2, ura3, trp1 | R Rothstein |
Fluorescence microscopy and immunofluorescence
Gfp-Met4 and Stp1-GFPVenus fusion protein signals were monitored in living cells on a Zeiss ‘Axioplan 2 Imaging' fluorescence microscope using a Zeiss GFP filter. Mup1-Gfp fusion protein was monitored on a laser scanning confocal microscope Leica TCS SP2. Immunofluorescence was assayed as described by Burke et al (2000). Cells expressing a functional tagged Stp13HA from the endogenous promoter were grown in B medium to an OD650nm of 0.8 and transferred to a B medium for 1 h without sulfur source. The culture was split into two equal cultures and 1 mM L-methionine was added to one of the two cultures for 40 min. Cells were fixed by adding formaldehyde directly to the cultures to a final concentration of 4.5% for 1 h. The primary antibody used was the mouse monoclonal Ha antibody from Santa Cruz diluted 1:75. The secondary antibody was Alexa Fluor 488 conjugated to goat anti-mouse IgG (H+L; Molecular Probes) diluted 1:500. Nuclei were stained using the dye Dapi.
ChIP and RNA analysis
Crosslinked chromatin preparation and immunoprecipitation (ChIP) was performed as described previously (Kuras et al, 2002; Barbey et al, 2005) The indicated IP/total ratio corresponds to the concentration of target DNA in the immunoprecipitated sample relative to that in the corresponding input sample. For each immunoprecipitation, the highest value obtained was set at 100, and other values were represented relative to this standard.
For mRNA level analyses, total RNA was extracted by the hot phenol method and reverse transcribed using Superscript II reverse transcriptase (Invitrogen) following the manufacturer's instructions. cDNA were analyzed by quantitative real-time PCR performed with the Light Cycler system (Roche) and normalized with U4 snRNA. For each gene tested, the higher transcription level measured in wild-type cells was arbitrarily set to 100 and all other values were represented relative to this standard. Values represent the average of two independent experiments, and error bars indicate standard deviations.
Protein analysis
Total proteins were extracted by a 20%-cold TCA procedure as described (Rouillon et al, 2000; Barbey et al, 2005) using a Fastprep apparatus (Qbiogen).
Supplementary Material
Supplementary Information
Acknowledgments
We thank Yolande Surdin-Kerjan for helpful discussions, Laurent Kuras for advice in ChIP and Jacqueline Loeper for her help in immunofluorescence experiments. This work was supported by funds from the Centre National de la Recherche Scientifique. AM is supported by thesis fellowships from the Ministère de la Recherche and the ‘Fondation pour la Recherche Médicale'.
References
- Abdel-Sater F, El Bakkoury M, Urrestarazu A, Vissers S, Andre B (2004b) Amino acid signaling in yeast: casein kinase I and the Ssy5 endoprotease are key determinants of endoproteolytic activation of the membrane-bound Stp1 transcription factor. Mol Cell Biol 24: 9771–9785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Sater F, Iraqui I, Urrestarazu A, Andre B (2004a) The external amino acid signaling pathway promotes activation of Stp1 and Uga35/Dal81 transcription factors for induction of the AGP1 gene in Saccharomyces cerevisiae. Genetics 166: 1727–1739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerik AY, Nowak J, Swaminathan S, Hochstrasser M (2000) The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol Biol Cell 11: 3365–3380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- André B (1995) An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11: 1575–1611 [DOI] [PubMed] [Google Scholar]
- Andreasson C, Ljungdahl PO (2002) Receptor-mediated endoproteolytic activation of two transcription factors in yeast. Genes Dev 16: 3158–3172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson C, Ljungdahl PO (2004) The N-terminal regulatory domain of Stp1p is modular and, fused to an artificial transcription factor, confers full Ssy1p-Ptr3p-Ssy5p sensor control. Mol Cell Biol 24: 7503–7513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda A, del Olmo ML (2004) Exposure of Saccharomyces cerevisiae to acetaldehyde induces sulfur amino acid metabolism and polyamine transporter genes, which depend on Met4p and Haa1p transcription factors, respectively. Appl Environ Microbiol 70: 1913–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey R, Baudouin-Cornu P, Lee TA, Rouillon A, Zarzov P, Tyers M, Thomas D (2005) Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium. EMBO J 24: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard F, Andre B (2001a) Genetic analysis of the signalling pathway activated by external amino acids in Saccharomyces cerevisiae. Mol Microbiol 41: 489–502 [DOI] [PubMed] [Google Scholar]
- Bernard F, Andre B (2001b) Ubiquitin and the SCF(Grr1) ubiquitin ligase complex are involved in the signalling pathway activated by external amino acids in. FEBS Lett 496: 81–85 [DOI] [PubMed] [Google Scholar]
- Blaiseau PL, Thomas D (1998) Multiple transcription activation complexes tether the yeast activator Met4 to DNA. EMBO J 17: 6327–6336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boban M, Zargari A, Andreasson C, Heessen S, Thyberg J, Ljungdahl PO (2006) Asi1 is an inner nuclear membrane protein that restricts promoter access of two latent transcription factors. J Cell Biol 173: 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles E, Andre B (2004) Role of transporter like sensors in glucose and amino-acid signalling in yeast. Topics Curr Genet 9: 121–153 [Google Scholar]
- Brivanlou AH, Darnell JE (2002) Signal transduction and the control of gene expression. Science 295: 813–818 [DOI] [PubMed] [Google Scholar]
- Burke D, Dawson D, Stearns T (2000) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, pp 133–135 [Google Scholar]
- Cantoni GL (1977) S-adenosylmethionine: present status and future perspectives. In The biochemistry of S-adenosylmethionine, Salvatore F, Borek E, Zappia V, Williams-Ashman HG and Schlenk F (eds) New York: Columbia University Press, pp 557–577 [Google Scholar]
- Didion T, Regenberg B, Jorgensen MU, Kielland-Brandt MC, Andersen HA (1998) The permease homologue Ssy1p controls the expression of amino acid and peptide transporter genes in Saccharomyces cerevisiae. Mol Microbiol 27: 643–650 [DOI] [PubMed] [Google Scholar]
- Fauchon M, Lagniel G, Aude JC, Lombardia L, Soularue P, Petat C, Marguerie G, Sentenac A, Werner M, Labarre J (2002) Sulfur sparing in the yeast proteome in response to sulfur demand. Mol Cell 9: 713–723 [DOI] [PubMed] [Google Scholar]
- Forsberg H, Gilstring CF, Zargari A, Martinez P, Ljungdahl PO (2001) The role of the yeast plasma membrane SPS nutrient sensor in the metabolic response to extracellular amino acids. Mol Microbiol 42: 215–228 [DOI] [PubMed] [Google Scholar]
- Forsberg H, Ljungdahl PO (2001) Genetic and biochemical analysis of the yeast plasma membrane Ssy1p-Ptr3p-Ssy5p sensor of extracellular amino acids. Mol Cell Biol 21: 814–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz D, St Jean A, Woods RA, Schiestl RH (1992) Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res 20: 1425–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG (1997) Translational regulation of yeast GCN4. A window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem 272: 21661–21664 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG (2005) Translational regulation of GCN4 and the general amino acid control of yeast. Annu Rev Microbiol 59: 407–450 [DOI] [PubMed] [Google Scholar]
- Horak J (1997) Yeast nutrient transporters. Biochim Biophys Acta 1331: 41–79 [DOI] [PubMed] [Google Scholar]
- Horak J (2003) The role of ubiquitin in down-regulation and intracellular sorting of membrane proteins: insights from yeast. Biochim Biophys Acta 1614: 139–155 [DOI] [PubMed] [Google Scholar]
- Iraqui I, Vissers S, Bernard F, de Craene JO, Boles E, Urrestarazu A, Andre B (1999) Amino acid signaling in Saccharomyces cerevisiae: a permease-like sensor of external amino acids and F-Box protein Grr1p are required for transcriptional induction of the AGP1 gene, which encodes a broad-specificity amino acid permease. Mol Cell Biol 19: 989–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isnard AD, Thomas D, Surdin-Kerjan Y (1996) The study of methionine uptake in Saccharomyces cerevisiae reveals a new family of amino acid permeases. J Mol Biol 262: 473–484 [DOI] [PubMed] [Google Scholar]
- Jorgensen MU, Bruun MB, Didion T, Kielland-Brandt MC (1998) Mutations in five loci affecting GAP1-independent uptake of neutral amino acids in yeast. Yeast 14: 103–114 [DOI] [PubMed] [Google Scholar]
- Kaiser P, Flick K, Wittenberg C, Reed SI (2000) Regulation of transcription by ubiquitination without proteolysis: Cdc34/SCF(Met30)-mediated inactivation of the transcription factor Met4. Cell 102: 303–314 [DOI] [PubMed] [Google Scholar]
- Klasson H, Fink GR, Ljungdahl PO (1999) Ssy1p and Ptr3p are plasma membrane components of a yeast system that senses extracellular amino acids. Mol Cell Biol 19: 5405–5416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuras L, Rouillon A, Lee T, Barbey R, Tyers M, Thomas D (2002) Dual regulation of the met4 transcription factor by ubiquitin-dependent degradation and inhibition of promoter recruitment. Mol Cell 10: 69–80 [DOI] [PubMed] [Google Scholar]
- Menant A, Barbey R, Thomas D (2006) Determinants of the ubiquitin-mediated degradation of the Met4 transcription factor. J Biol Chem 281: 11744–11754 [DOI] [PubMed] [Google Scholar]
- Patton EE, Peyraud C, Rouillon A, Surdin-Kerjan Y, Tyers M, Thomas D (2000) SCFMet30-mediated control of the transcriptional activator Met4 is required for the G1-S transition. EMBO J 19: 1613–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenberg B, During-Olsen L, Kielland-Brandt MC, Holmberg S (1999) Substrate specificity and gene expression of the amino-acid permeases in Saccharomyces cerevisiae. Curr Genet 36: 317–328 [DOI] [PubMed] [Google Scholar]
- Rouillon A, Barbey R, Patton EE, Tyers M, Thomas D (2000) Feedback-regulated degradation of the transcriptional activator Met4 is triggered by the SCFMet30 complex. EMBO J 19: 292–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouillon A, Surdin-Kerjan Y, Thomas D (1999) Transport of sulfonium compounds. Characterization of the S-adenosylmethionine and S-methylmethionine permeases from the yeast Saccharomyces cerevisiae. J Biol Chem 274: 28096–28105 [DOI] [PubMed] [Google Scholar]
- Schreve JL, Garrett JM (2004) Yeast Agp2p and Agp3p function as amino acid permeases in poor nutrient conditions. Biochem Biophys Res Commun 313: 745–751 [DOI] [PubMed] [Google Scholar]
- Thomas D, Kuras L, Barbey R, Cherest H, Blaiseau PL, Surdin-Kerjan Y (1995) Met30, a yeast transcriptional inhibitor that responds to S-Adenosylmethionine, is an essential protein with WD40 repeats. Mol Cell Biol 15: 6526–6534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Surdin-Kerjan Y (1997) Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 61: 503–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle D, Andre B (2001) A genomic view of yeast membrane transporters. Curr Opin Cell Biol 13: 389–398 [DOI] [PubMed] [Google Scholar]
- Yen JL, Su NY, Kaiser P (2005) The yeast ubiquitin ligase SCFMet30 regulates heavy metal response. Mol Biol Cell 16: 1872–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


