Abstract
Studies in the yeast Saccharomyces cerevisiae have shown that the inheritance of endoplasmic reticulum (ER), mitochondria, and vacuoles involves the capture of a tubular structure at the bud tip. Ptc1p, a serine/threonine phosphatase, has previously been shown to regulate mitochondrial inheritance by an unknown mechanism. Ptc1p regulates the high osmolarity glycerol mitogen-activated protein kinase (MAPK) pathway and has also been implicated in the cell wall integrity (CWI) MAPK pathway. Here we show that the loss of Ptc1p or the Ptc1p binding protein, Nbp2p, causes a prominent delay in the delivery of ER tubules to the periphery of daughter cells and results in a dramatic increase in the level of phosphorylated Slt2p, the MAPK in the CWI pathway. Either loss of Slt2p or inhibition of the CWI pathway by addition of sorbitol, suppresses the ER inheritance defect in the ptc1Δ and nbp2Δ mutants. Our findings indicate that Ptc1p and Nbp2p regulate ER inheritance through the CWI MAPK pathway by modulating the MAPK, Slt2p.
Keywords: ER inheritance, protein phosphorylation, Ptc1p, Slt2p, the MAPK cascade
Introduction
The yeast Saccharomyces cerevisiae has proven to be an excellent model system for studying the spatial and temporal control of endoplasmic reticulum (ER) structure and dynamics (Du et al, 2004). The peripheral ER of yeast forms a cortical network similar in structure to that found in animal cells (Voeltz et al, 2002). Cortical ER is inherited from mother to daughter cells in multiple stages that include the extension of linear tubules along the mother–bud axis, anchorage at the bud tip, and distribution along the bud periphery. This then leads to the formation of a polygonal network in the daughter cell. In neurons, linear tubule extension is the major mechanism driving peripheral ER expansion in the axoplasm (Du et al, 2004). The formation of tubular segregation structures is also important for the inheritance of mitochondria and vacuoles (Weisman, 2003; Boldogh et al, 2005). Following transfer into the yeast bud, mitochondrial and vacuolar tubular structures become anchored at the bud tip. Other organelles, such as secretory vesicles (Guo et al, 2000) and peroxisomes (Fagarasanu et al, 2005), are also anchored at the tip of the bud. Thus, the extension of tubular structures and anchorage at the bud tip may be a general method used by eukaryotic cells for the inheritance of complex organelles.
Several components required for cortical ER inheritance and maintenance have been identified in yeast through the use of genetic analysis. Actin and the type V myosin motor complex, Myo4-She3p, are needed for the extension of ER tubules (Estrada et al, 2003). Sec3p, a subunit of the exocyst complex that mediates the tethering of post-Golgi secretory vesicles, is a putative anchor at the bud tip for ER tubules (Wiederkehr et al, 2003). Additionally, two transmembrane ER proteins (Ice2p and Rtn1p) have been implicated in the formation and/or maintenance of the ER network (Estrada de Martin et al, 2005; De Craene et al, 2006; Voeltz et al, 2006). At least two of the proteins (Aux1p (Swa2p) and Sec3p) required for ER inheritance are phosphorylated, suggesting that protein phosphorylation and dephosphorylation may regulate this process (Gall et al, 2000; Ficarro et al, 2002; Ubersax et al, 2003). The protein kinases and phosphatases regulating cortical ER inheritance, however, have not been identified thus far.
Ptc1p, a type 2C serine/threonine protein phosphatase, is required for the temporal control of yeast mitochondrial inheritance (Roeder et al, 1998). The best understood role for Ptc1p is the regulation of the high osmolarity glycerol (HOG) pathway (Martin et al, 2005). The HOG pathway enables yeast cells to survive under hyperosmotic conditions (Westfall et al, 2004). Adaptation to high osmolarity is achieved by the activation of a mitogen-activated protein kinase (MAPK) cascade that leads to the phosphorylation and activation of the MAPK Hog1p. Ptc1p, which is recruited to the HOG MAPK cascade by its binding protein Nbp2p, inactivates Hog1p by dephosphorylating it after adaptation to osmostic stress (Warmka et al, 2001; Mapes and Ota, 2004). Additionally, both Ptc1p and Nbp2p have been implicated in regulating the CWI MAPK pathway (Huang and Symington, 1995; Ohkuni et al, 2003). Activation of the MAPK Slt2p in the CWI MAPK cascade is required for cell wall remodeling during cell proliferation, mating, and the maintenance of cell wall integrity (CWI) in response to environmental stimuli (Levin, 2005).
Here, we find a delay in the delivery of ER tubules from the bud tip to the bud periphery in yeast cells lacking Ptc1p or Nbp2p. Deletion of ptc1 or nbp2 leads to increased levels of phosphorylated Slt2p. Furthermore, inactivation of Slt2p, but not Hog1p, suppresses the ER inheritance defects in ptc1Δ and nbp2Δ mutants. We conclude that Ptc1p regulates cortical ER inheritance by inactivating the CWI pathway.
Results
Cells lacking Ptc1p display a severe delay in the release of ER tubules from the bud tip
To isolate mutants defective in cortical ER inheritance, we screened the 4857 mutants in the yeast deletion library for defects in ER inheritance as described earlier (Estrada et al, 2003). In addition to the mutants previously described (Estrada et al, 2003; De Craene et al, 2006), this screen revealed that buds of ptc1Δ cells consistently showed a dramatic delay in the delivery of cortical ER segregation tubules from the bud tip to the periphery of daughter cells. Polymerase chain reaction (PCR) analysis confirmed that the PTC1 gene was deleted in this strain. Deletion of ptc1 from our host laboratory strain also resulted in a qualitatively similar phenotype, confirming that the loss of Ptc1p was responsible for the observed ER inheritance defect in these different strain backgrounds.
The inheritance and distribution of two transmembrane ER markers, Hmg1p-GFP and Ssh1p-GFP, is shown in wild-type and ptc1Δ cells (see Figure 1A and B). The Hmg1p-GFP fusion used in this analysis contains the NH2-terminal transmembrane domain (amino acids 1–702) of HMG-CoA reductase isozyme 1 (Hmg1p) and is expressed from the constitutive TDH3 promoter (Du et al, 2001). Ssh1p, another ER transmembrane protein, was fused to GFP and expressed under the control of the endogenous SSH1 promoter and terminator as the sole copy of Ssh1p. The expression of these fusion proteins had no obvious effect on the growth rate of wild-type and mutant cells at all temperatures tested in this study (25–38.5°C). We observed that >80% of the new ptc1Δ and wild-type buds (diameter <0.3 of mother) acquired cortical ER segregation tubules as the only inherited ER elements (Figure 1A–C), indicating that bud-directed movement of ER tubules was normal in ptc1Δ cells. However, >70% of small ptc1Δ buds (diameter between 0.3 and 0.5 of mother), and a lower but still significant portion (∼30%) of medium-sized mutant buds (bud diameter greater than half the mother, yet before nuclear segregation) only contained ER tubules across the mother–bud axis (Figure 1A–C). In contrast, most small (81%) and medium (98%)-sized wild-type buds contained evenly distributed cortical ER that was indistinguishable from that of the mother cell. ER tubules in ptc1Δ buds appeared to have reached the bud tip and often formed a capped structure there, indicating that the capture of ER segregation tubules at the bud tip was unaffected (Supplementary Figure S1). In large budded M-phase cells (with undivided nucleus, see filled arrows), little cortical ER was observed in more than 70% of the mutant buds. ER tubules emanating from the nuclear envelope towards the cortex, as well as a capped structure, were detected in 35% of large ptc1Δ buds. These observations indicate that the deletion of ptc1 causes a severe delay in the delivery of ER segregation tubules from the bud tip to the bud periphery.
Figure 1.
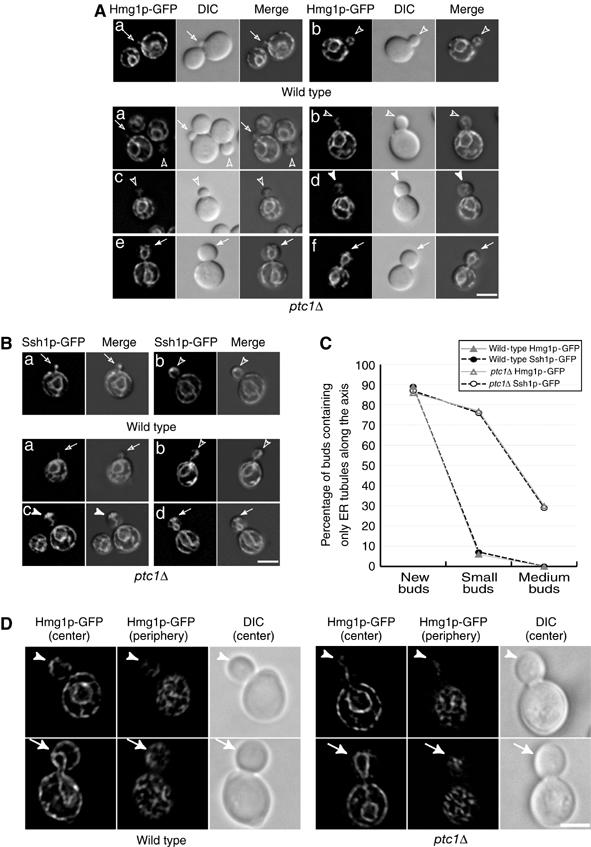
Deletion of the PTC1 gene causes a severe defect in the delivery of ER segregation tubules to the bud cortex. Cells were grown at 25°C in SC medium. Shown are representative wild-type and ptc1Δ cells expressing the ER markers Hmg1p-GFP (A) or Ssh1p-GFP (B). Merged DIC and fluorescence images reveal the localization of ER in the bud. Open arrows, open arrowheads, arrowheads, and arrows point to new, small, medium, and large buds, respectively. The majority of small ptc1Δ buds only contained ER tubules along the mother–bud axis (C). The ER distribution in 250 wild-type buds expressing Hmg1p-GFP ( ), 253 wild-type buds expressing Ssh1p-GFP (–•–), 264 ptc1Δ buds expressing Hmg1p-GFP (
), 253 wild-type buds expressing Ssh1p-GFP (–•–), 264 ptc1Δ buds expressing Hmg1p-GFP ( ), and 249 ptc1Δ buds expressing Ssh1p-GFP (–○–) was quantitated. The cortical ER tubular network is normal in ptc1Δ mother cells (D). A stack of images of wild-type (SFNY1625) and ptc1Δ (SFNY1624) diploid cells with focal planes 0.1-μm apart were obtained and deconvolved. Images obtained from the center of the cell and from the cell periphery are shown. Arrowheads point to medium buds and arrows point to large buds. Bars, 5 μm.
), and 249 ptc1Δ buds expressing Ssh1p-GFP (–○–) was quantitated. The cortical ER tubular network is normal in ptc1Δ mother cells (D). A stack of images of wild-type (SFNY1625) and ptc1Δ (SFNY1624) diploid cells with focal planes 0.1-μm apart were obtained and deconvolved. Images obtained from the center of the cell and from the cell periphery are shown. Arrowheads point to medium buds and arrows point to large buds. Bars, 5 μm.
Several mutants with disrupted cortical ER structure also display pronounced defects in cortical ER inheritance (Prinz et al, 2000; Estrada de Martin et al, 2005). It has been proposed that the delay in ER inheritance in these mutants might be caused by defects in the formation and/or maintenance of the cortical tubular network. To address this possibility, we investigated the structure of the ER network at the cell periphery in ptc1Δ cells expressing Hmg1p-GFP and compared it to wild type. The morphology of the cortical ER network appeared normal in ptc1Δ cells (Figure 1D), indicating that Ptc1p is required for ER inheritance but not for the maintenance of ER structure.
The phenotype observed in the ptc1Δ mutant may be the consequence of a defect in releasing ER tubules from the bud tip to the bud periphery. Alternatively, ER tubules may be delivered to the bud periphery normally, but fail to be retained there. To distinguish between these two possibilities, we used time-lapse video microscopy to follow ER tubule dynamics in wild-type and ptc1Δ mutant buds. In ptc1Δ cells, we consistently observed a severe delay in the release of ER tubules from the bud tip (Figure 2, bottom panels). In the first 30 min, no capped structure was detected at the bud tip. Between 30 and 100 min, a capped structure was observed at the tip, suggesting that ER tubules were stably anchored. After 100 min, tubules were detected along the bud periphery and remained there until the end of the time course. In contrast, ER tubules in wild-type buds spread along the bud cortex within 20 min (Figure 2, top panels). These observations demonstrate that the delivery of ER tubules from the bud tip to the bud periphery was disrupted in cells lacking Ptc1p.
Figure 2.
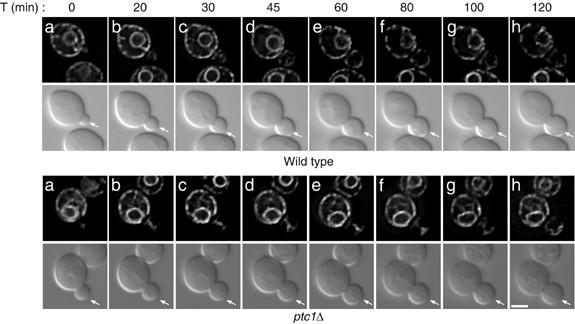
ER tubules are transported into newly developed ptc1Δ buds, but display a dramatic delay in their delivery to the bud cortex. Time-lapse images of wild-type (SFNY1625) and ptc1Δ (SFNY1624) diploid cells grown in SC medium at 25°C were acquired at 5 min intervals during 120-min time period as described in the Materials and methods. Ten cells were analyzed for each strain and the dynamics of the ER in representative cells are shown. These selected frames from the time series demonstrate that the release of ER tubules from the bud tip is severely delayed in ptc1Δ buds. Bar, 5 μm.
The inheritance of mitochondrial and vacuolar tubular structures is disrupted in the ptc1Δ mutant
Mitochondrial tubules are also captured at the bud tip before their delivery to the bud cortex (Yang et al, 1999). As Ptc1p is a potential regulator of mitochondrial transport into the bud (Roeder et al, 1998), we compared the distribution of the ER and mitochondria in ptc1Δ cells. Consistent with a previous report (Roeder et al, 1998), we observed that mitochondria were not present in 75% of small ptc1Δ buds (Figure 3A). Mitochondrial tubules along the mother–bud axis were observed in small and medium-sized wild-type buds that contained cortical ER. In ptc1Δ buds of the same size, which only contained ER tubules along the mother–bud axis, no mitochondria were detected. Interestingly, in mutant cells, mitochondria accumulated in the region of the mother cell distal to the bud (Figure 3A).
Figure 3.
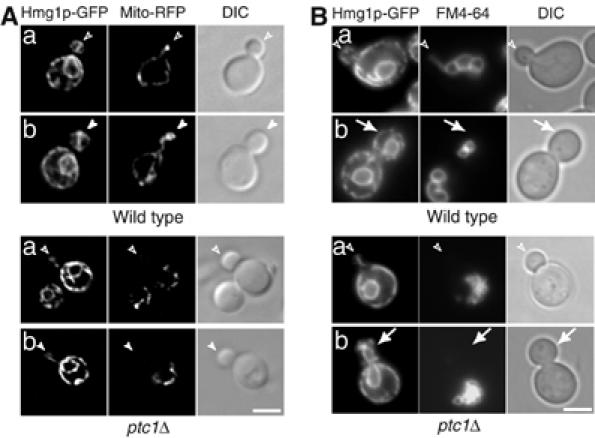
Mutant ptc1Δ cells display a severe delay in the formation of vacuolar and mitochondrial segregation structures. (A) A plasmid expressing the F0ATP synthase mitochondrial targeting sequence fused to RFP was transformed into wild-type (SFNY1255) and the ptc1Δ mutant (SFNY1610). Mitochondria in the transformed cells were visualized by RFP fluorescence. (B) SFNY1255 and SFNY1610 were stained with the vacuole-specific dye FM4-64. After 1 h of labeling, the cells were washed and incubated for 3 h in SC medium. Open arrowheads, arrowheads, and arrows point to small, medium and large buds, respectively. Bars, 5 μm.
The loss of PTC1 has previously been reported to cause vacuole fragmentation (Bonangelino et al, 2002). This observation prompted us to investigate Ptc1p's role in vacuole division during the cell cycle. To this end, the inheritance of FM4-64 labeled vacuoles was followed in cells expressing Hmg1p-GFP. Vacuole fragmentation was observed in all phases of the cell cycle including budded cells and cells with no buds, implying that the role of Ptc1p in vacuole morphology was not limited to a particular portion of the cell cycle (Figure 3B). In addition, we found that the majority of ptc1Δ buds (>80%) failed to acquire vacuoles (Figure 3B). Tubular vacuole structures were observed along the mother–bud axis in small wild-type buds that contained cortical ER (panel a). No such vacuolar structures were observed in ptc1Δ buds that only contained ER tubules along the mother–bud axis (panel a). The same observation was made in ptc1Δ buds that contained nuclear envelope and little cortical ER (panel b). The defect in vacuole inheritance was severe. All large wild-type buds contained vacuoles near the center of the bud (panel b), consistent with a previous report that vacuole inheritance ends before nuclear migration (Weisman, 2003). In contrast, the majority (∼ 80%) of large ptc1Δ buds did not contain any vacuole membranes. These results indicate that the transport or formation of mitochondrial and vacuole tubular structures was compromised in the ptc1Δ mutant.
The protein phosphatase activity of Ptc1p is required for ER inheritance
To determine whether the protein phosphatase activity of Ptc1p plays a role in cortical ER inheritance, we introduced a point mutation (ptc1D58N) in the metal-binding region of Ptc1p. This point mutation abolishes Ptc1p's ability to inactivate Hog1p, the MAP kinase in the HOG pathway (Warmka et al, 2001). Overexpression of PTC1, but not ptc1D58N, completely restored ER inheritance in ptc1Δ mutant cells (Figure 4A), indicating that the protein phosphatase activity of Ptc1p is required for ER inheritance.
Figure 4.
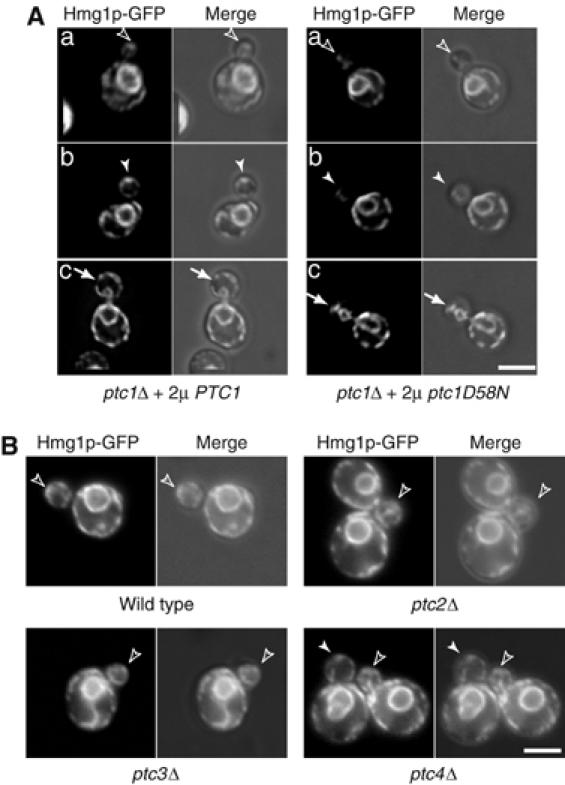
The phosphatase activity of Ptc1p is essential for its function in cortical ER inheritance. (A) Expression of PTC1 from a multicopy plasmid suppressed the ER inheritance defects of ptc1Δ (SFNY1610), whereas expression of the metal binding mutation ptc1D58N did not. The fluorescence and fluorescence-DIC merged images of representative cells with small (the first row), medium (the second row), and large buds (the third row) are shown. (B) ER inheritance is normal in cells lacking other PP2C members (Ptc2p, Ptc3p, and Ptc4p). Wild-type and mutant cells from the ResGen library, expressing Hmg1p-GFP, were grown in SC medium at 25°C and analyzed. PCR analysis confirmed the correct gene was disrupted in each of the strains used in this analysis. Bars, 5 μm.
The S. cerevisiae genome encodes three other members of the PP2C class of protein phosphatases, PTC2, PTC3, and PTC4 (Young et al, 2002). The catalytic domain of Ptc1p shows a high degree of similarity (38–51%) with these three phosphatases (Young et al, 2002). However, small budded cells (∼90%) lacking the entire coding sequence of PTC2, PTC3, or PTC4 did not display a defect in cortical ER inheritance (Figure 4B).
PTC1 displays genetic interactions with SEC3 and AUX1(SWA2)
To establish a functional link between PTC1 and other genes involved in cortical ER inheritance, we crossed ptc1Δ to mutants that display defects in cortical ER inheritance. The haploid double mutants, ptc1Δ sec3Δ and ptc1Δ aux1Δ, displayed either synthetic sickness or lethality (Figure 5A and B). No synthetic sickness or lethality was detected in ptc1Δ myo4Δ, ptc1Δ she3Δ, and ptc1Δ ice2Δ double mutants (our unpublished data). As sec3Δ haploid cells are not viable on YPD plates (Wiederkehr et al, 2003), ptc1Δ sec3Δ heterozygous diploids were dissected on synthetic complete (SC) plates. On SC plates, the ptc1Δ and sec3Δ single mutants formed colonies that varied in size, whereas ptc1Δ cells formed homogenously sized colonies on YPD plates (Figure 5). All ptc1Δ sec3Δ double mutants formed microcolonies. Although the ptc1Δ sec3Δ mutant colonies were somewhat variable in size (Figure 5A, top panel), they did not form single colonies when they were struck on SC plates and incubated at 30°C for 3 days. In contrast, cells containing only one mutation (ptc1Δ or sec3Δ) formed small colonies (bottom panel of Figure 5A). These findings demonstrate that PTC1 displays genetic interactions with SEC3 and AUX1(SWA2). Interestingly, these two genes encode proteins that are required for ER inheritance and are known to be phosphorylated (Gall et al, 2000; Ficarro et al, 2002; Ubersax et al, 2003).
Figure 5.
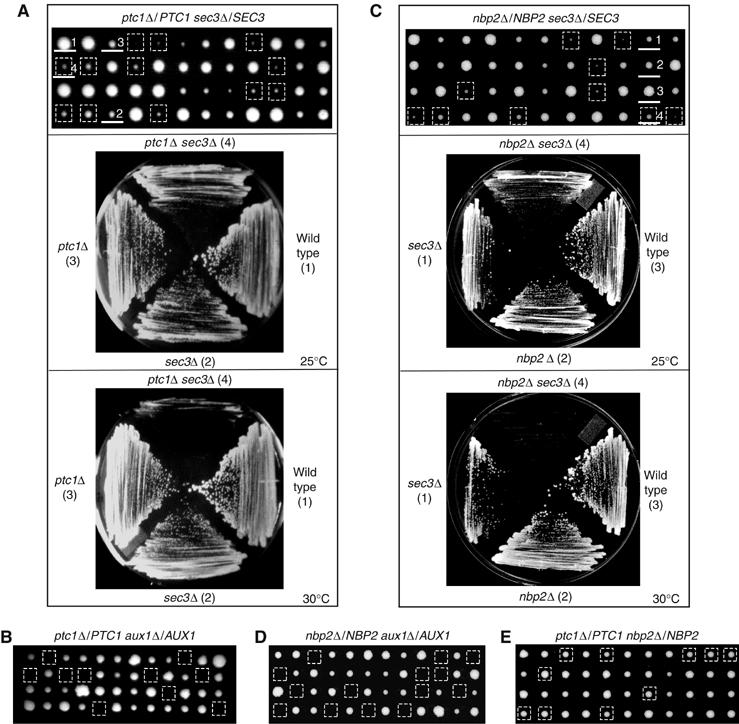
Both PTC1 and NBP2 display genetic interactions with AUX1(SWA2) and SEC3. Tetrads derived from the sporulation of diploid ptc1Δ/PTC1 sec3Δ/SEC3 (A, top panel), ptc1Δ/PTC1 aux1Δ/AUX1 (B), nbp2Δ/NBP2 sec3Δ/SEC3 (C, top panel), nbp2Δ/NBP2 aux1Δ/AUX1 (D), and ptc1Δ/PTC1 nbp2Δ/NBP2 (E) were dissected on YPD (B, D, E) or SC (A, C) plates and incubated at 25°C for 4 (B, D, E) or 6 days (A, C). The colonies enclosed in squares are the double-deletion mutants. The haploid cells (more than six for each genotype) derived from ptc1Δ/PTC1 sec3Δ/SEC3, or nbp2Δ/NBP2 sec3Δ/SEC3 were struck on SC plates and incubated at 25 and 30°C for 3 days. Representative plates are shown in A, C. The colonies on the representative plates are numbered and underlined on the dissection plates.
Cells lacking Nbp2p display the same defect in ER inheritance as the ptc1Δ mutant
Nbp2p is the only known binding partner for Ptc1p (Mapes and Ota, 2004). To determine whether Nbp2p regulates cortical ER inheritance at the same step as Ptc1p, we examined the distribution of two different ER proteins, Hmg1p-GFP (Figure 6A) and Ssh1p-GFP (Figure 6B), in the nbp2Δ mutant. The only inherited ER elements in nbp2Δ buds formed a capped structure at the bud tip. These structures were found in cells that were in the S to G2 phases of the cell cycle. As was observed with the ptc1Δ mutant, a severe delay in the delivery of ER tubules from the bud tip to the bud periphery was seen (Figure 6A and B, filled arrows). Quantitation of the data revealed that 68–75% of small budded nbp2Δ cells and 25% medium budded cells displayed defects in cortical ER inheritance. This is comparable to the defect in ptc1Δ cells (Figure 6C). The nbp2Δ and ptc1Δ mutants also showed qualitatively and quantitatively similar phenotypes in vacuole fragmentation and inheritance (our unpublished data). Thus, the loss of Nbp2p or Ptc1p leads to the same defects in organelle organization and inheritance. Like the ptc1Δ mutant, nbp2Δ displayed synthetic interactions with sec3Δ and aux1Δ (Figure 5C and D). Consistent with the hypothesis that Nbp2p interacts with Ptc1p to regulate ER inheritance, we found that nbp2Δ and ptc1Δ double mutants do not exhibit synthetic interactions at 25, 30, 34, or 37°C (Figure 5E and our unpublished data).
Figure 6.
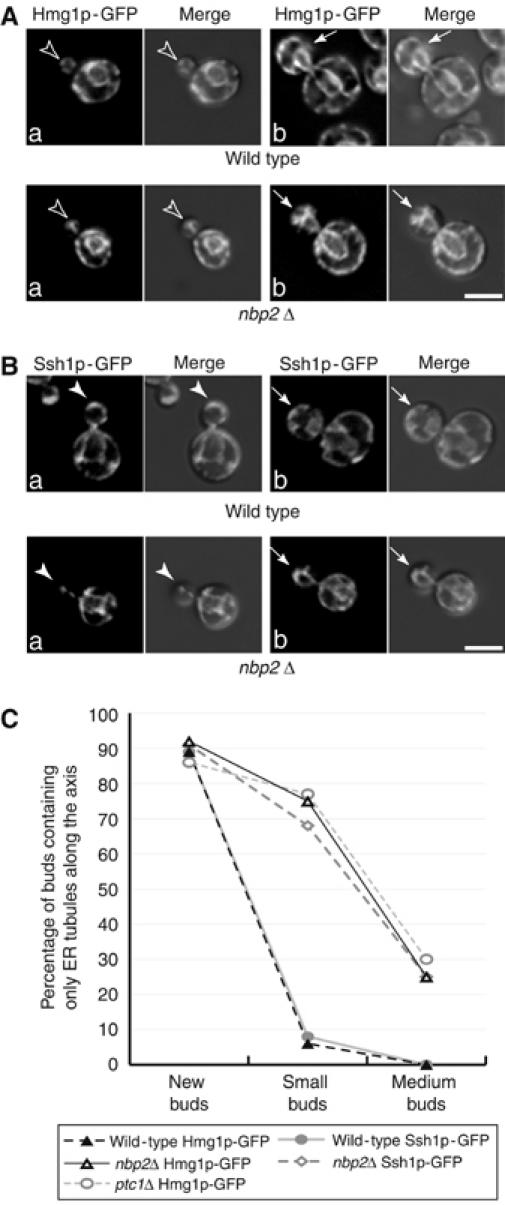
Deletion of nbp2 leads to a defect in cortical ER inheritance. Mutant cells lacking Nbp2p and expressing Hmg1p-GFP (A) or Ssh1p-GFP (B) were grown at 25°C in SC medium and assayed. The fluorescence and fluorescence-DIC merged images are shown. The open arrowheads, arrowheads, and arrows indicate small, medium, and large buds, respectively. The percentage of nbp2Δ budded cells that displayed cortical ER inheritance defects is comparable to ptc1Δ cells (C). All wild-type, nbp2Δ, and ptc1Δ strains used in this analysis are isogenic. The graph in (C) shows the ER distribution in 250 wild-type buds expressing Hmg1p-GFP (–▴–), 264 ptc1Δ buds expressing Hmg1p-GFP ( ), 253 nbp2Δ buds expressing Hmg1p-GFP (–▵–), 234 wild-type buds expressing Ssh1p-GFP (
), 253 nbp2Δ buds expressing Hmg1p-GFP (–▵–), 234 wild-type buds expressing Ssh1p-GFP ( ), and 260 nbp2Δ buds expressing Ssh1p-GFP (
), and 260 nbp2Δ buds expressing Ssh1p-GFP ( ).Bars, 5 μm.
).Bars, 5 μm.
Inactivation of Slt2p, but not Hog1p, restores ER inheritance in ptc1Δ and nbp2Δ mutants
Biochemical and genetic studies have shown that Ptc1p and Nbp2p are essential for the downregulation of Hog1p (Warmka et al, 2001; Mapes and Ota, 2004). Furthermore, both Ptc1p and Nbp2p have been implicated in the CWI pathway (Huang and Symington, 1995; Ohkuni et al, 2003 Figure 7A;) that maintains CWI during certain forms of stress (Levin, 2005). Within the CWI MAPK cascade, Slt2p plays a central role as the MAP kinase. The defect in cortical ER inheritance in the ptc1Δ mutant may reflect either overactivation of the HOG pathway or an increase in the level of activated Slt2p. To distinguish between these possibilities, we examined the distribution of ER in ptc1Δ hog1Δ and ptc1Δ slt2Δ double mutants. Although it was previously reported that a ptc1Δ slt2Δ double mutant is not viable (Huang and Symington, 1995), we were able to construct this mutant in our laboratory host strain (derived from S288C). Previous studies have shown that an nbp2Δ slt2Δ double mutant is only viable in the presence of a wild-type allele of SSD1, a polymorphic gene that regulates CWI in parallel with the CWI pathway (Ohkuni et al, 2003). Our host laboratory strain contains a wild-type allele of SSD1 (Du and Novick, 2002).
Figure 7.
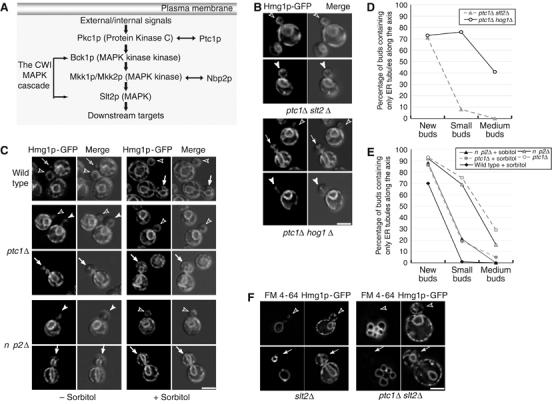
Inactivation of Slt2p, but not Hog1p, suppresses the ER inheritance defects in ptc1 and nbp2 mutant cells. (A) Both Ptc1p and Nbp2p have been implicated in the CWI MAPK pathway (Huang and Symington, 1995; Ohkuni et al, 2003). Protein kinase C (Pkc1p) acts as an upstream activator of this MAPK cascade. Deletion of ptc1 suppresses mutant phenotypes of pkc1–4 cells whereas deletion of either mkk1 or mkk2 suppresses the ts phenotype of nbp2Δ cells. (B) Deletion of slt2, but not hog1, restored ER inheritance in the ptc1Δ mutant. Shown are representative fluorescence and fluorescence-DIC merged images of the ER distribution in ptc1Δ slt2Δ buds and ptc1Δ hog1Δ cells grown to early log phase in SC medium at 25°C. (C) The ER inheritance defects in ptc1Δ and nbp2Δ cells was suppressed by sorbitol. Isogenic wild-type (SFNY1255), ptc1Δ (SFNY1610), and nbp2Δ (SFNY1685) strains were grown to early log phase and divided into two aliquots. Half the cells were grown at 25°C for 3 h in SC medium in the absence (−sorbitol) or presence of 1 M sorbitol (+sorbitol). Representative fluorescence and fluorescence-DIC merged images of cells with small (open arrow heads), medium (arrow heads), and large buds (arrows) are shown. The open arrow in the first row indicates a new wild-type bud. Quantitation revealed that the deletion of slt2 (D) and sorbitol treatment (E) leads to significant suppression. Key: 117 ptc1Δ slt2Δ buds ( ), 139 ptc1Δ hog1Δ buds (–○–), 278 wild-type buds cultured in SC+1 M sorbitol (–⧫–), 275 ptc1Δ buds grown in SC medium (
), 139 ptc1Δ hog1Δ buds (–○–), 278 wild-type buds cultured in SC+1 M sorbitol (–⧫–), 275 ptc1Δ buds grown in SC medium ( ), 284 ptc1Δ buds grown in SC+1 M sorbitol (
), 284 ptc1Δ buds grown in SC+1 M sorbitol ( ), 296 nbp2Δ buds grown in SC medium (–▵–), 284 nbp2Δ buds grown in SC+1 M sorbitol (–▴–) were analyzed. (F) Deletion of slt2 had no effect on vacuole distribution in ptc1Δ mutant and wild-type cells. The vacuole inheritance defect was analyzed as in Figure 3B. Bars, 5 μm.
), 296 nbp2Δ buds grown in SC medium (–▵–), 284 nbp2Δ buds grown in SC+1 M sorbitol (–▴–) were analyzed. (F) Deletion of slt2 had no effect on vacuole distribution in ptc1Δ mutant and wild-type cells. The vacuole inheritance defect was analyzed as in Figure 3B. Bars, 5 μm.
Deletion of the slt2 gene in ptc1Δ cells resulted in the restoration of cortical ER inheritance (Figure 7B). In contrast, deletion of hog1 in ptc1Δ cells showed no effect on ER distribution. Quantitation of the data revealed that the suppression was significant in small and medium buds (Figure 7D). Deletion of slt2 (Figure 7F) or hog1 alone (our unpublished data) did not lead to any delay in cortical ER inheritance. To confirm that the phenotype in ptc1Δ cells was caused by overactivation of the CWI pathway, we treated cells with 1 M sorbitol. Sorbitol is a known inhibitor of Slt2p and an activator of Hog1p (de Nobel et al, 2000; Hahn and Thiele, 2002). Isogenic wild-type, ptc1Δ, and nbp2Δ cells grown to early log phase were divided into two equal aliquots. One aliquot was incubated in a medium containing 1 M sorbitol and the other in a medium without sorbitol. Following a 3-h incubation at 25°C, cells were analyzed for defects in the distribution of ER. As shown earlier, in the absence of sorbitol, ptc1Δ and nbp2Δ cells displayed a severe delay in the delivery of ER tubules from the bud tip to the bud periphery (left panels, Figure 7C). In contrast, ptc1Δ and nbp2Δ cells treated with 1 M sorbitol showed normal cortical ER inheritance (right panels, Figure 7C). The phenotype of sorbitol-treated cells was comparable to that of slt2Δ (Figure 7D). Our results show that the inactivation of Slt2p rescues the ER inheritance defect in ptc1Δ and nbp2Δ mutants.
Interestingly, deletion of slt2 in ptc1Δ cells did not have any effect on the vacuole inheritance defect (Figure 7F). Vacuole inheritance was normal in slt2Δ cells, and the majority of large budded (>80%) ptc1Δ slt2Δ double-mutant cells did not obtain vacuoles from the mother cell. In addition, the deletion of hog1 in ptc1Δ cells did not suppress the vacuole inheritance defect (our unpublished data). These findings indicate that the inactivation of Slt2p does not suppress the vacuole inheritance defect in ptc1Δ cells, and are consistent with our observation that Ptc1p appears to have distinct execution points in cortical ER and vacuole inheritance.
The level of phosphorylated Slt2p is elevated in ptc1Δ and nbp2Δ cells
Our findings imply that, in cells lacking Ptc1p and Nbp2p, Slt2p is overactivated. As the phosphorylation of two conserved amino acids (Thr190 and Tyr192) in Slt2p is essential for its function (Martin et al, 2000), we examined whether Ptc1p and Nbp2p control the inactivation of the CWI pathway by limiting the level of phosphorylated Slt2p. The level of phosphorylated Slt2p was monitored with an antibody directed against the dually–phosphorylated p44/p42 MAPK. This antibody has been shown to specifically recognize phosphorylated Slt2p in yeast (Martin et al, 2000; Hahn and Thiele, 2002). Western blot analysis revealed a significant increase (5–7-fold) in the basal level of phosphorylated Slt2p in ptc1Δ and nbp2Δ cells (see 60 kDa band in Figure 8A). Phosphorylated Slt2p was not detected in the ptc1Δ mutant when slt2 was deleted (Figure 8A), confirming the specificity of the antibody. Using an antibody raised against Slt2p, we found that the expression of Slt2p was also significantly increased (∼3-fold) in ptc1Δ and nbp2Δ cells. This observation is consistent with previous reports showing that the activation of Slt2p also leads to an increase in its expression (Jung and Levin, 1999; Hahn and Thiele, 2002). The level of Bos1p, a 27 kDa integral membrane protein (Shim et al, 1991), was the same in all strains tested and served as a loading control for our studies. Overexpression of PTC1, but not ptc1D58N, decreased the level of phosphorylated Slt2p in ptc1Δ cells (Figure 8B). Thus, the protein phosphatase activity of Ptc1p, which is essential for modulating ER inheritance (Figure 4A), is required for Slt2p regulation. These findings support our hypothesis that the ER inheritance defects in the ptc1Δ and nbp2Δ mutants result from increased levels of activated Slt2p.
Figure 8.

Deletion of ptc1 and nbp2 leads to an increase in the level of phosphorylated Slt2p in vivo (A). Slt2p phosphorylation and expression was monitored as described in the Materials and methods. Bos1p (27 kDa) was used as a loading control. Expression of PTC1 from a multicopy plasmid decreased the level of phosphorylated Slt2p in ptc1Δ, whereas expression of the metal binding mutation ptc1D58N did not (B).
Discussion
The CWI MAPK cascade transduces various signals to downstream targets in budding yeast (Levin, 2005). It controls cell wall remodeling during cell proliferation and maintains CWI during adaptation to certain environmental stresses. Here, we report a role for this MAPK pathway in regulating the inheritance of ER during the cell cycle. Bud-directed transport of ER tubules appears to be normal in ptc1Δ and nbp2Δ mutants. However, the delivery of ER tubules from the bud tip to the bud periphery is severely delayed and there is an absence of cortical ER tubules lining the plasma membrane. Our results indicate that increased levels of the activated form of the MAPK in the CWI cascade accounts for the delay in the accurate delivery of ER to its proper location in daughter cells. These findings support the model that the inheritance of cortical ER is a highly regulated multistage process (Du et al, 2004).
The cortical ER, mitochondria, and vacuole are all inherited from mother to daughter cells via bud-directed movement of tubular structures (Yang et al, 1999; Weisman, 2003; Du et al, 2004). As previously reported (Roeder et al, 1998), we observed a role for Ptc1p in the temporal control of mitochondrial inheritance. Furthermore, we also saw a dramatic delay in vacuole inheritance in ptc1Δ and nbp2Δ mutants. Unlike the ER inheritance defect, the mitochondrial and vacuole inheritance defects appeared to be in the formation or transport of tubular structures. For both of these organelles, no segregation structures were found in ptc1Δ buds, and tubular structures were absent along the mother–bud axis in the mother cell. These findings indicate that the execution point for Ptc1p in ER inheritance is different from its execution point in mitochondrial and vacuole inheritance. In support of this proposal, we find that the deletion of slt2 restores defects in cortical ER inheritance but not in vacuole inheritance. Although the inheritance of ER, mitochondria, and vacuoles are all known to be dependent on actin (Estrada et al, 2003; Weisman, 2003; Boldogh et al, 2005), for several reasons, we think it is unlikely that the organelle inheritance defects in ptc1Δ and nbp2Δ cells results from a disruption of actin cables. First, actin cables and patches appear to be normal in ptc1Δ cells (Roeder et al, 1998 and our unpublished data). Second, Ptc1p has no obvious function in the actin-dependent extension of ER tubules into the bud. Third, the deletion of ptc1 has no detectable effects on the polarized growth of the bud or in the inheritance of the late Golgi marker Sec7p-GFP (our unpublished data), processes known to require intact actin cables (Rossanese et al, 2001).
In this study, SEC3 and AUX1(SWA2) are the only components of the cortical ER inheritance machinery that displayed genetic interactions with PTC1. Sec3p is a putative anchor at the bud tip for segregating ER tubules (Wiederkehr et al, 2003), whereas AUX1(SWA2) encodes a hydrophilic ER protein that contains a C-terminal J domain (Gall et al, 2000; Pishvaee et al, 2000). Both SEC3 and AUX1(SWA2) are known to encode phosphoproteins (Gall et al, 2000; Ficarro et al, 2002; Ubersax et al, 2003). The phosphorylation of Sec3p may play a role in its cell cycle regulation, as Sec3p is a possible substrate for the only known cyclin-dependent kinase in yeast, Cdc28p (Ubersax et al, 2003). The role of Aux1p (Swa2p) in ER inheritance and the significance of its hyperphosphorylation are currently unclear. The synthetic phenotypes we have observed may result from the disruption of parallel protein phosphorylation and dephosphorylation pathways that mediate ER inheritance. Alternatively, these genetic interactions may reflect the disruption of other essential processes.
Previous studies have suggested that Ptc1p and Nbp2p downregulate the CWI pathway (Figure 7A). For example, the loss of Ptc1p suppresses the temperature-sensitive (ts) growth defect of the pkc1–4 mutant (Huang and Symington, 1995). Additionally, the ts growth defect of nbp2Δ is suppressed by the deletion of mkk1 or mkk2 (Ohkuni et al, 2003). Here, we find that the inactivation of the CWI MAPK pathway in ptc1Δ and nbp2Δ cells, either by the deletion of slt2 or by sorbitol treatment, suppresses the cortical ER inheritance defect in these mutants. Furthermore, we show that the level of phosphorylated Slt2p is significantly increased in ptc1Δ and nbp2Δ mutants. These findings indicate that precise regulation of the CWI MAPK cascade by Ptc1p and Nbp2p is needed for the proper localization of ER tubules in the bud.
Many components of the CWI pathway, including Pkc1p, Mkk1p, Mkk2p, and Slt2p, localize in part to sites of polarized growth (Andrews and Stark, 2000; van Drogen and Peter, 2002). These proteins accumulate at the tip of newly developed buds, become delocalized in buds as they grow larger, and accumulate at the mother–bud neck region late in the mitosis. Slt2p is activated at early stages of the cell cycle in a Cdc28p-dependent manner (Zarzov et al, 1996), and accumulates at the bud tip approximately at the time when its activity peaks. We speculate that Ptc1p controls the timing of Slt2p inactivation, which in turn may play a role in transmitting a signal from the cell cycle machinery to the machinery that regulates ER tubule extension.
Animal cells contain proteins that share a high degree of sequence similarity with Ptc1p and components of the CWI pathway. Functional complementation studies have revealed that the CWI cascade is the MAPK cascade in yeast most similar to the ERK MAPK cascade in animal cells (Blumer et al, 1994; Lim et al, 1997). The ERK cascade has been implicated in regulating Golgi fragmentation in mitotic animal cells (Acharya et al, 1998; Shaul and Seger, 2006). Thus, protein phosphorylation and dephosphorylation by CWI kinases, PP2C phosphatases, and their homologs may be a general mechanism that is used to achieve accurate organelle partitioning during the cell cycle.
Materials and methods
Plasmid and strain construction
To tag the genomic copy of SSH1 with GFP, a PCR product containing the last 578 base pairs of coding sequence and the first 455 base pairs of the 3′ flanking sequence of SSH1 was digested with XhoI and XbaI and subcloned into pRS303 (CEN6, HIS3). Then the stop codon (TAA) of SSH1 was mutagenized to TCG using the Quick Change Site-Directed Mutagenesis Kit (Stratagene) to generate a SalI cutting site (ATG TCG ACA). The coding sequence of GFP was amplified by PCR, digested with SalI and inserted in-frame into the mutagenized stop codon of SSH1. The plasmid was linearized with EcoRI and transformed into yeast where SSH1-GFP was expressed as the sole copy of SSH1. The nucleotides that were mutagenized are in bold.
To overexpress PTC1, a 1.5 kb HindIII–SalI fragment was amplified by PCR and subcloned into pRS426 (2 μm, URA3) to generate pRS426-PTC1. To construct pRS426-ptc1D58N, the PTC1 gene in pRS426-PTC1 was mutagenized by mega-primer site-directed mutagenesis using the following oligonucleotides: 5′-CCCAGCATGTCCATTAAACACCGCG-3′. The mutated nucleotides are underlined.
SFNY1610 (MATα ptc1Δ) and SFNY1684 (MATa, ptc1Δ) were generated by replacing the PTC1 coding sequence in SFNY1255 (MATα GAL+his3-Δ200 ura3–52 leu2–3,112∷[LEU2, HMG1-GFP]) and SFNY1683 (MATα GAL+his3-Δ200, leu2–3,112 ura3–52∷[URA3, HMG1-GFP]), respectively, with the Schizosaccharomyces pombe his5+ gene using the PCR-mediated gene deletion method (Longtine et al, 1998). SFNY1624 (MATα/MATa ptc1Δ/ptc1Δ) was constructed by crossing SFNY1610 to SFNY1684. SFNY1625 (MATα/MATa PTC1/PTC1) was constructed by crossing SFNY1255 to SFNY1683. To construct SFNY1685 (MATα nbp2Δ), a 2.1-kb fragment containing the KanMX4 module was amplified by PCR using the Genome Deletion Library strain that is deleted for nbp2 as a template. This fragment contained 264 base pairs of the 5′ noncoding sequence of NBP2 and 267 base pairs of the 3′ noncoding sequence of NBP2. The primer pair NBP2primerA and NBP2primerD used in the PCR reaction was described before (Winzeler et al, 1999). The resulting PCR product replaced NBP2 in SFNY1255. The slt2 and hog1 gene disruptions were constructed by the same strategy.
Identification of ptc1Δ as a mutant defective in ER inheritance
The yeast MATa haploid deletion library from Research Genetics (ResGen-Invitrogene Corp) was screened for mutants in cortical ER inheritance as described previously (Estrada et al, 2003).
Microscopy
To visualize the ER and mitochondria, cells expressing GFP or RFP fusion proteins were grown overnight in SC medium at 25°C to log phase. Vacuoles labeled with 80 μM FM4-64 (Molecular Probes) were examined according to the method of Wang et al (1996). Fluorescence and differential interference contrast imaging was essentially performed as described previously (Du et al, 2001). To capture all of the fluorescence in a cell, seven images were acquired at 0.3-μm intervals along the z-axis. All images shown were deconvolved with Openlab software (Improvision). For the quantitation, cells were fixed at 25°C for 30 min in SC medium containing 3% formaldehyde. The cells were washed five times in phosphate-buffered saline (PBS) containing 1 mg/ml bovine serum albumin (BSA) (PBS-BSA) and mounted in PBS–BSA for visualization.
To visualize the cortical ER network (Figure 1D), 100 images were acquired at 0.1-μm intervals along the z-axis. Each image was deconvolved with Openlab software. Three to four images with focus planes close to the plasma membrane were merged to project the network structure.
Time-lapse microscopy of SFNY1625 (MATα/MATa PTC1/PTC1) and SFNY1624 (MATα/MATa ptc1Δ/ptc1Δ) was performed on cells grown to early log phase in SC medium at 25°C. Samples were mixed with an equal volume of SC medium containing 1.0% low melting temperature agarose. Then 10 μl of the mixture was placed on a glass slide and covered with a 22-mm square coverslip. To prevent the sample from dehydration, the edges of the coverslip were sealed with clear nail protector. Images were captured at 5 min intervals for 2 h with an AxioImager Z1 microscope (Zeiss) using AxioVision 4.5 software (Zeiss).
In order to study the effects of sorbitol treatment, cells were grown in SC medium to log phase. Cells were then divided into two aliquots and grown at 25°C for 3 h. One aliquot was grown in SC medium and the other in SC medium containing 1 M sorbitol. Cells were fixed and examined under the microscope as described above.
Detection of Slt2p
Yeast cells were grown at 25°C to early log phase in SC medium, harvested and washed in ice-cold water. Cell pellets from 12 A600 units of cells were broken by vortexing with glass beads in 350 μl of lysis buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM ethylene diaminetetra acetic acid, 10% glycerol, 1% Triton X-100, 0.1% sodium dodecyl sulfate) containing 1 × phosphatase inhibitor cocktail 1 (Sigma), 1 × phosphatase inhibitor cocktail 2 (Sigma), and protease inhibitors (170 μg/ml phenylmethylsulfonyl fluoride, 10 μM antipain, 1 μg/ml aprotinin, 30 μM leupeptin, 30 μM chymostatin, 20 μM pepstatin A). Samples (15 μg of protein) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (10%) followed by Western blot analysis using the enhanced chemiluminescence method. Phosphorylated Slt2p was detected with phospho-p44/42 MAP kinase (Thr202/Tyr204) antibody (Cell Signaling Technology) and Slt2p was detected with anti-Slt2p antibody (Santa Cruz Biotechnology). Both antibodies were preincubated with nitrocellulose membranes that contained immobilized lysate prepared from slt2Δ cells.
Supplementary Material
Figure S1
Acknowledgments
We thank Yueyi Zhang for technical assistance, and Felix Rivera-Molina for insightful discussions. This work was supported by grants from the National Institutes of Health, CA46128 (to S F-N) and GM73892 (to PN). Salary support for YD, LW, and SF-N was provided by the Howard Hughes Medical Institute.
References
- Acharya U, Mallabiabarrena A, Acharya JK, Malhotra V (1998) Signaling via mitogen-activated protein kinase kinase (MEK1) is required for Golgi fragmentation during mitosis. Cell 92: 183–192 [DOI] [PubMed] [Google Scholar]
- Andrews PD, Stark MJ (2000) Dynamic, Rho1p-dependent localization of Pkc1p to sites of polarized growth. J Cell Sci 113: 2685–2693 [DOI] [PubMed] [Google Scholar]
- Blumer KJ, Johnson GL, Lange-Carter CA (1994) Mammalian mitogen-activated protein kinase kinase kinase (MEKK) can function in a yeast mitogen-activated protein kinase pathway downstream of protein kinase C. Proc Natl Acad Sci USA 91: 4925–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldogh IR, Fehrenbacher KL, Yang HC, Pon LA (2005) Mitochondrial movement and inheritance in budding yeast. Gene 354: 28–36 [DOI] [PubMed] [Google Scholar]
- Bonangelino CJ, Chavez EM, Bonifacino JS (2002) Genomic screen for vacuolar protein sorting genes in Saccharomyces cerevisiae. Mol Biol Cell 13: 2486–2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craene J, Coleman J, Estrada de Martin P, Pypaert M, Anderson S, Yates JR, Ferro-Novick S, Novick P (2006) Rtn1p is involved in structuring the cortical ER. Mol Biol Cell 17: 3009–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nobel H, Ruiz C, Martin H, Morris W, Brul S, Molina M, Klis FM (2000) Cell wall perturbation in yeast results in dual phosphorylation of the Slt2/Mpk1 MAP kinase and in an Slt2-mediated increase in FKS2-lacZ expression, glucanase resistance and thermotolerance. Microbiology 146: 2121–2132 [DOI] [PubMed] [Google Scholar]
- Du LL, Novick P (2002) Pag1p, a novel protein associated with protein kinase Cbk1p, is required for cell morphogenesis and proliferation in Saccharomyces cerevisiae. Mol Biol Cell 13: 503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Ferro-Novick S, Novick P (2004) Dynamics and inheritance of the endoplasmic reticulum. J Cell Sci 117: 2871–2878 [DOI] [PubMed] [Google Scholar]
- Du Y, Pypaert M, Novick P, Ferro-Novick S (2001) Aux1p/Swa2p is required for cortical endoplasmic reticulum inheritance in Saccharomyces cerevisiae. Mol Biol Cell 12: 2614–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada de Martin P, Du Y, Novick P, Ferro-Novick S (2005) Ice2p is important for the distribution and structure of the cortical ER network in Saccharomyces cerevisiae. J Cell Sci 118: 65–77 [DOI] [PubMed] [Google Scholar]
- Estrada P, Kim J, Coleman J, Walker L, Dunn B, Takizawa P, Novick P, Ferro-Novick S (2003) Myo4p and She3p are required for cortical ER inheritance in Saccharomyces cerevisiae. J Cell Biol 163: 1255–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagarasanu M, Fagarasanu A, Tam YY, Aitchison JD, Rachubinski RA (2005) Inp1p is a peroxisomal membrane protein required for peroxisome inheritance in Saccharomyces cerevisiae. J Cell Biol 169: 765–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarro SB, McCleland ML, Stukenberg PT, Burke DJ, Ross MM, Shabanowitz J, Hunt DF, White FM (2002) Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat Biotechnol 20: 301–305 [DOI] [PubMed] [Google Scholar]
- Gall WE, Higginbotham MA, Chen C, Ingram MF, Cyr DM, Graham TR (2000) The auxilin-like phosphoprotein Swa2p is required for clathrin function in yeast. Curr Biol 10: 1349–1358 [DOI] [PubMed] [Google Scholar]
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P (2000) Protein complexes in transport vesicle targeting. Trends Cell Biol 10: 251–255 [DOI] [PubMed] [Google Scholar]
- Hahn JS, Thiele DJ (2002) Regulation of the Saccharomyces cerevisiae Slt2 kinase pathway by the stress-inducible Sdp1 dual specificity phosphatase. J Biol Chem 277: 21278–21284 [DOI] [PubMed] [Google Scholar]
- Huang KN, Symington LS (1995) Suppressors of a Saccharomyces cerevisiae pkc1 mutation identify alleles of the phosphatase gene PTC1 and of a novel gene encoding a putative basic leucine zipper protein. Genetics 141: 1275–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung US, Levin DE (1999) Genome-wide analysis of gene expression regulated by the yeast cell wall integrity signalling pathway. Mol Microbiol 34: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Levin DE (2005) Cell wall integrity signaling in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 69: 262–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YM, Tsuda L, Inoue YH, Irie K, Adachi-Yamada T, Hata M, Nishi Y, Matsumoto K, Nishida Y (1997) Dominant mutations of Drosophila MAP kinase kinase and their activities in Drosophila and yeast MAP kinase cascades. Genetics 146: 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961 [DOI] [PubMed] [Google Scholar]
- Mapes J, Ota IM (2004) Nbp2 targets the Ptc1-type 2C Ser/Thr phosphatase to the HOG MAPK pathway. EMBO J 23: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H, Flandez M, Nombela C, Molina M (2005) Protein phosphatases in MAPK signalling: we keep learning from yeast. Mol Microbiol 58: 6–16 [DOI] [PubMed] [Google Scholar]
- Martin H, Rodriguez-Pachon JM, Ruiz C, Nombela C, Molina M (2000) Regulatory mechanisms for modulation of signaling through the cell integrity Slt2-mediated pathway in Saccharomyces cerevisiae. J Biol Chem 275: 1511–1519 [DOI] [PubMed] [Google Scholar]
- Ohkuni K, Okuda A, Kikuchi A (2003) Yeast Nap1-binding protein Nbp2p is required for mitotic growth at high temperatures and for cell wall integrity. Genetics 165: 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pishvaee B, Costaguta G, Yeung BG, Ryazantsev S, Greener T, Greene LE, Eisenberg E, McCaffery JM, Payne GS (2000) A yeast DNA J protein required for uncoating of clathrin-coated vesicles in vivo. Nat Cell Biol 2: 958–963 [DOI] [PubMed] [Google Scholar]
- Prinz WA, Grzyb L, Veenhuis M, Kahana JA, Silver PA, Rapoport TA (2000) Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J Cell Biol 150: 461–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AD, Hermann GJ, Keegan BR, Thatcher SA, Shaw JM (1998) Mitochondrial inheritance is delayed in Saccharomyces cerevisiae cells lacking the serine/threonine phosphatase PTC1. Mol Biol Cell 9: 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossanese OW, Reinke CA, Bevis BJ, Hammond AT, Sears IB, O'Connor J, Glick BS (2001) A role for actin, Cdc1p, and Myo2p in the inheritance of late Golgi elements in Saccharomyces cerevisiae. J Cell Biol 153: 47–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul YD, Seger R (2006) ERK1c regulates Golgi fragmentation during mitosis. J Cell Biol 172: 885–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Newman AP, Ferro-Novick S (1991) The BOS1 gene encodes an essential 27-kD putative membrane protein that is required for vesicular transport from the ER to the Golgi complex in yeast. J Cell Biol 113: 55–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, Morgan DO (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864 [DOI] [PubMed] [Google Scholar]
- van Drogen F, Peter M (2002) Spa2p functions as a scaffold-like protein to recruit the Mpk1p MAP kinase module to sites of polarized growth. Curr Biol 12: 1698–1703 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA (2006) A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 124: 573–586 [DOI] [PubMed] [Google Scholar]
- Voeltz GK, Rolls MM, Rapoport TA (2002) Structural organization of the endoplasmic reticulum. EMBO Rep 3: 944–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YX, Zhao H, Harding TM, Gomes de Mesquita DS, Woldringh CL, Klionsky DJ, Munn AL, Weisman LS (1996) Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol Biol Cell 7: 1375–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmka J, Hanneman J, Lee J, Amin D, Ota I (2001) Ptc1, a type 2C Ser/Thr phosphatase, inactivates the HOG pathway by dephosphorylating the mitogen-activated protein kinase Hog1. Mol Cell Biol 21: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisman LS (2003) Yeast vacuole inheritance and dynamics. Annu Rev Genet 37: 435–460 [DOI] [PubMed] [Google Scholar]
- Westfall PJ, Ballon DR, Thorner J (2004) When the stress of your environment makes you go HOG wild. Science 306: 1511–1512 [DOI] [PubMed] [Google Scholar]
- Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P (2003) Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum. Mol Biol Cell 14: 4770–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler EA, Shoemaker DD, Astromoff A, Liang H, Anderson K, Andre B, Bangham R, Benito R, Boeke JD, Bussey H, Chu AM, Connelly C, Davis K, Dietrich F, Dow SW, El Bakkoury M, Foury F, Friend SH, Gentalen E, Giaever G, Hegemann JH, Jones T, Laub M, Liao H, Liebundguth N, Lockhart DJ, Lucau-Danila A, Lussier M, M'Rabet N, Menard P, Mittmann M, Pai C, Rebischung C, Revuelta JL, Riles L, Roberts CJ, Ross-MacDonald P, Scherens B, Snyder M, Sookhai-Mahadeo S, Storms RK, Veronneau S, Voet M, Volckaert G, Ward TR, Wysocki R, Yen GS, Yu K, Zimmermann K, Philippsen P, Johnston M, Davis RW (1999) Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285: 901–906 [DOI] [PubMed] [Google Scholar]
- Yang HC, Palazzo A, Swayne TC, Pon LA (1999) A retention mechanism for distribution of mitochondria during cell division in budding yeast. Curr Biol 9: 1111–1114 [DOI] [PubMed] [Google Scholar]
- Young C, Mapes J, Hanneman J, Al-Zarban S, Ota I (2002) Role of Ptc2 type 2C Ser/Thr phosphatase in yeast high-osmolarity glycerol pathway inactivation. Eukaryot Cell 1: 1032–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov P, Mazzoni C, Mann C (1996) The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO J 15: 83–91 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1


