Abstract
We describe a new member of the F-box family, Pof14, which forms a canonical, F-box dependent SCF (Skp1, Cullin, F-box protein) ubiquitin ligase complex. The Pof14 protein has intrinsic instability that is abolished by inactivation of its Skp1 interaction motif (the F-box), Skp1 or the proteasome, indicating that Pof14 stability is controlled by an autocatalytic mechanism. Pof14 interacts with the squalene synthase Erg9, a key enzyme in ergosterol metabolism, in a membrane-bound complex that does not contain the core SCF components. pof14 transcription is induced by hydrogen peroxide and requires the Pap1 transcription factor and the Sty1 MAP kinase. Pof14 binds to and decreases Erg9 activity in vitro and a pof14 deletion strain quickly loses viability in the presence of hydrogen peroxide due to its inability to repress ergosterol synthesis. A pof14 mutant lacking the F-box and an skp1-3 ts mutant behave as wild type in the presence of oxidant showing that Pof14 function is independent of SCF. This indicates that modulation of ergosterol level plays a key role in adaptation to oxidative stress.
Keywords: Erg9, fission yeast, hydrogen peroxide, sterol
Introduction
Oxidative stress occurs when cellular defences are unable to cope with existing reactive oxygen species (ROS), including hydrogen peroxide (H2O2), the superoxide anion (O2−) and the hydroxyl radical (OH−). In respiring cells, the primary source of ROS is incomplete reduction of oxygen to water, leading to leakage of electrons from the respiratory chain. ROS are also generated extracellularly by stimulated host phagocytes, exposure to UV and other environmental agents. This causes damage to numerous cellular components, including nucleic acids and lipids, which can result in a number of cardiovascular diseases, cancer or age-related pathologies (Busciglio and Yankner, 1995; Costa and Moradas-Ferreira, 2001). Therefore, it is not surprising that cells developed a variety of defences, including production of detoxifying enzymes (catalases, peroxidases and superoxide dismutases) and synthesis of molecular scavengers such as glutathione, ubiquinol and vitamins (Moradas-Ferreira and Costa, 2000). The response is adaptative, with exposure to a low dose leading to resistance to a higher dose that would otherwise be lethal (Marini et al, 1996).
Although it has been assumed that H2O2 could diffuse freely across biological membranes, recent data support the fact that their permeability is under active regulation. A stress-induced decrease in permeability could impose an extra-intracellular gradient attenuating the toxicity of subsequent exposure to a higher dose of ROS, and that could be a major determinant of the adaptative response (Branco et al, 2004; Sousa-Lopes et al, 2004). However, no molecular mechanism supports this hypothesis, and the strongest evidence that membrane permeability plays a key role in adaptation is that mutants producing altered sterols show higher permeability to and are more sensitive to oxidative stress-generating compounds (Higgins et al, 2003; Branco et al, 2004; Thorpe et al, 2004). The predominant sterol in yeast is ergosterol, which is identical to cholesterol except for the presence of double bonds in C7 and C22 and a methyl group at C28. Its synthesis and modifications are very similar to those occurring in mammalian cells for cholesterol and it likely acts in a similar manner with regard to both membrane fluidity and microdomain functions (Sturley, 2000). All cellular sterols are derived from farnesyl diphosphate (FPP) through the intermediate squalene. The membrane-bound squalene synthase Erg9 therefore represents an ideal site to regulate sterol formation selectively without interfering with other FPP derivatives such as isoprenoids (Robinson et al, 1993; Sturley, 2000). Deletion of erg9 is lethal in budding yeast, except in anaerobic growth where extracellular uptake of ergosterol is possible (Henneberry and Sturley, 2005). In the pathogenic fungus Candida glabrata, depletion of squalene synthase is also lethal but growth in mice is not affected due to incorporation of cholesterol from the serum (Nakayama et al, 2000). By contrast, many mutations occurring in genes acting downstream of erg9 in the pathway are not lethal but lead to other final products that can substitute for ergosterol in unchallenging conditions. Ergosterol synthesis is regulated by intracellular sterol and oxygen levels, and some regulations are known to occur at the transcriptional level for many ERG genes encoding enzymes implicated in the pathway (Kennedy and Bard, 2001; Hongay et al, 2002). However, direct links between that control and the oxidative response have not been reported so far.
The fission yeast Schizosacharromyces pombe responds to a wide range of stresses by a multistep phosphorelay activating the Sty1 SAPK (stress-activated protein kinase) (Millar et al, 1995; Degols et al, 1996; Shieh et al, 1997; Shiozaki et al, 1998; Nguyen et al, 2000), similarly to the activation of JNK and p38 stress-activated kinases in metazoans (reviewed in Toone and Jones, 1998). In turn, Sty1 regulates the transcription of stress response genes through a b-Zip transcription factor Atf1 (Takeda et al, 1995). However, the atf1 deletion mutant only displays a subset of the sty1 deletion phenotypes, suggesting that other factors might act downstream of Sty1 (Toone and Jones, 1999; Toone et al, 2001). Although another b-Zip transcription factor, Pap1, does not seem to be a direct target of Sty1, H2O2 dose-dependent changes in its subcellular localisation are impaired in a sty1 deleted strain (Wilkinson et al, 1996; Shiozaki et al, 1998; Toone et al, 1998). The reason appears to be that at a low dose of oxidant, an H2O2 induced disulphide bond directly activates Pap1, while at a higher dose, Sty1 needs to be activated first to reach the lower activation range of Pap1 (Vivancos et al, 2004). The current model is that Pap1 is primarily required for transcription of target genes in response to low levels of oxidant, while Atf1 becomes predominant at higher doses and only the double inactivation of atf1 and pap1 mimics the sty1 deletion defect in both acute and adaptative responses (Quinn et al, 2002).
Here we describe a new target of Pap1 regulated transcription in S. pombe, namely the F-box protein Pof14 that is required for survival to H2O2 stress. F-box proteins constitute a large family of proteins (Cenciarelli et al, 1999; Regan-Reimann et al, 1999; Winston et al, 1999; Jin et al, 2004) that are thought to provide substrate specificity to the SCF (Skp1, Cullin, F-box protein) E3 ubiquitin ligases by interacting with the core component Skp1 through their F-box motif, and by recruiting substrates (often after their phosphorylation) to be ubiquitinilated (Bai et al, 1996; Skowyra et al, 1997; Patton et al, 1998). A dynamic equilibrium has been proposed to exist because several F-box proteins are degraded by an autocatalytic mechanism (Galan and Peter, 1999). We show that Pof14 exemplifies such an F-box protein that is degraded in a proteasome and SCF-dependent manner. We also show that Pof14 plays an essential, SCF-independent role in the stress response to peroxide by negatively regulating ergosterol synthesis most likely by directly binding the squalene synthase Erg9.
Results and discussion
Pof14 is a component of the SCF ubiquitin ligase
We screened 107 clones from a fission yeast two-hybrid cDNA library using Skp1 as bait. Ninety-four positive candidates representing 19 proteins (Supplementary Table I) were isolated. Unsurprisingly, several previously characterised F-box proteins were recovered, but the most frequently isolated clone encoded an unknown protein (SPAC13D6.01) lacking known motifs. However, dissection of the protein sequence with the help of the Blocks software (Henikoff et al, 2000) revealed the presence of a putative F-box between residues 172 and 214. To test this possibility, a Gal4-AD (Gal4 activation domain) fusion protein bearing deletion of the first 14 residues (172–186) of the predicted F-box was constructed and tested against Skp1 in the two-hybrid system. Figure 1A–C shows that in spite of similar expression levels, only the full-length protein retained ability to interact with Skp1. We therefore named the new protein Pof14 (S.pombe F-box protein). We checked the ability of Pof14 to form an SCF complex in vivo. Co-immunoprecipitations from fission yeast extract revealed that Pof14 forms a complex with the SCF core components Skp1 and Pcu1 (Figure 1D). Together with the two-hybrid data, this demonstrates that a canonical SCFPof14 complex is present in fission yeast. We noted that Pof14 could precipitate two forms of Pcu1 most likely corresponding to the unmodified and the neddylated forms based on published data (Osaka et al, 2000; Harrison et al, 2005).
Figure 1.
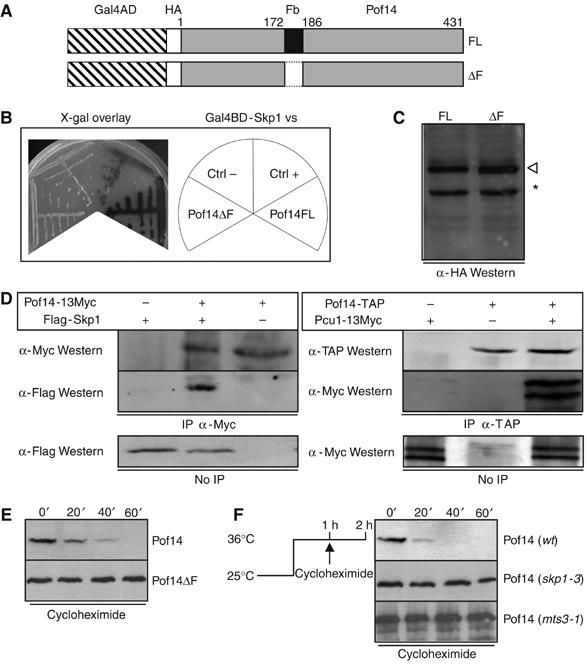
Pof14 is a component of SCF and is degraded by an autocatalytic mechanism. (A) Schematic representation of Pof14 deletion mutants. FL: full length, ΔF: deletion of the first 14 residues of the predicted F-box motif (172–186). (B) Two-hybrid interaction between Skp1 and Pof14. The MAV103 two-hybrid strain expressing Gal4DB-Skp1 was transformed with pACTII harbouring full-length pof14 or F-box deletion mutant. Controls consist of MAV103 expressing Gal4DB-Skp1 and transformed with pACTII-pop2 (Ctrl+) or empty pACTII (Ctrl−). Strains were streaked and overlaid with X-Gal containing medium to test activation of lacZ reporter gene. (C) Expression of Pof14 variants. Protein extracts from strains used in B were analysed by Western blot using α-HA antibody. An open arrowhead indicates gal4AD-HA-Pof14FL and −ΔF. An asterisk indicates a cross-reaction band. (D) Protein extracts from cells expressing the indicated tagged proteins from the endogenous locus (except for Skp1 expressed from a plasmid) were prepared and used for immunoprecipitation using the indicated antibodies. After SDS–PAGE, immunoblotting was performed as indicated. Total extracts are shown as control. (E) Cycloheximide was added to cultures expressing HA-tagged Pof14 or a mutant lacking the F-box from the locus. Samples were collected every 20 min and analysed by anti-HA Western blotting. (F) Cultures expressing HA-tagged Pof14 in wt, skp1-3 or mts3-1 background were shifted for 1 h to 36°C in order to inactivate Skp1 or the proteasome. Cycloheximide was added and samples collected every 20 min. Protein extracts were analysed by anti-HA Western blotting.
The pof14 open reading frame is not essential and no obvious phenotype was noted in the deleted strain (data not shown). The protein is very unstable with a half-life of less than 20 min, as determined after cycloheximide addition, which blocks proteins synthesis and allows the half-life of the present pool to be estimated (Figure 1E). The instability was fully dependent on the presence of the F-box (Figure 1E) and therefore on the interaction with SCF through Skp1. This suggests that Pof14 could be degraded by an autocatalytic mechanism, as reported for other F-box proteins (Galan and Peter, 1999). This possibility is further supported by the fact that wild-type Pof14 was stabilised by inactivation of either SCF (using the skp1-3 ts allele) or the proteasome (using the mts3-1 ts allele) (Figure 1F). We conclude that Pof14 is part of an SCF, which regulates its own stability.
Pof14 interacts with the squalene synthase Erg9 independently of SCF
To gain insight into the function of Pof14, a second two-hybrid screen with Pof14 as bait was performed and 61 candidates representing five proteins were isolated (Supplementary Table II). Two clones were recovered at similar high frequency: Skp1, as expected, and Erg9 (Figure 2A and B). Erg9 is the squalene synthase, a membrane-bound enzyme that plays a pivotal role in sterol metabolism. Analysis of overlapping interacting clones revealed that the C-terminal part of Erg9 was sufficient for interaction with Pof14 (Figure 2B).
Figure 2.
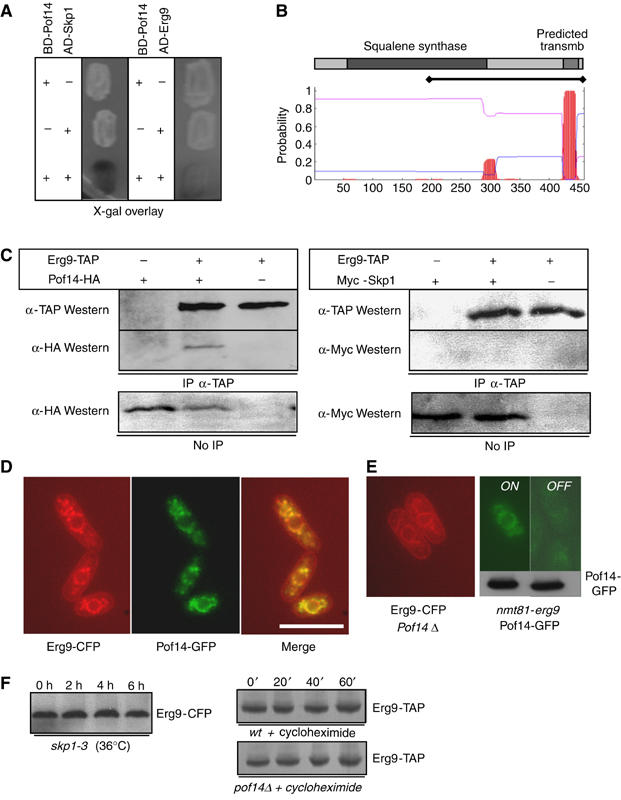
Pof14 interacts with Erg9 in a membrane bound complex. (A) Two-hybrid interaction between Pof14 and Erg9. The MAV103 two-hybrid strain expressing Gal4DB-Pof14 was transformed with pACTII harbouring skp1 (left) or erg9 (right). Strains were patched and overlaid with X-Gal containing medium to test activation of lacZ reporter gene. (B) Schematic representation of Erg9 catalytic domain (squalene synthase) and predicted transmembrane domain by a hydropathy plot (www.cbs.dtu.dk/services/TMHMM/). The bar indicates the fragment of Erg9 interacting with Pof14 in the two-hybrid screen. (C) Protein extracts from cells expressing the indicated tagged proteins from the endogenous locus (except for Skp1 expressed from a plasmid) were prepared and used for immunoprecipitation using the indicated antibodies. After SDS–PAGE, immunoblotting was performed as indicated. Total extracts are shown as control. (D) Cells expressing Pof14-GFP and Erg9-CFP were grown exponentially, patched on EMM agarose pads and observed for fluorescence microscopy. Bar: 10 μm. (E) Left: Cells deleted for pof14 and expressing Erg9-CFP were grown exponentially, patched on EMM agarose pads and observed for fluorescence microscopy. Right: identical except nmt81-erg9 pof14-GFP were grown in the absence (ON) or presence (OFF) of thiamine to repress erg9 expression. Bottom: Western blot with anti-GFP antibodies. (F) Left panel: cultures expressing CFP-tagged Erg9 in skp1-3 background were shifted for 6 h to 36°C in order to inactivate Skp1. Samples were collected every 2 h and protein extracts were analysed by anti-CFP Western blotting. Right panel: cycloheximide was added to cultures expressing TAP tagged Erg9 in wt and pof14Δ backgrounds and samples collected every 20 min. Protein extracts were analysed by anti-TAP Western blotting.
Pof14 and Erg9 could be co-immunoprecipitated from fission yeast extracts (Figure 2C), supporting the fact that the two-hybrid interaction is physiologically relevant. However, neither Skp1 (Figure 2C) nor Pcu1 (data not shown) could be co-immunoprecipitated with Erg9, raising the possibility that two Pof14-containing complexes might coexist in vivo, one of them being a canonical SCF and the other one containing Erg9. To further investigate this possibility, subcellular localisation of both Erg9 and Pof14 was determined by fusing the proteins to CFP and GFP, respectively. As reported for the budding yeast homologue of Erg9 (Kumar et al, 2002), SpErg9 associated with vesicle-like and ER (including a nuclear rim) structures (Figure 2D, left panel), consistent with the presence of a conserved transmembrane domain in Erg9 (Figure 2B; Robinson et al, 1993). Pof14 displayed a very similar localisation (Figure 2D, central panel), and colocalisation was confirmed (Figure 2D, right panel). No change in Erg9 localisation was observed when pof14 was absent (Figure 2E). In the reverse experiment, erg9 was placed under the control of the weak nmt81 promoter, which is turned off in the presence of thiamine. Upon thiamine addition, Pof14 localisation was largely lost although Pof14 protein level was unchanged (Figure 2E).
We first hypothesised that Erg9 could be a substrate of SCFPof14. However, the inability of Erg9 to coimmunoprecipitate Skp1 or Pcu1 (Figure 2C) with the fact that neither Skp1 nor Pcu1 present the typical subcellular localisation of Erg9 (D Hermand, unpublished data) did not support the hypothesis. Moreover, inactivation of Skp1 did not affect the level of Erg9 in vivo and its half-life was unchanged in a strain deleted for pof14 (Figure 2F). Altogether, these data led us to consider that Pof14 could regulate Erg9 independently of its proteolysis.
Pof14 and repression of ergosterol synthesis are required for adaptation to hydrogen peroxide
Genome-wide microarray analysis of the stress response in fission yeast showed that transcription of the pof14 gene is induced after exposure to 0.5 mM hydrogen peroxide (Chen et al, 2003). We expanded these data by analysing the expression of pof14 during a time course in two oxidative stress conditions: 0.2 and 1 mM based on previous works (Quinn et al, 2002). This was performed in a wild-type strain or in strains deleted for the stress response kinase Sty1 or transcription factors Atf1 and Pap1 (Figure 3A). In the conditions tested, an induction peak fully dependent upon the presence of Sty1 and Pap1 was observed within 1 h (Figure 3A), consistent with the published microarray data (Chen et al, 2003). We noticed that the pof14 promoter contains a TCTTTCAT motif reminiscent of the TCTTNCTT consensus of the stress response genes activated independently of Atf1 (Chen et al, 2003). The relevance of this motif was not tested experimentally in this study.
Figure 3.
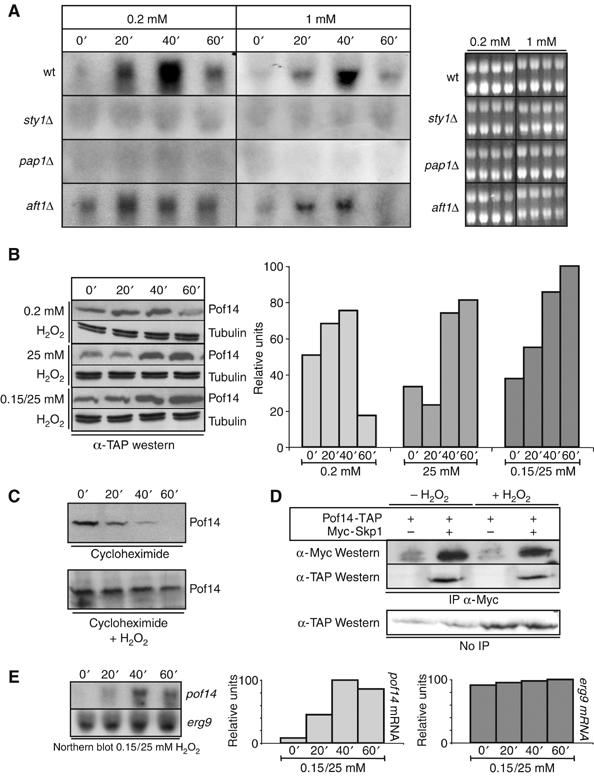
Pof14 expression is induced during oxidative stress. (A) Cultures of wt cells or cells deleted for either sty1, pap1 or atf1 were grown exponentially and H2O2 was added at 0.2 or 1 mM. Samples were taken every 20 min and RNA prepared. After separation on agarose gels, the blots were hybridised with a probe corresponding to pof14 open reading frame. An ethidium bromide staining of the gels is shown on the right as loading control. (B) Samples from wt cultures expressing Pof14-TAP and treated as indicated were collected and protein extracts were analysed by anti-TAP Western blotting. Anti-tubulin Western blotting is used as loading control. Quantification of Pof14 expression is shown on the right. (C) Cycloheximide was added a culture expressing HA-tagged Pof14 after a 20 min treatment with 0.2 mM H2O2. Control cells grown in the absence of H2O2 (identical to Figure 1E) are also presented. Samples were collected every 20 min and analysed by anti-HA Western blotting. (D) Protein extracts from cells expressing tagged Pof14 from the endogenous locus (except for Skp1 expressed from a plasmid) and grown in the presence or absence of H2O2 (40 min; 0.2 mM) were prepared and used for immunoprecipitation using the indicated antibodies. After SDS–PAGE, immunoblotting was performed as indicated. Total extracts are shown as control. (E) The blotting membrane used in Figure 3A was stripped and rehybridised with a probe corresponding to erg9 open reading frame. The result with a pof14 probe (identical to part of Figure 3A) is also shown. Right panel: quantification of the signal.
Western blot analysis and quantification of Pof14 protein under several stress conditions (Figure 3B) was also performed and a similar induction was observed. Moreover, the half-life of Pof14 was increased under stress conditions (Figure 3C), raising the possibility that beside transcriptional induction, Pof14 stability is also regulated in these circumstances to increase its level further. This might be achieved through dissociation of Pof14 from the core SCF as reported for the F-box protein Met30 (Barbey et al, 2005). However, it is not likely to be the case because when immunoprecipitating Skp1 in the presence or absence of stress (40 min, 0.2 mM H2O2), a similar amount of Pof14 was co-precipitated (Figure 3D), although the total Pof14 concentration was elevated as shown above (Figure 3B). This rather suggests that the SCF might be saturated when Pof14 is induced, therefore leading to a stabilisation of the Pof14 pool present in the cell. Pof14 could then only reach its normal level after transcriptional induction has been turned off.
We next compared the effect of hydrogen peroxide addition on Erg9 transcription, and Figure 3E shows that Erg9 does not appear to be regulated at this level. The Erg9 protein level was also stable under these conditions (data not shown).
The induction of pof14 transcription by hydrogen peroxide prompted us to analyse the Pof14–Erg9 interaction under oxidative stress conditions (H2O2 0.2 mM for 40 min). An increased amount of Pof14, proportional to its elevated level in total cell extract, was co-precipitated by Erg9, suggesting that after induction, the newly synthesised Pof14 proteins bind to Erg9 (Supplementary Figure 1).
To test the physiological importance of pof14 induction, we compared the sensitivity of a wild type and pof14 deletion treated with 0.2 mM H2O2, 25 mM H2O2 (acute stress) or 1 h with 0.15 mM H2O2 followed by 25 mM H2O2 (adaptative stress). Deletion of pof14 led to a marked decrease in viability in both acute and adaptative oxidative stress (Figure 4A). Surprisingly, the Pof14 mutant lacking the F-box could maintain viability similarly to the wild type, indicating that this function of Pof14 is independent of SCF. An skp1 ts mutant incubated at restrictive temperature during oxidative shock also maintained viability, confirming this conclusion (Figure 4A). In that case, we also checked that the heat shock did not affect the wild-type response (data not shown). Together with the fact that Pof14 and Erg9 form a complex that does not include Skp1 and Pcu1 (Figure 2), this suggests a link between the requirement of Pof14 to maintain viability in oxidative stress and ergosterol metabolism through its interaction with Erg9. To test this, ergosterol level was measured in the same strains used in Figure 4A. In the wild type, exposure to a low level of peroxide (0.15 mM) enabling adaptation led to a 25% decrease in ergosterol content within 1 h, indicating that cells repress ergosterol metabolism and decrease their ergosterol content to adapt to oxidative stress (Figure 4B). The loss of viability in the pof14 deletion in the same conditions correlates with stable ergosterol content while a mutant lacking the F-box or a mutant affected in SCF behaved as wild type.
Figure 4.
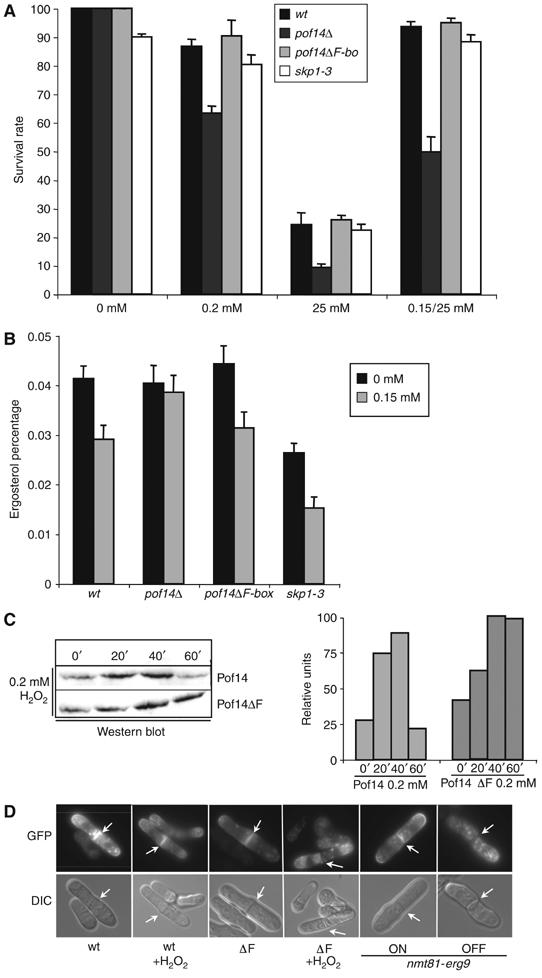
Pof14 mediates decrease in ergosterol content following oxidative stress. (A) The sensitivity of a wild-type strain (wt), a strain deleted for pof14 (pof14Δ), a strain expressing a pof14 mutant lacking the F-box (pof14ΔF) and an skp1 ts mutant (skp1-3) were analysed. After incubation of the cultures for 1 h with indicated H2O2 treatment, cells were diluted and plated. In the case of skp1-3, temperature was raised to 36°C at the time of peroxide addition. Survival was measured as a percentage of colony number of untreated cells. Mean survival rate is from three experiments. (B) Ergosterol content expressed as a percentage of wet weight was measured from a wild-type strain (wt), a strain deleted for pof14 (pof14Δ), a strain expressing a pof14 mutant lacking the F-box (pof14ΔF) and an skp1 ts mutant (skp1-3) grown for 1 h in the presence or absence of 0.15 mM hydrogen peroxide. In the case of skp1-3, temperature was raised to 36°C at the time of peroxide addition. Mean ergosterol content is from three experiments. (C) Samples from strains expressing integrated tagged Pof14 and Pof14 ΔF and treated for indicated times with 0.2 mM H2O2 were collected and protein extracts were analysed by Western blotting. Quantification of Pof14 expression is shown on the right. (D) 1–4: Indicated strains expressing the cell surface marker ScFus1-GFP from pREP41 were grown in the presence or absence of 0.2 mM H2O2 for 100 min and observed by fluorescence microscopy (GFP) or DIC. 5–6: nmt81-erg9 cells expressing the cell surface marker ScFus1-GFP from pREP41 were grown in the presence (OFF) or absence (ON) of thiamine for 6 h and observed by fluorescence microscopy. Arrows indicate the septum.
The behaviour of the F-box mutant raised the question of the physiological significance of Pof14 instability. To investigate this further, we compared the level of wild-type versus F-box mutant during oxidative stress. Although total Pof14 protein level was elevated in the mutant, the main feature is persistence (even at the 80 and 100 min time point, data not shown) of the F-box mutant at a high level after transcriptional induction is switched off (Figure 4C). We reasoned that a long-term decrease in the ergosterol level, consequent to high level of Pof14, might be deleterious for the cell. This hypothesis was suggested by recent data establishing a central role for ergosterol in correct and efficient cell surface delivery in yeast (Proszynski et al, 2005). Defective ergosterol synthesis results in inhibition of trafficking and sorting of proteins to the cell surface as exemplified by defects in the targeting of the tryptophan permease Tat2 to the cell surface in the absence of ergosterol (Umebayashi and Nakano, 2003). We tested a potential effect of long-term stabilisation of Pof14 on the delivery of the previously characterised cell surface marker ScFus1-GFP, a raft-associated protein that is missorted in mutants defective in ergosterol synthesis (Proszynski et al, 2005; Tanaka et al, 2005). In fission yeast, the reporter localises mainly to the septum (Tanaka et al, 2005). As shown in Figure 4D, the addition of oxygen peroxide (100 min, 1 mM) did not modify Fus1 localisation. By contrast, when the ΔF-box mutant was used, proper delivery of Fus1 to the septum was inhibited and instead it accumulated intracellularly in dot-like structures (Figure 4D and Supplementary Figure 2). Interestingly, inhibition of erg9 synthesis using a repressible promoter (nmt81) leads to similar defects (Figure 4D) in the absence of stress. However, the ΔF-box mutant does not show obvious phenotype, suggesting that in the conditions tested, cells might tolerate this effect.
Taken together, these data suggest that a short-term downregulation of the activity of Erg9 and the synthesis of ergosterol is required to set up an adaptative response to oxidative stress, and this requires Pof14. If this model is correct, transcriptional induction of pof14 in the absence of peroxide might mimic the adaptation following exposure to low dose. To test this, pof14 was overexpressed from a plasmid under the control of the strong nmt1 promoter, which is turned on in the absence of thiamine. Figure 5 shows that induction of Pof14 expression led to a decrease in ergosterol content and to a marked increase in viability following an acute stress with 25 mM H2O2. We also wanted to test the effect of higher level of erg9 on the response, but overexpression from the nmt1 promoter turned out to be toxic for the cell (Supplementary Figure 3).
Figure 5.
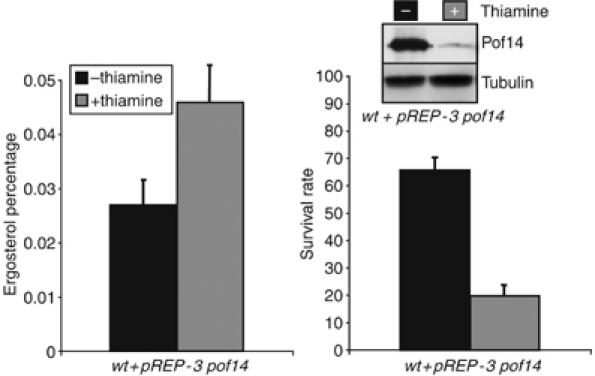
Pof14 directly regulates Erg9 activity. A strain harbouring a pREP-3 pof14 vector allowing overexpression of pof14 in the absence of thiamine was grown in the presence or absence of thiamine for 14 h and both ergosterol content and survival rate were determined following an acute stress with 25 mM H2O2 as in Figure 4A and B. Right panel: A Western blot showing Pof14 levels, anti-tubulin was used as loading control.
These data constitute the first molecular evidence supporting that membrane composition is altered to adapt permeability to H2O2. The two-hybrid interaction and the colocalisation are in favour of a direct effect of Pof14 on Erg9, although the way in which Pof14 affects Erg9 activity remains to be determined. To gain further insight on this aspect, we set up an activity assay for Erg9 in fission yeast based on previous works (Jennings et al, 1991; Shechter et al, 1992; Kribii et al, 1997). It consists in isolating Erg9 bound microsomes and measuring the amount of radiolabelled squalene produced in vitro in the presence of {3H} FPP and required co-factors. We first determined the squalene synthase activity in wild-type strain grown in the presence or absence of oxygen peroxide. Remarkably, a decrease in activity consistent with the reduction in ergosterol content (Table I) was observed after treatment with H2O2 and this was not observed in a pof14Δ strain. To assess a direct effect on Erg9 activity in vitro, GST or GST-Pof14 was expressed and purified from bacteria (Supplementary Figure 4) and increasing amounts of the recombinant proteins were added to the squalene synthase assay. As shown in Table I, addition of GST-Pof14 but not GST alone inhibited Erg9 in vitro. With the physical association detected in vivo, these in vitro data strongly support that Pof14 can directly affect Erg9 activity.
Table 1.
Squalene synthase activity assay in microsomal and cytosolic fractions
| Strain | Specific activity (nmol min−1 mg−1) |
Relative activity (microsomes) | |
|---|---|---|---|
| Cytosol | Microsomes | ||
| wt | 0.08 | 0.32 | 100 |
| wt+H2O2 | 0.1 | 0.21 | 66 |
| pof14Δ | 0.005 | 0.32 | 100 |
| pof14Δ+H2O2 | 0.005 | 0.29 | 90 |
| wt+GST 1 ng | ND | 0.35 | 109 |
| wt+GST-Pof14 1 ng | ND | 0.32 | 100 |
| wt+GST 10 ng | ND | 0.34 | 106 |
| wt+GST-Pof14 10 ng | ND | 0.25 | 78 |
| wt+GST 100 ng | ND | 0.31 | 97 |
| wt+GST-Pof14 100 ng | ND | 0.17 | 53 |
| Fractions were assayed for squalene synthase activity in triplicate assays with standard deviation within a 0.03 range. The mean specific activity found in wild-type strain (0.32) was assigned a relative activity of 100. ND: not determined. | |||
In this study, we report the role of the F-box protein Pof14 in directly regulating the activity of a key enzyme in sterol metabolism as a way for the cell to maintain viability following oxidative stress. This constitutes a molecular mechanism for the change in plasma membrane permeability induced by exposure to oxygen peroxide (Branco et al, 2004). Although the ability of the Pof14ΔF mutant and the skp1-3 ts mutant to behave as wild type shows that the role of Pof14 in this context does not require association with SCF, we do not rule out the possibility that SCFPof14 regulates the stability of proteins other than its own.
Materials and methods
Yeast strains, media and techniques
All standard protocols for fission yeast have been described previously (Moreno et al, 1991). Cycloheximide (Sigma) was used at 100 μg/ml.
Two-hybrid screens
The budding yeast strain used for the two-hybrid screen is MAV103 (MATa, gal4Δgal80Δ, SPAL10∷URA3, GAL1-LacZ, lys2∷GAL1-HIS3, ade2-101, ura3-52, leu2-3,112, trp1-901, his3Δ200; Vidal et al, 1996). The two-hybrid screens were performed according to the ‘TRAFO' protocol (see the Gietz lab website http://www.umanitoba.ca/faculties/medicine/units/biochem/gietz/2HS.html) based on the Matchmaker-II Two-Hybrid system (see the Clontech website and handbooks for complete protocols). Sequences and maps of the bait vectors harbouring Skp1 and Pof14 are available upon request. Expression was checked by Western blot using anti-Gal4DB antibody (Clontech).
Expression vectors, gene deletion and tagging
Flag-tagged Skp1 was expressed from pAAUN. Flag-tag sequence: MDYKDDDDK.
Myc-tagged Skp1 has been described previously (Hermand et al, 2003). pREP41-ScFus1-GFP is a kind gift from Kaoru Takegawa (Tanaka et al, 2005).
Deletion, locus-specific integration of the regulatable weak nmt81 and strong nmt1 promoters and C-term tagging were performed by the PCR method exactly as described (Bahler et al, 1998). All primer sequences are available upon request. For selection of natMX6, clonNAT was purchased from Werner Bioagent. All pof14 deletion mutants were created by combining PCR products; all primer sequences and detailed cloning sites are available upon request.
Western blotting, immunoprecipitations and GST-fusion proteins purification
For Western blotting, boiled extracts were prepared as described (Moreno et al, 1991). Cells were broken using 1 ml glass beads (Sigma) and a Fastprep device (Qbiogen). For immunoprecipitation, soluble protein extracts were prepared from log phase cultures, as described (Bamps et al, 2004). For IgG precipitation (IP-TAP), 20 μl of IgG agarose (Sigma A2909) was added to 1 mg of soluble extracts prepared with 1% Triton X-100. For Myc immunoprecipitation, 1 μl of anti-Myc (9E10, BabCo) coated to 50 μl of proteinA-sepharose beads (Sigma) was added to 1 mg of soluble extracts prepared with 1% Triton X-100. After three washes, proteins were separated on SDS–PAGE and blotted onto nitrocellulose membranes. Western blotting using anti-Myc (BabCo 9E10), anti-Flag (M5 monoclonal antibody, Stratagene), PAP (Peroxydase anti-peroxydase, Sigma P1291), anti-GFP isoforms (JL8, BD Biosciences), antitubulin (Sigma) and anti-HA (16B12, BabCo) were performed according to the manufacturer's instruction (ECL, Amersham Pharmacia Biotech). NIH Image (National Institutes of Health, Bethesda, MD) was used for quantification of protein levels after immunoblotting. The GST-Pof14 fusion was expressed from pGEX-4T-1 in the Escherichia coli BL21 strain according to the manufacturer's instructions (GE Healthcare). Purified proteins were concentrated on vivaspin (VivaScience).
Hydrogen peroxide sensitivity tests
For H2O2 sensitivity stress, overnight cultures were diluted at OD595=0.1. After 1 h recovery, H2O2 was added to cultures at indicated concentration. Cells were taken at various time points, diluted, then plated on YES plates to determine CFU, expressed as a percentage of CFU of untreated cells.
Ergosterol quantification method
Ergosterol level was measured using the method described by Arthington-Skaggs et al (1999). Briefly, cells were centrifuged and washed with water. The net weight of the cell pellet was determined, 3 ml of 25% alcoholic potassium hydroxide solution was added to each pellet and vortex mixed for 1 min. Cells were incubated at 85°C for 1 h and allowed to cool to room temperature. Sterols were extracted by the addition of a mixture of 1 ml of water and 3 ml of n-heptane followed by vortexing for 3 min. The heptane layer was recovered, diluted fivefold in ethanol and scanned spectrophotometrically between 240 and 300 nm. The presence of ergosterol was detected at 281.5 nm and ergosterol content was calculated as a percentage of the wet weight as reported (Arthington-Skaggs et al, 1999).
Yeast microsomal extracts and squalene synthase activity (Jennings et al, 1991; Shechter et al, 1992; Kribii et al, 1997)
Yeast cells grown in EMM were harvested by centrifugation, washed in 50 mM potassium phosphate pH 7.5 and resuspended in breakage buffer (50 mM potassium phosphate pH 7.5, 1 mM dithiothreitol and protease inhibitors cocktail). Cells were disrupted by vigorous vortexing in glass beads and the extract was centrifuged at 12 000 g for 30 min. The supernatant was centrifuged again at 105 000 g for 40 min. The supernatant (cytosolic fraction) was collected and the pellet (microsomal fraction) was resuspended in breakage buffer and centrifuged again at 105 000 g for 40 min. The pelleted mircosomes were resuspended in 50 mM potassium phosphate pH 7.5 at a concentration of 1 mg/ml.
SQS activity was assayed for 30 min at 30°C in a reaction mixture (0.5 ml) containing 50 mM potassium phosphate pH 7.5, 5 mM MgCl2, 10 mM KF, 1 mM NADPH, 4 mM glucose-6-phosphate, 2 U glucose-6-phosphate dehydrogenase and 25 μM (48 nCi, 20 μCi/mmol) {1-3H} farnesyl-P2 (farnesyl pyrophosphate triammonium salt, Perkin Elmer) and either microsomal or cytosolic fractions (100 μg protein) supplemented by various amounts of either GST or GST-Pof14 as indicated. The reaction was stopped by adding 0.25 ml 40% KOH and 0.25 ml ethanol. Saponification was allowed to proceed for 30 min at 60°C the nonsaponifiable lipids were extracted three times with 1 ml hexane. The radioactivity present in the hexane extract was quantified by liquid scintillation counting. The lower detection limit under these conditions was 0.002 nmol of squalene/min/mg protien, which corresponds to about 10 dpm above background.
In vivo fluorescence imaging
Cells expressing pof14-GFP and erg9-CFP were cultured overnight, washed twice with PBS and resuspended in 100 μl PBS. Two microlitres of cells were placed on EMM-agarose pads and covered with a coverslip as described in Hermand et al (2003). Living cells were then observed with a Nikon microscope (Eclipse E1000), using a × 100/1.40 Nikon oil immersion objective lens. Images were obtained on a cooled CCD camera (Hamamatsu ORCA-ER) with an exposure time of 2 s. Images were processed using Simple PCI software.
Northern blots
Aliquots (50 ml) of cultures were collected and centrifuged, and then rapidly frozen. Total RNA was extracted using a hot-phenol protocol (Chen et al, 2003). Thirty micrograms were separated on a 1.2% agarose-formaldehyde gel and blotted on a nylon membrane. RNA was probed with 32P-labeled DNA fragments (obtained by a radioactive PCR) specific to the target mRNA. NIH Image (National Institutes of Health, Bethesda, MD) was used for the quantification of mRNA level after revelation.
Supplementary Material
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Tables
Acknowledgments
We are grateful to Caroline Wilkinson, Nic Jones, Takashi Toda, Kaoru Takegawa, Marc Vidal and Steve Elledge for strains and reagents. We thank Tomi Mäkelä, Takashi Toda and Damien Coudreuse for a critical reading of the manuscript. LT was supported by a FRIA fellowship, and DH is supported by the Human Frontier Science Program (LT00665/2003). This work was supported by FNRS Grant 1.5.076.02 (to DH) and FRFC Grant 2.4504.00/1999 (to JVDH and DH).
References
- Arthington-Skaggs BA, Jradi H, Desai T, Morrison CJ (1999) Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J Clin Microbiol 37: 3332–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J, Wu JQ, Longtine MS, Shah NG, McKenzie A III, Steever AB, Wach A, Philippsen P, Pringle JR (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14: 943–951 [DOI] [PubMed] [Google Scholar]
- Bai C, Sen P, Hofmann K, Ma L, Goebl M, Harper JW, Elledge SJ (1996) SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F-box. Cell 86: 263–274 [DOI] [PubMed] [Google Scholar]
- Bamps S, Westerling T, Pihlak A, Tafforeau L, Vandenhaute J, Makela TP, Hermand D (2004) Mcs2 and a novel CAK subunit Pmh1 associate with Skp1 in fission yeast. Biochem Biophys Res Commun 325: 1424–1432 [DOI] [PubMed] [Google Scholar]
- Barbey R, Baudouin-Cornu P, Lee TA, Rouillon A, Zarzov P, Tyers M, Thomas D (2005) Inducible dissociation of SCF(Met30) ubiquitin ligase mediates a rapid transcriptional response to cadmium. EMBO J 24: 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Marinho HS, Cyrne L, Antunes F (2004) Decrease of H2O2 plasma membrane permeability during adaptation to H2O2 in Saccharomyces cerevisiae. J Biol Chem 279: 6501–6506 [DOI] [PubMed] [Google Scholar]
- Busciglio J, Yankner BA (1995) Apoptosis and increased generation of reactive oxygen species in Down's syndrome neurons in vitro. Nature 378: 776–779 [DOI] [PubMed] [Google Scholar]
- Cenciarelli C, Chiaur DS, Guardavaccaro D, Parks W, Vidal M, Pagano M (1999) Identification of a family of human F-box proteins. Curr Biol 9: 1177–1179 [DOI] [PubMed] [Google Scholar]
- Chen D, Toone WM, Mata J, Lyne R, Burns G, Kivinen K, Brazma A, Jones N, Bahler J (2003) Global transcriptional responses of fission yeast to environmental stress. Mol Biol Cell 14: 214–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa V, Moradas-Ferreira P (2001) Oxidative stress and signal transduction in Saccharomyces cerevisiae: insights into ageing, apoptosis and diseases. Mol Aspects Med 22: 217–246 [DOI] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P (1996) Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol 16: 2870–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galan JM, Peter M (1999) Ubiquitin-dependent degradation of multiple F-box proteins by an autocatalytic mechanism. Proc Natl Acad Sci USA 96: 9124–9129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison C, Katayama S, Dhut S, Chen D, Jones N, Bahler J, Toda T (2005) SCF(Pof1)-ubiquitin and its target Zip1 transcription factor mediate cadmium response in fission yeast. EMBO J 24: 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff JG, Greene EA, Pietrokovski S, Henikoff S (2000) Increased coverage of protein families with the blocks database servers. Nucleic Acids Res 28: 228–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberry AL, Sturley SL (2005) Sterol homeostasis in the budding yeast, Saccharomyces cerevisiae. Semin Cell Dev Biol 16: 155–161 [DOI] [PubMed] [Google Scholar]
- Hermand D, Bamps S, Tafforeau L, Vandenhaute J, Makela TP (2003) Skp1 and the F-box protein Pof6 are essential for cell separation in fission yeast. J Biol Chem 278: 9671–9677 [DOI] [PubMed] [Google Scholar]
- Higgins VJ, Beckhouse AG, Oliver AD, Rogers PJ, Dawes IW (2003) Yeast genome-wide expression analysis identifies a strong ergosterol and oxidative stress response during the initial stages of an industrial lager fermentation. Appl Environ Microbiol 69: 4777–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongay C, Jia N, Bard M, Winston F (2002) Mot3 is a transcriptional repressor of ergosterol biosynthetic genes and is required for normal vacuolar function in Saccharomyces cerevisiae. EMBO J 21: 4114–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SM, Tsay YH, Fisch TM, Robinson GW (1991) Molecular cloning and characterization of the yeast gene for squalene synthetase. Proc Natl Acad Sci USA 88: 6038–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Cardozo T, Lovering RC, Elledge SJ, Pagano M, Harper JW (2004) Systematic analysis and nomenclature of mammalian F-box proteins. Genes Dev 18: 2573–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MA, Bard M (2001) Positive and negative regulation of squalene synthase (ERG9), an ergosterol biosynthetic gene, in Saccharomyces cerevisiae. Biochim Biophys Acta 1517: 177–189 [DOI] [PubMed] [Google Scholar]
- Kribii R, Arro M, Del Arco A, Gonzalez V, Balcells L, Delourme D, Ferrer A, Karst F, Boronat A (1997) Cloning and characterization of the Arabidopsis thaliana SQS1 gene encoding squalene synthase—involvement of the C-terminal region of the enzyme in the channeling of squalene through the sterol pathway. Eur J Biochem 249: 61–69 [DOI] [PubMed] [Google Scholar]
- Kumar A, Agarwal S, Heyman JA, Matson S, Heidtman M, Piccirillo S, Umansky L, Drawid A, Jansen R, Liu Y, Cheung KH, Miller P, Gerstein M, Roeder GS, Snyder M (2002) Subcellular localization of the yeast proteome. Genes Dev 16: 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini M, Frabetti F, Musiani D, Franceschi C (1996) Oxygen radicals induce stress proteins and tolerance to oxidative stress in human lymphocytes. Int J Radiat Biol 70: 337–350 [DOI] [PubMed] [Google Scholar]
- Millar JB, Buck V, Wilkinson MG (1995) Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev 9: 2117–2130 [DOI] [PubMed] [Google Scholar]
- Moradas-Ferreira P, Costa V (2000) Adaptive response of the yeast Saccharomyces cerevisiae to reactive oxygen species: defences, damage and death. Redox Rep 5: 277–285 [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P (1991) Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol 194: 795–823 [DOI] [PubMed] [Google Scholar]
- Nakayama H, Izuta M, Nakayama N, Arisawa M, Aoki Y (2000) Depletion of the squalene synthase (ERG9) gene does not impair growth of Candida glabrata in mice. Antimicrob Agents Chemother 44: 2411–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AN, Lee A, Place W, Shiozaki K (2000) Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol Biol Cell 11: 1169–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaka F, Saeki M, Katayama S, Aida N, Toh EA, Kominami K, Toda T, Suzuki T, Chiba T, Tanaka K, Kato S (2000) Covalent modifier NEDD8 is essential for SCF ubiquitin-ligase in fission yeast. EMBO J 19: 3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Willems AR, Tyers M (1998) Combinatorial control in ubiquitin-dependent proteolysis: don't Skp the F-box hypothesis. Trends Genet 14: 236–243 [DOI] [PubMed] [Google Scholar]
- Proszynski TJ, Klemm RW, Gravert M, Hsu PP, Gloor Y, Wagner J, Kozak K, Grabner H, Walzer K, Bagnat M, Simons K, Walch-Solimena C (2005) A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc Natl Acad Sci USA 102: 17981–17986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn J, Findlay VJ, Dawson K, Millar JB, Jones N, Morgan BA, Toone WM (2002) Distinct regulatory proteins control the graded transcriptional response to increasing H(2)O(2) levels in fission yeast Schizosaccharomyces pombe. Mol Biol Cell 13: 805–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan-Reimann JD, Duong QV, Jackson PK (1999) Identification of novel F-box proteins in Xenopus laevis. Curr Biol 9: R762–R763 [DOI] [PubMed] [Google Scholar]
- Robinson GW, Tsay YH, Kienzle BK, Smith-Monroy CA, Bishop RW (1993) Conservation between human and fungal squalene synthetases: similarities in structure, function, and regulation. Mol Cell Biol 13: 2706–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter I, Klinger E, Rucker ML, Engstrom RG, Spirito JA, Islam MA, Boettcher BR, Weinstein DB (1992) Solubilization, purification, and characterization of a truncated form of rat hepatic squalene synthetase. J Biol Chem 267: 8628–8635 [PubMed] [Google Scholar]
- Shieh JC, Wilkinson MG, Buck V, Morgan BA, Makino K, Millar JB (1997) The Mcs4 response regulator coordinately controls the stress-activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev 11: 1008–1022 [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P (1998) Heat stress activates fission yeast Spc1/StyI MAPK by a MEKK-independent mechanism. Mol Biol Cell 9: 1339–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skowyra D, Craig KL, Tyers M, Elledge SJ, Harper JW (1997) F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91: 209–219 [DOI] [PubMed] [Google Scholar]
- Sousa-Lopes A, Antunes F, Cyrne L, Marinho HS (2004) Decreased cellular permeability to H2O2 protects Saccharomyces cerevisiae cells in stationary phase against oxidative stress. FEBS Lett 578: 152–156 [DOI] [PubMed] [Google Scholar]
- Sturley SL (2000) Conservation of eukaryotic sterol homeostasis: new insights from studies in budding yeast. Biochim Biophys Acta 1529: 155–163 [DOI] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N (1995) Schizosaccharomyces pombe atf1+ encodes a transcription factor required for sexual development and entry into stationary phase. EMBO J 14: 6193–6208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka N, Fujita Y, Suzuki S, Morishita M, Giga-Hama Y, Shimoda C, Takegawa K (2005) Characterization of O-mannosyltransferase family in Schizosaccharomyces pombe. Biochem Biophys Res Commun 330: 813–820 [DOI] [PubMed] [Google Scholar]
- Thorpe GW, Fong CS, Alic N, Higgins VJ, Dawes IW (2004) Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc Natl Acad Sci USA 101: 6564–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone WM, Jones N (1998) Stress-activated signalling pathways in yeast. Genes Cells 3: 485–498 [DOI] [PubMed] [Google Scholar]
- Toone WM, Jones N (1999) AP-1 transcription factors in yeast. Curr Opin Genet Dev 9: 55–61 [DOI] [PubMed] [Google Scholar]
- Toone WM, Kuge S, Samuels M, Morgan BA, Toda T, Jones N (1998) Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (Exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev 12: 1453–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toone WM, Morgan BA, Jones N (2001) Redox control of AP-1-like factors in yeast and beyond. Oncogene 20: 2336–2346 [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Nakano A (2003) Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol 161: 1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal M, Brachmann RK, Fattaey A, Harlow E, Boeke JD (1996) Reverse two-hybrid and one-hybrid systems to detect dissociation of protein–protein and DNA–protein interactions. Proc Natl Acad Sci USA 93: 10315–10320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivancos AP, Castillo EA, Jones N, Ayte J, Hidalgo E (2004) Activation of the redox sensor Pap1 by hydrogen peroxide requires modulation of the intracellular oxidant concentration. Mol Microbiol 52: 1427–1435 [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toone WM, Shieh JC, Toda T, Millar JB, Jones N (1996) The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev 10: 2289–2301 [DOI] [PubMed] [Google Scholar]
- Winston JT, Koepp DM, Zhu C, Elledge SJ, Harper JW (1999) A family of mammalian F-box proteins. Curr Biol 9: 1180–1182 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Tables


