Abstract
In eukaryotic translation initiation, the eIF2·GTP/Met-tRNAiMet ternary complex (TC) binds the eIF3/eIF1/eIF5 complex to form the multifactor complex (MFC), whereas eIF2·GDP binds the pentameric factor eIF2B for guanine nucleotide exchange. eIF5 and the eIF2Bɛ catalytic subunit possess a conserved eIF2-binding site. Nearly half of cellular eIF2 forms a complex with eIF5 lacking Met-tRNAiMet, and here we investigate its physiological significance. eIF5 overexpression increases the abundance of both eIF2/eIF5 and TC/eIF5 complexes, thereby impeding eIF2B reaction and MFC formation, respectively. eIF2Bɛ mutations, but not other eIF2B mutations, enhance the ability of overexpressed eIF5 to compete for eIF2, indicating that interaction of eIF2Bɛ with eIF2 normally disrupts eIF2/eIF5 interaction. Overexpression of the catalytic eIF2Bɛ segment similarly exacerbates eIF5 mutant phenotypes, supporting the ability of eIF2Bɛ to compete with MFC. Moreover, we show that eIF5 overexpression does not generate aberrant MFC lacking tRNAiMet, suggesting that tRNAiMet is a vital component promoting MFC assembly. We propose that the eIF2/eIF5 complex represents a cytoplasmic reservoir for eIF2 that antagonizes eIF2B-promoted guanine nucleotide exchange, enabling coordinated regulation of translation initiation.
Keywords: eIF, GTPase activation, guanine nucleotide exchange, ribosomal preinitiation complex, translation initiation
Introduction
In eukaryotic translation initiation, the eukaryotic initiation factor 2 (eIF2)·GTP/Met-tRNAiMet ternary complex (TC) sequentially binds to the 40S ribosomal subunit and eIF4F/m7G-capped mRNA complex to mediate the formation of 43S and 48S preinitiation complexes, respectively. The multisubunit factor eIF3 and other factors promote these assembly processes by binding directly or indirectly to the 40S subunit (for review, see Hershey and Merrick, 2000). The 48S complex then searches or ‘scans' for the first AUG codon in the mRNA. Correct base pairing between Met-tRNAiMet anticodon and the AUG codon triggers the hydrolysis of GTP bound to eIF2. This is mediated by the GTPase-activating protein eIF5 (Huang et al, 1997) and the subsequent Pi release is coupled to eIF1 dissociation (Algire et al, 2005). These events lead to dissociation of pre-assembled eIFs and formation of the 40S initiation complex. The second guanine nucleotide-binding (G) protein, eIF5B, then stimulates joining of the 60S subunit with the 40S initiation complex to form the 80S complex. Polypeptide chain elongation starts from the methionine linked to the 80S initiation complex.
eIF2 is a trimeric factor whose γ subunit carries a typical G protein fold and binds a guanine nucleotide. Regeneration of the GTP form of eIF2, catalyzed by the guanine nucleotide exchange factor (GEF) eIF2B, is essential for TC formation with Met-tRNAiMet (Hershey and Merrick, 2000). The affinity of eIF2 for GDP is much greater than GTP, so guanine nucleotide exchange is strongly dependent on the GEF activity of eIF2B. eIF2B comprises regulatory and catalytic subcomplexes containing α/β/δ and γ/ɛ subunits, respectively (Pavitt et al, 1998). Each subcomplex has independent eIF2-binding sites. The regulatory subcomplex binds eIF2α (Krishnamoorthy et al, 2001), whereas the catalytic subcomplex binds eIF2β and stimulates release of the guanine nucleotide bound to eIF2γ (Gomez et al, 2002). Of the catalytic subcomplex, the C-terminal domain (CTD) of eIF2Bɛ (residues 518–712) is folded into a HEAT domain composed of eight α-helices, with the GEF catalytic center at the first two α-helices and a strong eIF2β-binding site at the last three α-helices (Boesen et al, 2004). This conserved eIF2β-binding motif within eIF2Bɛ has been termed aromatic/acidic (AA-) boxes 1 and 2 and interacts with the lysine-rich boxes (K-boxes) found in the eIF2β N-terminal domain (NTD) (Asano et al, 1999).
Different stress stimuli activate four eIF2 kinases in mammals and Gcn2p in yeast Saccharomyces cerevisiae, all of which phosphorylate the conserved serine residue of eIF2α (Ser-51) (Dever, 2002). The phosphorylation of eIF2α increases its affinity for the eIF2B α/β/δ subcomplex, thereby rendering eIF2 into a competitive inhibitor of eIF2B, and reducing the level of functional TC (Krishnamoorthy et al, 2001). Although this effect is inhibitory to general protein synthesis, it paradoxically promotes translation of GCN4 (in yeast) or ATF4 (in mammals), both encoding transcription factors. This translational derepression is dependant upon regulatory upstream open reading frames (uORFs) in the leader regions of these regulated mRNAs (also see Results). Upregulated Gcn4p or Atf4 levels promote transcription of genes required to overcome the original stress stimuli: the general control response in yeast and the integrated stress response in mammals (Hinnebusch, 1997; Dever, 2002). As the cellular concentration of eIF2B is much lower than that of eIF2, phosphorylation of only a portion of eIF2 can significantly inhibit the guanine nucleotide exchange catalyzed by eIF2B.
After guanine nucleotide exchange, eIF2·GTP binds Met-tRNAiMet to form the TC that subsequently binds eIFs 1, 3, and 5 to form a multifactor complex (MFC). The C-terminal HEAT domain of eIF5, containing conserved AA-boxes, serves as an important core of the MFC by binding concurrently to eIF1 and the NTDs of eIF2β and eIF3c (Asano et al, 2000; Singh et al, 2005; Yamamoto et al, 2005). As with the eIF2Bɛ-CTD described above, K-boxes in eIF2β are necessary for binding to the eIF5-CTD AA-boxes (Asano et al, 1999). Genetic suppression studies of eIF5-CTD mutants suggested that the eIF2 TC/eIF5 complex formation is the rate-limiting step of the MFC assembly (Singh et al, 2005). In agreement with this idea, the affinity of eIF5-CTD for eIF3c-NTD is greatly enhanced by the prior association of the former with eIF2β-NTD (Singh et al, 2004b).
How eIF2·GTP and eIF2·GDP are efficiently incorporated into the correct multiprotein complexes for GTP hydrolysis and binding is not understood. Translation initiation schemes depict eIF2B interacting with eIF2·GDP and eIF5 with TC. However, recent evidence suggests that eIF5 interacts with eIF2 independently of its nucleotide-bound state in vitro (Algire et al, 2005) (CR Singh and K Asano, unpublished data). How potential competition between eIF2B and eIF5 for eIF2 interaction is resolved in vivo is an important unanswered question. We recently used quantitative blotting techniques to determine the relative expression levels of individual eIFs and, using this information, examined the stoichiometry of multiprotein complexes containing eIF2 in vivo by immunoprecipitation from S. cerevisiae cell extracts. We found that eIF1, eIF2, and eIF5 are nearly stoichiometric whereas the level of eIF3 is about half the level of these MFC components. In contrast, eIF2B is present at only ∼7% of the level of eIF2. Moreover, while only ∼15% of eIF2 is associated with tRNAiMet as TC, nearly half of the entire cellular eIF2 is bound to eIF5 in a complex devoid of tRNAiMet (Singh et al, manuscript in preparation).
To study the role of this novel eIF2/eIF5 complex and examine potential competition between eIF2B and eIF5 for binding to eIF2, we examined the effect of eIF5 overexpression on MFC and eIF2/eIF2B interactions. We show that eIF5 overexpression from a high-copy (hc) plasmid increases the abundance of this novel complex and stabilizes an eIF5/TC complex, thereby impeding eIF2B reaction and MFC formation. Further genetic and biochemical experiments reveal that the natural eIF5 and eIF2B factor expression levels are optimized, presumably to enable effective translational regulation by eIF2. Hence, we propose that the eIF2/eIF5 complex serves as a cytoplasmic reservoir for eIF2 that antagonizes guanine nucleotide exchange, such that this step of the reaction is rate-limiting for translational control. We discuss the function of eIF5 as part of a novel complex with eIF2, possibly in its GDP-bound state.
Results
Overexpression of eIF5 derepresses the general amino-acid control response, promotes eIF2/eIF5 complex formation, and reduces TC abundance
In this report, we used the GCN4-depednent general (amino acid) control response as a sensitive indicator of in vivo eIF2 activity. Under non-starvation conditions, GCN4 mRNA translation is repressed by a series of four short uORFs in its 5′ leader. Amino-acid starvation signals activation of the protein kinase Gcn2p, which then phosphorylates eIF2, resulting in inhibition of eIF2B. A reduction in TC level owing to eIF2B inhibition allows the ribosome on the GCN4 leader to bypass the uORFs hence to translate GCN4. Derepression of GCN4 activity in the absence of Gcn2p is therefore a sensitive in vivo measure for impairment of the eIF2B activity. GCN4 translation can also be derepressed by eIF mutations delaying TC binding to ribosomes migrating on GCN4 mRNA (see below).
We previously observed that ∼20-fold overexpression of eIF5 from an hc plasmid causes GCN2-independent derepression of GCN4 expression in the absence of amino-acid starvation: a Gcd− phenotype (Asano et al, 1999). The Gcd− phenotype suggested that the excess eIF5 somehow inhibits the eIF2B activity or delays TC binding to the ribosome in vivo. As indicated in Introduction, we recently found that a large fraction of eIF2 and eIF5 form a complex devoid of tRNAiMet. eIF5 overexpression can theoretically increase the abundance of the novel complex by mass action. We therefore reasoned that examination of the biochemical basis for the Gcd− phenotype may provide clues to the normal function of this novel eIF2/eIF5 complex. Thus, we first examined the effect of eIF5 overexpression (hc eIF5) on the formation of eIF5- and eIF2-containing complexes with our immunoprecipitation assays (to be described elsewhere). Briefly, the experimental approach was to perform immunoprecipitation reactions from extracts of cells expressing a FLAG epitope-tagged protein and to use Western and Northern blotting to quantitate the amount of co-precipitating proteins and tRNAiMet, respectively. When strains contained FLAG-tagged eIF2β (FL-eIF2), the amount of eIF5 associated with FL-eIF2 increased 1.7(±0.3)-fold (P=0.016) in hc eIF5 cells, as shown as an example in Figure 1A, second and third panels. Thus, excess eIF5 increased the abundance of complexes containing FL-eIF2 and eIF5, as expected.
Figure 1.
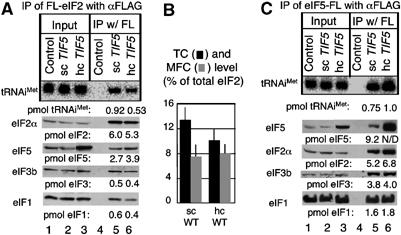
Determination of TC and eIF assembly intermediate levels in vivo. (A) and (C) Quantitative immunoprecipitation of FL-eIF2 and eIF5-FL complexes, respectively. A 1 mg portion of whole-cell extract (WCE) prepared from (A) KAY17 (Control), KAY128 (sc TIF5), and KAY482 (hc TIF5), and (C) KAY24 (Control), KAY35 (sc TIF5), and KAY39 (hc TIF5) was used for anti-FLAG immunoprecipitation, and 80% (top panel) and 20% (bottom panels) of the precipitated fractions (lanes under IP) were analyzed by Northern and Western blotting, respectively, with 2% input amount (lanes under Input) and purified tRNAiMet and eIF2 as the standard (not shown), as described in Materials and methods. The molar amounts of precipitated components, calculated in comparison with the band densities of purified components, are listed below each panel. N/D, not determined as the bond density was out of linearity range. (B) TC and MFC levels. Percentage of tRNAiMet- or eIF3-bound eIF2 compared to total eIF2, observed in KAY128 (sc TIF5) and KAY482 (hc TIF5), is represented by bars with s.d. in lines.
In contrast, Northern blotting of FL-eIF2 immune complexes with the probe specific for tRNAiMet (Figure 1A, top panel, lane 6) indicated that the tRNAiMet co-precipitated with FL-eIF2 was reduced to 75±6% (P=0.015, n=7) in hc eIF5 cells compared to wild-type cells (Figure 1B). This provides evidence for a modest reduction in steady-state TC abundance in these cells. As reduced TC levels can cause a Gcd− phenotype, this is a potentially important observation. eIF3 and eIF1 associate with eIF2 as part of MFC and 43S/48S complexes. eIF3b and eIF1 were probed for as markers for the abundance of these complexes in the reactions. The experiments indicated that the amount of eIF3b and eIF1 associated with FL-eIF2 was unchanged by hc eIF5 (Figure 1A, lower panels, compare lanes 5 and 6). Thus, overexpression of eIF5 increases the steady-state abundance of an eIF2/eIF5 complex, decreases TC levels, and leaves MFC and 40S-bound eIF2 complex concentrations unchanged.
Excess eIF5 binds aberrantly to its MFC partners in vivo
To examine the effect of hc eIF5 on eIF5 interactions with MFC components, we performed quantitative co-immunoprecipitations with eIF5-FL. Analysis of the eIF5-FL complexes by anti-eIF2α antisera confirmed that hc eIF5 increases the abundance of eIF2/eIF5 (-FL) complexes (Figure 1C, second and third panels). Probing immunoprecipitates with anti-eIF3b antibodies (Figure 1C, fourth panel) showed that 41% (3.8 pmol of 9.2 pmol) of total eIF5-FL was bound to eIF3. Because the level of eIF3 is half the level of eIF5 (Singh et al, manuscript in preparation), this indicated that nearly all of eIF3 was bound to eIF5 in wild-type (sc eIF5) cells, consistent with the tight copurification of eIF5 with eIF3 (Phan et al, 1998). Accordingly, hc eIF5 did not further increase the amount of eIF3 bound to eIF5-FL, as shown in Figure 1C. We also observed that 17% of eIF5-FL (1.6 pmol of 9.2 pmol) was associated with eIF1 (Figure 1C, bottom panel). Because the interaction between eIF1 and eIF5 is much weaker than that between eIF1 and eIF3 (Singh et al, 2004b), it seemed likely that the eIF5/eIF1 interaction occurred within the context of MFC or eIF1/eIF3/eIF5 complex. If so, this suggests that at least half of the eIF3/eIF5-containing complex is bound to eIF1 (lane 5). The abundance of the eIF1/eIF5-containing complex was not increased significantly by hc eIF5 (lane 6).
In contrast, Northern blotting with the tRNAiMet probe indicated that the amount of tRNAiMet associated with eIF5-FL was significantly increased by 29% (P=0.034, n=7; Figure 1C, top gel, lanes 5 and 6). Considering that the total TC level was rather decreased from ∼13.5 to ∼11% by hc eIF5 and that the total MFC level was unaltered at ∼8% of total eIF2 (Figure 1A and B), this observation suggests that the abundance of a TC/eIF5-FL complex free from the other MFC factors tested is dramatically increased by hc eIF5.
To confirm this last point, we resolved eIF complexes from cycloheximide-treated single-copy (sc) and hc eIF5-FL cells by sucrose gradient-velocity sedimentation and immunoprecipitated eIF5-FL complexes in each fraction by anti-FLAG. As shown in Figure 2A and B, eIF2(α) and eIF5-FL, but not eIF3(b), comigrated in ribosome-free, top fractions 1 and 2. The eIF2(α) in these fractions efficiently co-precipitated with eIF5-FL, confirming formation of eIF2/eIF5 complexes free of MFC (Figure 2C and D, top gels, lanes 1 and 2). Northern blotting of the precipitated complexes indicated that tRNAiMet was associated with the eIF2/eIF5-FL complex in these fractions isolated from hc eIF5 cells (Figure 2D, bottom gel), but not from sc eIF5 cells (Figure 2C, bottom gel). These results support the idea that the binary TC/eIF5 complexes are almost negligible in wild-type cells but are produced in hc eIF5 cells by mass action, explaining the increase in tRNAiMet precipitation by eIF5-FL (Figure 1C).
Figure 2.
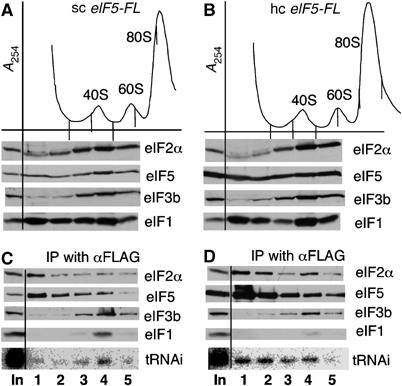
Analyses of eIF complexes by sucrose gradient-velocity sedimentation. KAY35 (TIF5-FL) (A, C) and KAY39 (hc TIF5-FL) (B, D) were grown in YPD at 30°C, treated with cycloheximide for 5 min, and then harvested for preparation of WCE, as described previously (Asano et al, 2001). Twenty five A260 units of the WCE were fractionated on 5–45% sucrose gradients by centrifugation at 39 000 r.p.m. for 2 h. Top panels: A254 profile, indicating the positions of ribosomal species. A quarter of the first five fractions was precipitated with ethanol and analyzed by SDS–PAGE and immunoblotting with antibodies indicated to the left (middle to bottom panels in (A) and (B)). The reminder of the fractions was incubated with anti-FLAG affinity resin (Sigma) for 30 min, followed by washing four times. A third of the immunoprecipitated fractions was analyzed similarly by immunoblotting (C and D, top to second to the last panels). The reminder was analyzed by Northern blotting for tRNAiMet (C and D, bottom panels). For accurate comparison, proteins in the gradient samples, either ethanol or immunoprecipitated, were analyzed on the same gel and with each antibody simultaneously. In, 1% of the in-put amount, except that 0.5% was used for Northern blotting (bottom gels).
In the experiment shown in Figure 2, increased eIF5 is evident in all fractions of the sucrose gradients tested (Figure 2B, panel 2). One possible explanation for this is that excess eIF5 is incorporated into both MFC and 40S-bound initiation complexes. Excess eIF5 bound to ribosomes is potentially deleterious to the efficiency of the initiation pathway. We therefore tested whether excess eIF5 would interact with eIF3 by precipitating eIF3i-HA from hc eIF5 cells using anti-HA antisera. Figure 3A and B shows that the amount of eIF5 associated with HA-eIF3 was increased 2.1 (±0.5)-fold (P=0.008, n=7) in hc eIF5 cells (Figure 3B). These results strongly suggest that a significant subpopulation of eIF5 present in MFC and/or the 43S complex is hyper-stoichiometric with respect to other MFC components in hc eIF5 cells.
Figure 3.
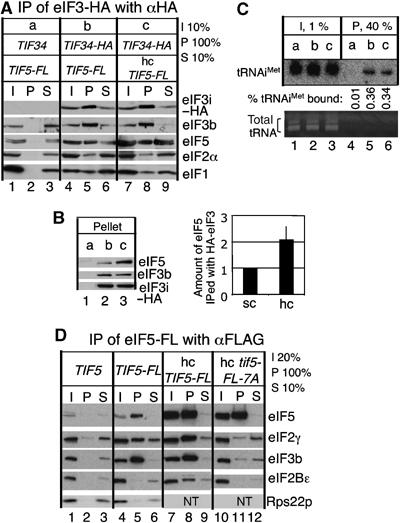
Analyses of different eIF complexes by co-immunoprecipitation. A 200 μg portion of WCE prepared from the following strains, grown in YPD, was subjected to HA-eIF3 or eIF5-FL immunoprecipitation with anti-HA (A, B) or anti-FLAG (D) antibodies, respectively, as described previously (Asano et al, 1999; Singh et al, 2004b). The entire pellet fractions (P) were analyzed by immunoblotting with antibodies indicated to the left of each panel. Percentage of input (I), pellet (P), and supernatant (S) fractions used to analyze the reaction is indicated besides the panel. (A–C) Extra copies of eIF5 are found in MFC formed in hc eIF5 cells. To preserve intact eIF association, we added formaldehyde to the culture before cell disruption, as described previously (Nielsen et al, 2004). Strains used are KAY35 (a), KAY50 (b), and KAY471 (c). In (B), the P fractions from a repeat of the experiment in (A) were analyzed side by side to show the increase in eIF5 binding to HA-eIF3. The graph to the right shows the relative amount of eIF5 found in the P fraction from KAY471 (hc WT) compared to that in KAY50 bearing sc TIF5 allele with s.d. in line. In (C), 1 mg of WCE was subjected to anti-HA precipitation and 40% of the P fraction was analyzed by Northern blotting with the probe specific to tRNAiMet, as in Figure 1A. Bottom gel: The EtBr staining pattern of total tRNAs in these fractions. The position of tRNA was determined by running side by side with purified total yeast tRNA (Sigma). (D) Formation of aberrant eIF2/eIF5/eIF2B complex in hc eIF5 cells. Strains used are KAY24 (lanes 1–3), KAY35 (lanes 4–6), KAY39 (lanes 7–9), and KAY40 (lanes 10–12). Data from top to third panels of lanes 1–9 are adapted from Asano et al (1999). NT, not tested.
hc eIF5 does not promote formation of MFC lacking tRNAiMet
Co-immunoprecipitation of eIF2(α) (Figure 3A, fourth panel, compare lanes 5 and 8) and tRNAiMet (Figure 3C) with eIF3i-HA confirmed that hc eIF5 did not alter TC recruitment into MFC, despite the observation that at least one extra copy of eIF5 was included in the majority of MFC formed (see above). Considering the large abundance of eIF2/eIF5 complex devoid of tRNAiMet in normal cells and its increase caused by hc eIF5 (Figure 1A–C), these results are not consistent with the idea that an eIF2/eIF5/eIF3 complex lacking Met-tRNAiMet can form even when eIF5 is overexpressed. Perhaps MFC lacking tRNAiMet is unstable. Instead, these findings are in accordance with our previous observation that tRNAiMet and eIF2 in the MFC are stoichiometric (Asano et al, 2000) and further suggest that tRNAiMet is a critical determinant for MFC formation. This observation raises the interesting possibility that tRNAiMet binding to MFC components in addition to eIF2 drives MFC formation (see Discussion).
hc eIF5 Gcd− phenotype is dependant upon the acidic eIF2β binding face of eIF5-CTD
To determine whether interaction of eIF5 with eIF2 is critical for the hc eIF5 Gcd− phenotype, we next performed a genetic analysis using eIF5-CTD ‘surface' mutations created in our recent study (Yamamoto et al, 2005). Based upon a homology modeled 3-D structure using the crystal structure of eIF2Bɛ C-terminal HEAT domain (Boesen et al, 2004), several clusters of surface residues were independently mutated and their effects on interaction with MFC-binding partners and eIF4G were analyzed (Yamamoto et al, 2005). The eIF5 alleles used and the interactions affected are summarized in Figure 4A and schematically in Figure 4B. W391Δ (deleting W391 onwards) and AN1 E396Δ (altering D354, E358, E359, and E360 to serine and deleting E396 onwards) both change the charged acidic region termed area I (Figure 4B). Both BN1 (altering K367, K370, K371, K375, K379, and R382 to glutamine) and BN2 (changing H336 and K337 to glutamine) affect the charged basic area termed area II (Figure 4B). Mutations altering area I interfere with eIF2 binding, whereas those impairing area II diminish eIF1 and eIF3 interactions.
Figure 4.
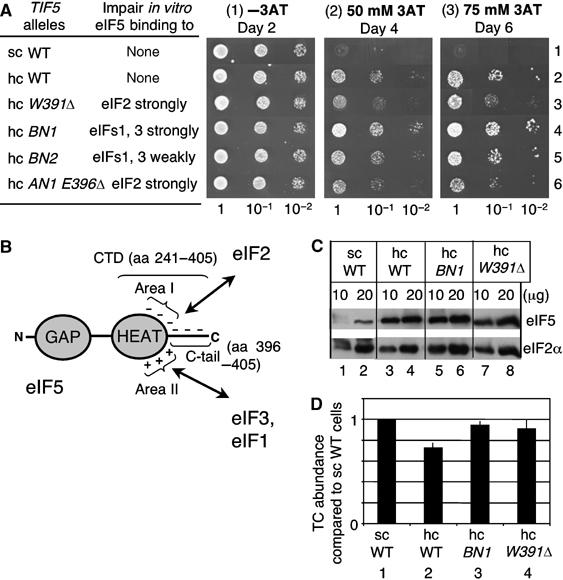
Effect of eIF5-CTD mutations on the Gcd− phenotype caused by hc eIF5. (A) Yeast growth assay on 3AT media. The gcn2 deletion strains KAY128 (sc WT) and KAY482 (hc WT) and its derivatives (Supplementary Tables S2) overexpressing different mutant forms of eIF5 were grown in the YPD medium and diluted to A600=0.15. Then, 5 μl of this and indicated dilutions were spotted onto SD medium containing uracil with (panels 2 and 3) or without (panel 1) 3AT (concentration shown) and incubated for the time indicated. Hc WT cells are 3AT resistant owing to general control derepression independent of Gcn2p (Gcd− phenotype) (panels 2 and 3, row 2). Column 1, TIF5 alleles expressed from sc or hc plasmid. Column 2 indicates the factor(s) whose in vitro interaction with eIF5-CTD was perturbed for each of eIF5 mutants, based on the data from Yamamoto et al (2005). (B) Structure and interactions of eIF5. Gray ovals, the NTD involved in GTPase activation (GAP) and the C-terminal HEAT domain (HEAT) as a core for MFC assembly. Thick lines with ‘N' and ‘C' at the ends, unstructured regions indicating amino and carboxyl termini, respectively. The positively and negatively charged areas II and I, respectively, of eIF5-CTD are indicated by + and − signs. Arrows denote interaction with designated partners at the corresponding surface. (C) Expression levels of eIF5-CTD mutants. Indicated amounts of WCE prepared from strains used in (A), grown in YPD, were subjected to immunoblotting with antibodies specific to eIF5 and eIF2α, as indicated. (D) Summary of TC levels in indicated strains (defined as in (A)). All of the strains tested carry FL-SUI3 allele as the sole source of eIF2β, for anti-FLAG immunoprecipitation of FL-eIF2. Presented are the average (thick bar) and s.d. (line) for relative TC levels compared to sc WT strain (KAY128) from three independent experiments for each strain.
To assess the effects of these surface mutations on the Gcd− phenotype conferred by hc eIF5, gcn2 cells containing each hc eIF5 mutant allele were grown on medium containing 3 aminotriazole (3AT). 3AT is an inhibitor of His3p, and Gcn2p-independent growth on this medium owing to Gcn4p-directed gene transcription is a hallmark of the Gcd− phenotype. As shown in Figure 4A, panels 2 and 3, each of the acidic site mutations W391Δ (row 3) and the AN1 E396Δ (row 6), but not the basic site mutations BN1 and BN2 (rows 4 and 5), significantly compromised the Gcd− phenotype caused by overexpression of eIF5. This shows that the eIF5 interaction with eIF2 at the acidic surfaces is responsible for the Gcd− phenotype. Immunoblot analyses in Figure 4C revealed that the effects of BN1 and W391Δ were not due to altered eIF5 expression levels. Importantly, quantitative immunoprecipitation as in Figure 1A indicated that W391Δ restored the TC level that had been reduced by hc wild-type eIF5 (P=0.05, n=3) (Figure 4D, column 4), confirming that eIF2/eIF5 interaction is responsible for GEF inhibition.
It is important to note that, despite almost a full restoration of eIF2B activity, hc eIF5 mutant W391Δ (Figure 4A, row 3) showed a residual 3AT resistance or Gcn2p-independent general control response, as compared to sc eIF5 control (row 1). As mentioned above, Gcd− phenotype can result from delay in TC binding to the ribosome, even though the TC level is normal. We therefore suggest that the residual activity is due to this mechanism, because the hc eIF5 mutant can still bind and thereby sequester other MFC partners, such as eIF1 and eIF3, inhibiting efficient MFC formation.
Like W391Δ, BN1 also restored TC level (P=0.04, n=3) (Figure 4D, column 3), consistent with the idea that in vivo, this mutation impairs eIF5 binding to eIF2 as well as to eIF3/eIF1 complex by disrupting mutually cooperative interactions (Yamamoto et al, 2005). Curiously, this mutation modestly enhanced the hc eIF5 Gcd− phenotype (Figure 4A, panels 2 and 3, row 4). It is conceivable that the binding of the eIF5-BN1/eIF2 TC complex to the 40S subunit is significantly delayed owing to its defective interaction with eIF3. Taken together with the residual general control response observed with hc eIF5-W391Δ (Figure 4A, row 3), this finding further supports the idea that inhibition of MFC formation contributes to the hc eIF5 Gcd− phenotype, in addition to a reduced TC abundance caused by the competitive GEF inhibition.
The eIF2Bɛ subunit is required for overcoming the GEF inhibition by the eIF2/eIF5 complex
As shown in Figure 1A and B, hc eIF5 decreases the TC abundance, indicating the GEF inhibition. To investigate the mechanism of this phenomenon, we examined the FL-eIF2 precipitated from sc and hc eIF5 cells using anti-eIF2Bɛ antibodies to measure the level of eIF2/eIF2B complex. Curiously, the amount of eIF2B associated with FL-eIF2β complex was not affected by hc eIF5 (data not shown). When eIF2Bɛ antisera were used to probe anti-FLAG immunoprecipitates from hc eIF5-FL extracts, we found that hc eIF5 also promoted formation of an unusual eIF5/eIF2B complex (Figure 3D, gel 4, lane 8). This complex was dependent upon eIF5 AA-box 2, as co-immunoprecipitation using hc eIF5-FL-7A (altering AA-box 2) cells eliminated this interaction (Figure 3D, compare lanes 8 and 11). Although the AA-boxes of eIF2Bɛ and eIF5-CTD interact with K-boxes of eIF2β, eIF2B is a large multisubunit complex and also interacts with eIF2α via its α/β/δ regulatory subcomplex (Krishnamoorthy et al, 2001) and with eIF2γ via the catalytic domain of eIF2Bɛ (Alone and Dever, 2006). Thus, our observations are consistent with the idea that eIF2 bridges eIF5 and eIF2B, forming an eIF2B/eIF2/eIF5 complex in hc eIF5 cells. This in turn indicates that the level of eIF2/eIF2B complex free from eIF5 was reduced by hc eIF5, consistent with the lowered TC abundance noted above (Figure 1B). We will discuss later the physiological relevance of the eIF2B/eIF2/eIF5 complex that was observed in hc eIF5 cells (see Discussion).
To further investigate the interplay between eIF5 abundance and eIF2B activity, we examined the effect of hc eIF5 on yeast mutants altering different eIF2B interfaces to eIF2. We first tested gcd6-7A altering the AA-box 2 of eIF2Bɛ. This mutation weakly derepresses general amino-acid control, thereby conferring 3AT resistance to gcn2Δ mutant yeast (Asano et al, 1999) (Figure 5A, panels 2 and 3, row 3). As expected, the hc eIF5 allele enhanced the Gcd− phenotype of this mutant (row 2 versus 4) without altering the level of overproduced eIF5, as examined by immunoblotting with anti-eIF5 antibodies (Figure 5B). Overexpression of eIF5 in the gcd6-7A strain also led to a slow-growth phenotype in the absence of amino-acid deprivation (panel 1, row 4), indicative of a partial inhibition of general protein synthesis. On amino-acid starvation, Gcn4p activates the transcription of HIS4 encoding a histidine synthesis enzyme (Hinnebusch, 1997). As shown in Figure 5C, β-galactosidase activity expressed from the HIS4-lacZ allele encoded in each strain confirms that the observed enhancement of 3AT resistance is due to corresponding increase in gene transcription under the general control program. A relatively high basal expression of HIS4-lacZ observed in GCD6 sc eIF5 cells (Figure 5C, row 1) is due to Gcn4p-independent transcription from an adjacent transcription start site. These results indicate that eIF5 produced in excess competes more effectively with eIF2B for eIF2, when gcd6-7A alters the AA-box of eIF2Bɛ.
Figure 5.
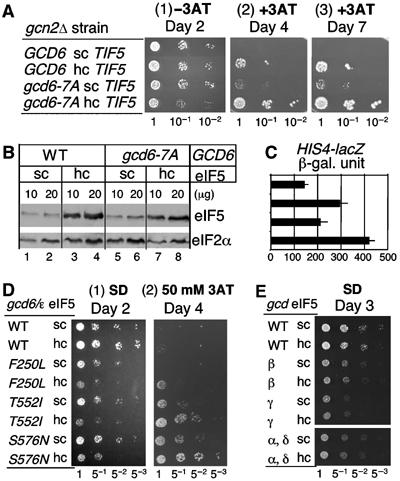
The effect of hc eIF5 on eIF2Bɛ mutations. (A) Transformants of KAY33 (gcn2Δ GCD6) and KAY34 (gcn2Δ gcd6-7A) carrying YEplac195 (rows 1 and 3) and YEpU-TIF5 (Supplementary Tables S2) (rows 2 and 4) were grown overnight in the SC-ura medium and diluted and spotted, as in Figure 5A, onto SD medium with (panels 2 and 3) or without (panel 1) 20 mM 3AT and incubated for times indicated on the top. (B) Expression levels of eIF5 in the strains used in (A) were measured and presented, as in Figure 4C, except that yeast was grown in SC-ura. (C) HIS4-lacZ expression. Yeast strains used in (A) were grown in the SC-ura medium and assayed for β-galactosidase as described previously (Hannig and Hinnebusch, 1988). The enzyme activities from strains at corresponding rows in (A) were presented with s.d. in line. (D) Transformants of GP3751 (WT, wild type) and its designated eIF2Bɛ mutant derivatives (listed to the left) carrying an empty vector (sc eIF5) or hc eIF5 plasmid were grown to A600=0.3 and 2 μl of five-fold serial dilutions spotted onto SD medium and incubated at 30°C for 2 days (panel 1). The same cells were spotted onto SD medium with 50 mM 3AT and incubated at 30°C for 4 days (panel 2). (E) Transformants of GP4214 (WT) and GP4203, F98, and H625 altering indicated eIF2B subunits (Supplementary Tables S2) were assayed exactly as in (D), panel 1, except that the spots at the top six rows were incubated at 37°C.
We then examined other eIF2Bɛ mutations. These alleles used in Figure 5D cause significant impairment of eIF2B GEF activity and impart a slow-growth phenotype and 3AT resistance (Gomez and Pavitt, 2000) that was enhanced here by hc eIF5. Interestingly, hc eIF5 did not alter the growth rate of slow-growing strains analyzed bearing mutations in eIF2Bβ (gcd2), γ (gcd1), and δ (gcd7) genes (Figure 5E). These results indicate that eIF2B binding to eIF2 in the presence of eIF2/eIF5 complexes requires the direct eIF2Bɛ/eIF2 interaction. The inability of hc eIF5 to further impair growth of other eIF2B mutant cells supports the idea that multiple interfaces between eIF2B and eIF2 facilitate their interaction and that the eIF2Bɛ/eIF2 interface for which eIF5 in excess can compete is critical for efficient eIF2B GEF activity.
Competition between eIF5 and the catalytic eIF2Bɛ segment
In light of the genetic interaction between eIF5 and eIF2B, we investigated whether competition between eIF5 and eIF2Bɛ might offer an explanation for the observation that overexpression of eIF2Bɛ, similar to hc eIF5, derepressed general amino-acid control (Richardson et al, 2004) (also see Figure 6A, row 3). The minimal catalytic domain of eIF2B (here called meIF2Bɛ) has been identified at the CTD of the ɛ subunit (aa 518–712) that retains eIF2-binding and GEF activities, but is not able to stably interact with the other eIF2B subunits (Gomez et al, 2002). As shown in Figure 6A, we found that the overexpression of both the FLAG-His-tagged and GST-fusion forms of meIF2Bɛ from the GAL promoter derepressed the general control response, thereby giving a gcn2Δ strain resistance to 3AT (rows 4 and 6). A truncated version (GST-meIF2BɛΔ containing aa 581–712) with a reduced affinity for eIF2 and inactive in GEF assays (Gomez et al, 2002) was expressed at the same level as GST-meIF2Bɛ (Figure 6B). However, the expression of this construct did not derepress the general control response (Gcd+), confirming that the phenotype is likely due to the interaction with eIF2 (Figure 6A, row 7). To provide further insight into the mechanism by which meIF2Bɛ derepresses general control, we first examined TC abundance by Northern blotting of FL-eIF2β immunoprecipitates from cultures of cells overexpressing GST-meIF2Bɛ or GST alone in galactose medium (SGal). We found no difference in the levels of tRNAiMet precipitated (Figure 6C), suggesting that the overexpressed polypeptide does not interfere with the GEF activity of the normal cellular five-subunit eIF2B in these cells. Instead, it is possible that the excess meIF2Bɛ competes with eIF5 for binding to TC. In support of this latter idea, we found that the Gcd− phenotype of GST-eIF2Bɛ was exacerbated by tif5-7A altering eIF5-CTD (Figure 6D, panel 2), with concomitant decrease in the growth rate (Figure 6D, panel 1). Thus, the minimal catalytic eIF2Bɛ-CTD segment can compete effectively with eIF5 and the eIF5-CTD mutant is more susceptible to growth inhibition from competition by the minimal eIF2Bɛ present in excess.
Figure 6.
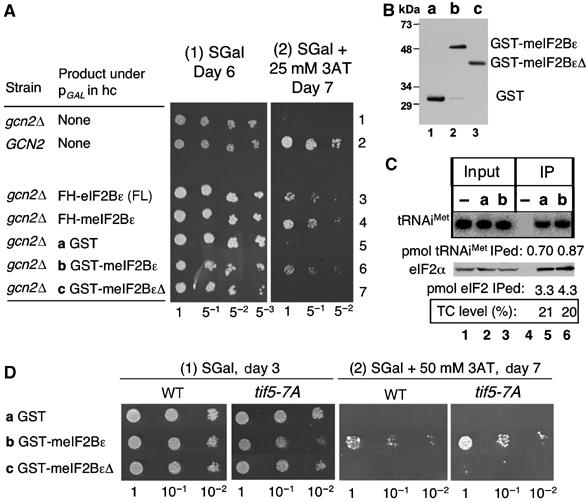
The mini-eIF2Bɛ derepresses the general control without inhibiting eIF2B activity. (A) Transformants of KAY16 carrying p(LEU2 GCD6) (row 1) and p(LEU2 GCN2) (row 2) and those of GP3889 carrying pAV1427 (row 3), pAV1689 (row 4), pEG(KT) (row 5), pAV1694 (row 6), and pAV1695 (row 7) encoding indicated products under the GAL promoter (see Supplementary Tables S1 and S2) were spotted onto synthetic galactose/raffinose (SGal) medium with (2) or without (1) 25 mM 3AT for indicated times. Spots are five-fold dilutions starting at A600=0.02. (B) A 10 μg portion of WCE prepared from GP3889 transformants carrying plasmids a–c as defined in the table in (A), which had been grown in SGal medium to A600∼1, was subjected to immunoblotting with αGST mouse monoclonal antibodies. (C) TC level (% in square) was determined for KAY128 (FL-SUI3) transformants carrying pEG(KT) (a) and pAV1694 (b). −, negative control using KAY113 (SUI3) transformant carrying a URA3 vector. (D) Transformants of KAY113 (wild type) and KAY282 (tif5-7A) (Singh et al, 2005) carrying pEG(KT) (a), pAV1694 (b), and pAV1695 (c) were spotted onto SGal medium with (2) or without (1) 50 mM 3AT for indicated times. Spots are 10-fold dilutions starting at A600=0.15.
Discussion
In depictions of the translation initiation pathway established in early 1990s, eIF5 is shown joining the 48S translation initiation complex late, just before stimulating hydrolysis of eIF2-bound GTP. Two key observations changed this view: (i) the fact that eIF5 plays a role in AUG codon selection, as shown by eIF5-NTD mutations relaxing the stringency of start codon selection (Sui− phenotype) in yeast (Huang et al, 1997); and (ii) the identification of a multifactor eIF5/3/1/TC complex (Asano et al, 2000). Subsequent work has established that eIF5-CTD makes critical contacts with eIF1, eIF2, eIF3, and eIF4G (Asano et al, 2001; Singh et al, 2004a, 2005; Yamamoto et al, 2005). A current translation initiation model therefore depicts an early association of TC with eIF5 to form MFC, as shown by solid lines in Figure 7. However, the fact that eIF5 and eIF2Bɛ bind the same site of eIF2 (eIF2β K-boxes) (Asano et al, 1999) and that eIF5 can interact with equal affinity to eIF2·GDP, eIF2·GTP, or TC in vitro (Algire et al, 2005) (CR Singh and K Asano, unpublished data) provides a potential conundrum for cells, as eIF5/eIF2·GDP complexes could be antagonistic to the function of the GEF eIF2B. The idea that eIF2B and eIF5 might compete for eIF2 in vivo was also stimulated by our recent findings that a large proportion of eIF2 and eIF5 were found together in a complex devoid of tRNAiMet. In the experiments described here, we used a combination of genetic and biochemical methods to address the function of this novel eIF2/eIF5 complex and further tested the idea that eIF2B and eIF5 compete for interaction with eIF2 in vivo.
Figure 7.
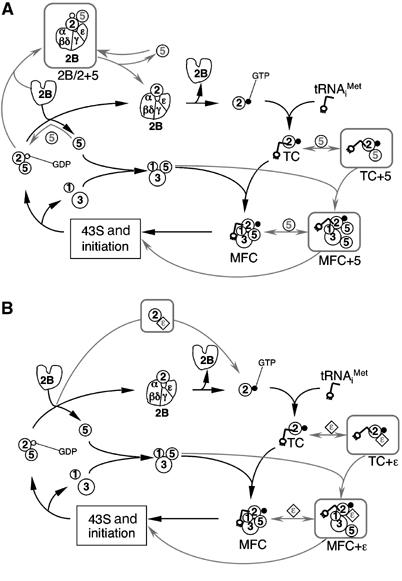
Impact of excess eIF5 or eIF2Bɛ on MFC and eIF2/eIF2B complex assembly. Numbers in circles represent eIFs. (A, B) A standard initiation cycle is presented focusing on eIF2–factor interactions. Factors are numbered in black and connected in a currently accepted sequence with black arrows, but with the addition of an eIF2·GDP/eIF5 complex that is shown here as forming upon eIF release from initiating ribosomes. (A) Additional competing complexes, identified in this study, form in the presence of excess eIF5 (gray circles labeled 5) and are shown in gray outlined square boxes with connecting gray arrows to indicate possible connection routes with the main pathway. However, TC+5 complex may transiently form as a natural MFC intermediate (Singh et al, 2004b). Likewise, it is possible that the eIF2B/eIF2/eIF5 complex is a transient intermediate before eIF5 displacement from the eIF2/eIF5 complex (see Discussion). (B) By analogy with hc eIF5, proposed complexes formed by excess eIF2Bɛ (gray diamonds labeled ɛ) are shown in gray outlined boxes connected with gray arrows to the main pathway (black factors and arrows).
The eIF2/eIF5 complex antagonizes eIF2B
Evidence indicating that the eIF2/eIF5 complex antagonizes the eIF2 TC recycling catalyzed by eIF2B is as follows. First, we found that the increase in the eIF2/eIF5 complex abundance by hc eIF5 led to a significant reduction in TC level (Figure 1A and B), which is restored by the eIF5 mutation W391Δ that specifically impairs the eIF5 interaction with eIF2 (Figure 4D). Second, the observed reduction in TC abundance was accompanied by corresponding changes in the level of Gcn4p-dependent general control response (Figure 4A), supporting physiological relevance for the biochemical result. Third, we provided genetic evidence that eIF2Bɛ-CTD, but not other eIF2B interfaces for eIF2, is required to overcome competitive inhibition by the eIF2/eIF5 complex (Figure 5).
Taken together, these observations are consistent with the idea that eIF5 binding to eIF2 can antagonize the guanine nucleotide exchange function of eIF2B. A recent study examining the release of initiation factors from 48S complexes during ribosomal subunit joining using in vitro reactions with purified components suggested that, following GTP hydrolysis, both eIF2 and eIF5 are released from the initiation complex before the release of eIF1 and eIF3 (Unbehaun et al, 2004). This would potentially provide an abundant source of eIF2·GDP/eIF5 complexes. Given the high abundance of a similar complex (Figure 1), we propose that the eIF2/eIF5 complex devoid of tRNAiMet serves as the cytoplasmic reservoir for eIF2, providing the means to coordinately regulate translation initiation (also see below).
As in our hc eIF5 experiments (Figures 1, 2, 3, 4, and 5), we found that the eIF2Bɛ catalytic segment expressed outside of eIF2B derepresses the general control response (Gcd− phenotype) (Figure 6A). The Gcd− phenotype here is likely due to the catalytic segment interfering with MFC formation, because the expression of the catalytic segment did not lower TC level (Figure 6C) and an eIF5-CTD mutation enhanced this phenotype (Figure 6D). These latter results suggest strongly that the initial steps in MFC and eIF2/eIF2B complex formation are mutually competitive (Figure 7A) and that the eIF2Bɛ segment disturbs MFC formation, possibly by competing directly with eIF5 for one or more eIF2 contacts within MFC or its intermediates including TC (Figure 7B).
Does the eIF2/eIF5 complex antagonize MFC formation?
If the eIF5/eIF2 complex inhibits the eIF2B activity by sequestering eIF2·GDP from eIF2B, the same complex might reduce the efficiency of MFC formation by sequestering eIF5 away from TC. However, this is probably not the case in vivo, because eIF5 is an abundant factor, stoichiometric to the level of eIF2 (Singh et al, manuscript in preparation). Accordingly, hc eIF5 did not reduce overall MFC level (Figure 1A and B), although it attenuated 43S complex formation by producing eIF5/TC complex free of MFC (Figures 1C and 2) and consequently aberrant MFC containing excess eIF5 (Figure 3A and B). Therefore, our ability to observe MFC attenuation was obscured by the fact that these cells contain presumably less productive MFC with at least an extra copy of eIF5. Because hc eIF5 substantially increased the level of eIF5/TC complex, an immediate MFC precursor (Figures 1C and 2), the production of hyperstoichiometric MFC at the expense of more functional, stoichiometric MFC by hc eIF5 is apparently not the direct consequence of increased competition from the eIF5/eIF2 complex, but rather due to the increased interaction of eIF3/eIF1 complex with excess eIF5 and the resulting upset of the canonical MFC assembly pathway (Figure 7A).
Our observation that the increase in the eIF2/eIF5 complex did not lead to direct inhibition of MFC formation suggests that the biological significance of this novel complex is to primarily regulate the guanine nucleotide exchange. Because eIF5 and eIF2 are stoichiometric to each other and 12 times more abundant than eIF2B in yeast (Singh et al, manuscript in preparation), it is conceivable that eIF2B has a strong ability to outcompete the stable eIF2/eIF5 complex, utilizing the eIF2 interface similar to eIF5-CTD, for which we provided evidence in Figure 5. Thus, it could be proposed that the conserved surface of eIF2Bɛ-CTD not only promotes substrate-docking function for eIF2 but also enables eIF2B to compete effectively with eIF5 for eIF2·GDP. In this context, it is possible that the eIF2B/eIF2/eIF5 complex that we observed in Figure 3D is a natural transient intermediate before eIF5 displacement promoted by eIF2Bɛ-CTD and that its abundance in hc eIF5 cells is due to preventing eIF5 dissociation by mass action (Figure 7A). We also hypothesize that the additional interaction of eIF2Bα/β/δ with eIF2α and/or eIF2Bɛ with eIF2γ might prevent competition with eIF5 for TC by discriminating eIF2·GDP against TC. This idea is consistent with the model that tRNAiMet is crucial for stable MFC assembly, as discussed next.
It is important to note that restricting access of eIF2B to eIF2·GDP by the eIF2/eIF5 complex devoid of tRNAiMet would further make the nucleotide exchange step rate-limiting for translation initiation in actively growing cells. As mentioned above, eIF2 phosphorylation increases its affinity for the eIF2Bα/β/δ interface, thereby changing it into a competitive inhibitor of eIF2B (Krishnamoorthy et al, 2001). Therefore, the downregulation of eIF2B activity by the eIF2/eIF5 complex might also provide the basis for coordinated regulation of the eIF2B GEF reaction in response to eIF2 phosphorylation.
tRNAiMet is a critical factor for MFC assembly
Here we also showed that MFC lacking tRNAiMet is not produced in hc eIF5 cells (Figures 1, 3A and C) as well as in normal cells (Asano et al, 2000). Thus, eIF2/eIF5 complexes do not stably associate with eIF3 or eIF1 in the absence of tRNAiMet binding to eIF2. This suggests either that tRNAiMet binding to eIF2 alters the latter's conformation so that it can now interact with eIF3 subunits eIF3a and eIF3c (Valásek et al, 2002), or that the eIF2-bound tRNAiMet itself directly interacts with another MFC partner to stabilize MFC. X-ray structural analyses of archaeal eIF2γ homologues suggest that eIF2 conformation undergoes only minimal changes when bound to GDP or GTP ligands (Schmitt et al, 2002; Roll-Mecak et al, 2004). The eIF3b and eIF3g subunits contain RNA recognition motifs that could be candidates for subunits with a direct tRNA-binding role. Taken together, we speculate that these findings make direct tRNAiMet recognition by eIF3 likely.
Is eIF5 a GDI?
Finally, the high level of the eIF2/eIF5 binary complex as the cytoplasmic reservoir for eIF2 is reminiscent of the finding that the cytoplasmic pools for the Rho and Rab GTPases exist in complexes with guanine nucleotide dissociation inhibitors (GDIs) (Collins, 2003; Der Mardirossian and Bokoch, 2005). For these GTPases, interaction with a GDI antagonizes the function of the GEF. A requirement for a GDI in eIF2 TC recycling would be consistent with the ‘ping-pong' mechanism for the eIF2B catalysis, postulating that a bound GDP is required for an efficient GEF catalysis (Hinnebusch, 2000). The potential interaction of the eIF5 GAP domain (NTD) (Das et al, 2001; Paulin et al, 2001) with eIF2γ before the GAP activation on AUG recognition might help eIF2 retain the guanine nucleotide. In addition, the similarity of eIF5-CTD with eIF2Bɛ-CTD (Boesen et al, 2004; Yamamoto et al, 2005; Wei et al, 2006) extends beyond the eIF2β interacting interface. The first two α-helices of eIF2Bɛ-CTD are involved in catalysis and the domain interacts with eIF2γ (Alone and Dever, 2006). By analogy, the first two helices of eIF5-CTD may also interact with a portion of eIF2γ when bound to GDP.
Conclusion
Based on all the data presented, we conclude that the normal eIF5 and eIF2Bɛ levels have likely evolved to coordinate the formation of their multiprotein complexes with the common substrate eIF2 and enable efficient regulation of the latter. Disruption of this balance by overexpression of eIF5 or eIF2Bɛ impedes the multiprotein complex formation by competition mechanisms. Resistance to eIF5 overexpression is apparently dependent on the ability of eIF2B to outcompete a novel eIF2/eIF5 complex. This complex in turn likely restricts eIF2B access to the GDP-bound substrate in normal cells, which might provide the basis for control of the GEF activity by eIF2 phosphorylation. Further work on the novel eIF2/eIF5 complex is obviously required to test these interesting ideas.
Materials and methods
Plasmid and yeast strains
Plasmids and yeast S. cerevisiae strains used in this study are listed in Supplementary Tables S1 and S2, respectively, with their constructions described in detail in Supplementary data. The standard genetic/molecular biology techniques were used throughout.
Quantitative immunoprecipitation
To measure the steady-state levels of eIF2- or eIF5-containing complexes, we grew yeast strains in YPD until the mid-exponential phase for WCE preparation. Details of immunoprecipitation experiments will be described elsewhere. P-values were calculated at http://calculators.stat.ucla.edu/twosamp/.
Supplementary Material
Supplementary Materials
Acknowledgments
We are indebted to Alan Hinnebusch for sharing results before publication, Ernie Hannig and Jim Anderson for purified eIF2 and tRNAiMet, Peter Reid for technical help, and Beth Montelone for comments on the manuscript. This work was supported by the NIH COBRE award P20 RR15563, the NCRR K-INBRE pilot grant P20 RR016475, matching support from the State of Kansas and the KSU, and NIH grant R01 GM64781 to KA and project grants from The Wellcome Trust to GDP.
References
- Algire MA, Maag D, Lorsch JR (2005) Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell 20: 1–12 [DOI] [PubMed] [Google Scholar]
- Alone PV, Dever TE (2006) Direct binding of translation initiation factor eIF2γ-G domain to its GTPase-activating and GDP–GTP exchange factors eIF5 and eIF2Beplsilon. J Biol Chem 281: 12636–12644 [DOI] [PubMed] [Google Scholar]
- Asano K, Clayton J, Shalev A, Hinnebusch AG (2000) A multifactor complex of eukaryotic initiation factors eIF1, eIF2, eIF3, eIF5, and initiator tRNAMet is an important translation initiation intermediate in vivo. Genes Dev 14: 2534–2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K, Krishnamoorthy T, Phan L, Pavitt GD, Hinnebusch AG (1999) Conserved bipartite motifs in yeast eIF5 and eIF2Bɛ, GTPase-activating and GDP–GTP exchange factors in translation initiation, mediate binding to their common substrate eIF2. EMBO J 18: 1673–1688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano K, Shalev A, Phan L, Nielsen K, Clayton J, Valasek L, Donahue TF, Hinnebusch AG (2001) Multiple roles for the carboxyl terminal domain of eIF5 in translation initiation complex assembly and GTPase activation. EMBO J 20: 2326–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesen T, Mohammad SS, Pavitt GD, Andersen GR (2004) Structure of the catalytic fragment of translation initiation factor 2B and identification of a critically important catalytic residue. J Biol Chem 279: 10584–10592 [DOI] [PubMed] [Google Scholar]
- Collins RN (2003) ‘Getting it on'—GDI displacement and small GTPase membrane recruitment. Mol Cell 12: 1064–1066 [DOI] [PubMed] [Google Scholar]
- Das S, Ghosh R, Maitra U (2001) Eukaryotic translation initiation factor 5 functions as a GTPase activating protein. J Biol Chem 276: 6720–6726 [DOI] [PubMed] [Google Scholar]
- Der Mardirossian C, Bokoch GM (2005) GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol 15: 356–363 [DOI] [PubMed] [Google Scholar]
- Dever TE (2002) Gene-specific regulation by general translation factors. Cell 108: 545–556 [DOI] [PubMed] [Google Scholar]
- Gomez E, Mohammad SS, Pavitt GP (2002) Characterization of the minimal catalytic domain within eIF2B: the guanine-nucleotide exchange factor for translation initiation. EMBO J 21: 5292–5301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E, Pavitt GD (2000) Identification of domains and residues within the ε subunit of eukaryotic translation initiation factor 2B (eIF2Bε) required for guanine nucleotide exchange reveals a novel activation function promoted by eIF2B complex formation. Mol Cell Biol 20: 3965–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannig EM, Hinnebusch AG (1988) Molecular analysis of GCN3, a translational activator of GCN4: evidence for posttranslational control of GCN3 regulatory function. Mol Cell Biol 8: 4808–4820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey JWB, Merrick WC (2000) Pathway and mechanism of initiation of protein synthesis. In Translational Control of Gene Expression, Sonenberg N, Hershey JWB, Mathews MB (eds) pp 33–88. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Hinnebusch AG (1997) Translational regulation of yeast GCN4: a window on factors that control initiator-tRNA binding to the ribosome. J Biol Chem 272: 21661–21664 [DOI] [PubMed] [Google Scholar]
- Hinnebusch AG (2000) Mechanism and regulation of initiator methionyl-tRNA binding to ribosomes. In Translational Control of Gene Expression, Sonenberg N, Hershey JWB, Mathews MB (eds) pp 185–243. Cold Spring Harbor Laboratory Press, NY: Cold Spring Harbor [Google Scholar]
- Huang H, Yoon H, Hannig EM, Donahue TF (1997) GTP hydrolysis controls stringent selection of the AUG start codon during translation initiation in Saccharomyces cerevisiae. Genes Dev 11: 2396–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamoorthy T, Pavitt GD, Zhang F, Dever TE, Hinnebusch AG (2001) Tight binding of the phosphorylated α subunit of initiation factor 2 (eIF2α) to the regulatory subunits of guanine nucleotide exchange factor eIF2B is required for inhibition of translation initiation. Mol Cell Biol 21: 5018–5030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen KH, Szamecz B, Valasek L, Jivotovskaya A, Shin BS, Hinnebusch AG (2004) Functions of eIF3 downstream of 48S assembly impact AUG recognition and GCN4 translational control. EMBO J 23: 1166–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulin FE, Campbell LE, O'Brien K, Loughlin J, Proud CG (2001) Eukaryotic translation initiation factor 5 (eIF5) acts as a classical GTPase-activator protein. Curr Biol 11: 55–59 [DOI] [PubMed] [Google Scholar]
- Pavitt GD, Ramaiah KVA, Kimball SR, Hinnebusch AG (1998) eIF2 independently binds two distinct eIF2B subcomplexes that catalyze and regulate guanine-nucleotide exchange. Genes Dev 12: 514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan L, Zhang X, Asano K, Anderson J, Vornlocher HP, Greenberg JR, Qin J, Hinnebusch AG (1998) Identification of a translation initiation factor 3 (eIF3) core complex, conserved in yeast and mammals, that interacts with eIF5. Mol Cell Biol 18: 4935–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JP, Mohammad SS, Pavitt GD (2004) Mutations causing childhood ataxia with central nervous system hypomyelination reduce eukaryotic initiation factor 2B complex formation and activity. Mol Cell Biol 24: 2352–2363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, Cao C, Alone TE, Dever TE, Burley SK (2004) X-ray structures of translation initiation factor eIF2γ: implications for tRNA and eIF2α binding. J Biol Chem 279: 10634–10642 [DOI] [PubMed] [Google Scholar]
- Schmitt E, Blanquet S, Mechulam Y (2002) The large subunit of initiation factor aIF2 is a close structural homologue of elongation factors. EMBO J 21: 1821–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CR, Curtis C, Yamamoto Y, Hall NS, Kruse DS, Hannig EM, Asano K (2005) eIF5 is critical for the integrity of the scanning preinitiation complex and accurate control of GCN4 translation. Mol Cell Biol 25: 5480–5491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh CR, Hui H, Ii M, Yamamoto Y, Asano K (2004a) Efficient incorporation of eIF1 into the multifactor complex is critical for formation of functional ribosomal preinitiation complexes in vivo. J Biol Chem 279: 31910–31920 [DOI] [PubMed] [Google Scholar]
- Singh CR, Yamamoto Y, Asano K (2004b) Physical association of eukaryotic initiation factor 5 (eIF5) carboxyl terminal domain with the lysine-rich eIF2β segment strongly enhances its binding to eIF3. J Biol Chem 279: 49644–49655 [DOI] [PubMed] [Google Scholar]
- Unbehaun A, Borukhov SI, Hellen CU, Pestova TV (2004) Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon–anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev 18: 3078–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valásek L, Nielsen KH, Hinnebusch AG (2002) Direct eIF2–eIF3 contact in the multifactor complex is important for translation initiation in vivo. EMBO J 21: 5886–5898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Xue Y, Xu H, Gong W (2006) Crystal structure of the C-terminal domain of S. cerevisiae eIF5. J Mol Biol 359: 1–9 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Singh CR, Marintchev A, Hall NS, Hannig EM, Wagner G, Asano K (2005) The eukaryotic initiation factor (eIF) 5 HEAT domain mediates multifactor assembly and scanning with distinct interfaces to eIF1, eIF2, eIF3 and eIF4G. Proc Natl Acad Sci USA 102: 16164–16169 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materials


