Abstract
Congenital amegakaryocytic thrombocytopenia (CAMT) is a rare disorder expressed in infancy and characterized by isolated thrombocytopenia and megakaryocytopenia with no physical anomalies. Our previous hematological analysis indicated similarities between human CAMT and murine c-mpl (thrombopoietin receptor) deficiency. Because the c-mpl gene was considered as one of the candidate genes for this disorder, we analyzed the genomic sequence of the c-mpl gene of a 10-year-old Japanese girl with CAMT. We detected two heterozygous point mutations: a C-to-T transition at the cDNA nucleotide position 556 (Q186X) in exon 4 and a single nucleotide deletion of thymine at position 1,499 (1,499 delT) in exon 10. Both mutations were predicted to result in a prematurely terminated c-Mpl protein, which, if translated, lacks all intracellular domains essential for signal transduction. Each of the mutations was segregated from the patient’s parents. Accordingly, the patient was a compound heterozygote for two mutations of the c-mpl gene, each derived from one of the parents. The present study suggests that at least a certain type of CAMT is caused by the c-mpl mutation, which disrupts the function of thrombopoietin receptor.
Congenital amegakaryocytic thrombocytopenia (CAMT) is a rare disorder regularly expressed in infancy and characterized by isolated thrombocytopenia and the absence of megakaryocytes in the bone marrow with no physical anomalies (1). This disorder has been classified as a form of constitutional marrow-failure syndrome, because aplastic bone marrow evolves with time (1, 2). Recently, thrombopoietin (TPO), the ligand for the c-Mpl receptor (3), has been identified (4–6). Our previous study of a patient with CAMT identified a defective response to TPO in megakaryocyte-colony formation, decreased numbers of erythroid and myelocytic progenitors in clonal cultures, a lack of c-mpl mRNA in bone-marrow mononuclear cells, and an elevated serum level of TPO (7). In animal studies, a deficiency of the c-mpl gene results in amegakaryocytic thrombocytopenia, decreased numbers of hematopoietic progenitors, and increased concentrations of circulating TPO with no physical or developmental abnormalities (8–11). Thus, considerable similarities between human CAMT and murine c-mpl deficiency prompted us to analyze the c-mpl gene in a patient with CAMT.
In this report, we describe the mutations in the c-mpl gene that caused CAMT. The findings confirm the importance of the TPO receptor for the development and maturation of the megakaryocytic lineage and early hematopoietic progenitor cells.
Case Report.
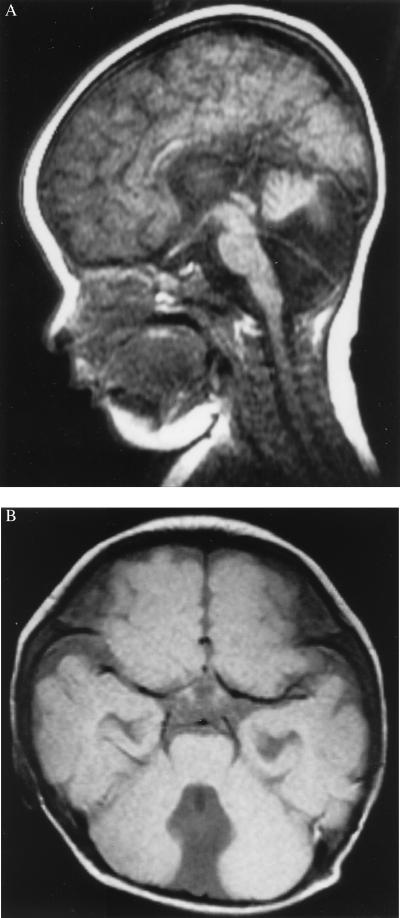
The 10-year-old patient with CAMT has been described (7). In brief, the patient was born to nonconsanguineous Japanese parents and was the first girl in the family. Her mother has a history of miscarriage: two miscarriages both before and after this patient’s birth. Her two siblings, a brother and a sister, have been healthy. She was born at full term with a birth weight of 2,905 g. On physical examination, she appeared to be healthy at birth. However, laboratory data showed an isolated thrombocytopenia in the peripheral blood and an absence of megakaryocytes in the bone marrow. The platelet count was 2,000 per μl; the white blood cell (WBC) count was 10,700 per μl with 64% neutrophils, 29% lymphocytes, and 6% monocytes; the Hb level was 10.8 g/dl. An MRI of the brain showed a hypoplastic cerebellar vermis with a communication between the fourth ventricle and the cisterna magna (Fig. 1). Despite this malformation, the child exhibited normal neurological development and normal physical and developmental growth. The karyotype of the lymphocytes was normal. In addition to thrombocytopenia, WBC and red blood cell counts have gradually decreased with age. At 5 years of age, the patient’s WBC count was 4,200 per μl; the Hb level was 9.1 g/dl; the platelet count was 8,000 per μl. At 7.5 years of age, the WBC count was 3,400 per μl; the Hb level was 8.2 g/dl; the platelet count was 6,000 per μl. At the age of 6, the serum TPO level was significantly higher than that in healthy controls (18.5 fmol/ml, whereas the normal range is 0.44 to 1.0 fmol/ml). c-mpl was not detected in the bone mononuclear cells by reverse transcription–PCR or by Northern blot analysis, performed as described (7).
Figure 1.
T1-weighted brain MRI of the patient at 10 months of age. The MRI showed a hypoplastic cerebellar vermis with a communication between the fourth ventricle and cisterna magna. (A) Sagittal section. (B) Axial section.
MATERIALS AND METHODS
Genomic Sequencing.
Genomic DNA was prepared from peripheral blood cells by using standard protocols. A whole c-mpl coding genomic region containing exons 1 to 6 and exons 11 to 12 was amplified by PCR in 1× buffer (10 mM Tris⋅HCl, pH 8.3/50 mM KCl/2.5 mM MgCl2) by using 100 ng of template DNA, 40 μM dNTP, 50 pmol of each primer (see below), and 1 unit of Takara LA Taq polymerase (Takara Shuzo, Otsu, Japan) in a total reaction volume of 50 μl. PCR was performed at 94°C for 5 min, followed by 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 3 min, with a final extension at 72°C for 10 min. Exons 7–8, 9, and 10 were amplified in 1× buffer (10 mM Tris⋅HCl, pH 9.0/50 mM KCl/2 mM MgCl2/0.1% Triton X-100) by using 100 ng of template genomic DNA, 20 μM dNTP, 50 pmol of each primer (see below), and 1 unit of Promega Taq polymerase (Promega) in a total reaction volume of 50 μl. PCR conditions were 94°C for 3 min, followed by 30 cycles at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, with a final extension for 7 min at 72°C with a Takara PCR machine (Takara). PCR products were purified on a QIAquick column (Qiagen, Tokyo, Japan). Direct sequencing of PCR products from the patient and carriers was performed by using a fluorescent dideoxy terminator method and analyzed with an ABI 310 DNA sequencer (Perkin—Elmer).
PCR primers were 1–6F (primer for exons 1 to 6 forward; 5′-CATGCGCAAAGTCACAGAAC-3′), 1–6R (primer for exons 1 to 6 reverse; 5′-TGGGGAATACATTGGAGGTG-3′), 7–8F (5′-CAGGCCATCGTTCTTGTAGGA-3′), 7–8R (5′-CTAACAGGCATGGCCAACC-3′), 9F (5′-TCTTTGTGGGAATCTCCGAC-3′), 9R (5′-AGGCGCTGTGCGGCTTTGGT-3′), 10F (5′-AGTAGGGGCTGGCTGGATGA-3′), 10R (5′-GAGATCTGGGGTCACAG-3′), 11–12F (5′-GTGTTTCAGGCAAGGTAGTCA-3′), and 11–12R (5′-CCCAATTTGAGTGTGTCATCT-3′). Internal primers for direct sequencing of exons 1 to 6 and 11 to 12 were 1–3F (5′-TGTATCTGACAGGAACCTGAG-3′), 1–3R (5′-GGCACAGGCAAGTCTGATTC-3′), 4F (5′-GGTACTCAGAGTTCTGATGTG-3′), 4R (5′-GAAGGTAGGAGATAAGAGGTG-3′), 5–6F (5′-CAGACCTAGATTGTGAAGCTG-3′), 5–6R (5′-GCTCACTCCCATGACACCAA-3′), 11F (5′-TATCTCCAAGCCTTACTCCC-3′), and 11R (5′-GTCTGGGGTGCTTGTGTTGT-3′).
Restriction-Enzyme Analysis.
The mutations in the family were assessed by PCR amplification followed by restriction-enzyme digestion. Exons 4 and 10 were amplified by PCR with primers 4F and 4R, and 10F and 10R, respectively. Amplified products were purified by using a QIAquick purification kit (Qiagen). PCR fragments of exons 4 and 10 were incubated at 37°C for 2 h with PvuII (Boehringer Mannheim) and Bfa I (New England Biolabs), respectively, in accordance with the suppliers’ instructions. The digests were analyzed by electrophoresis in 1× TBE (90 mM Tris/64.6 mM boric acid/2.5 mM EDTA, pH 8.3) on 4% agarose (GIBCO/BRL) in parallel with a 100-bp ladder marker (New England Biolabs) and visualized with ethidium bromide staining.
RESULTS
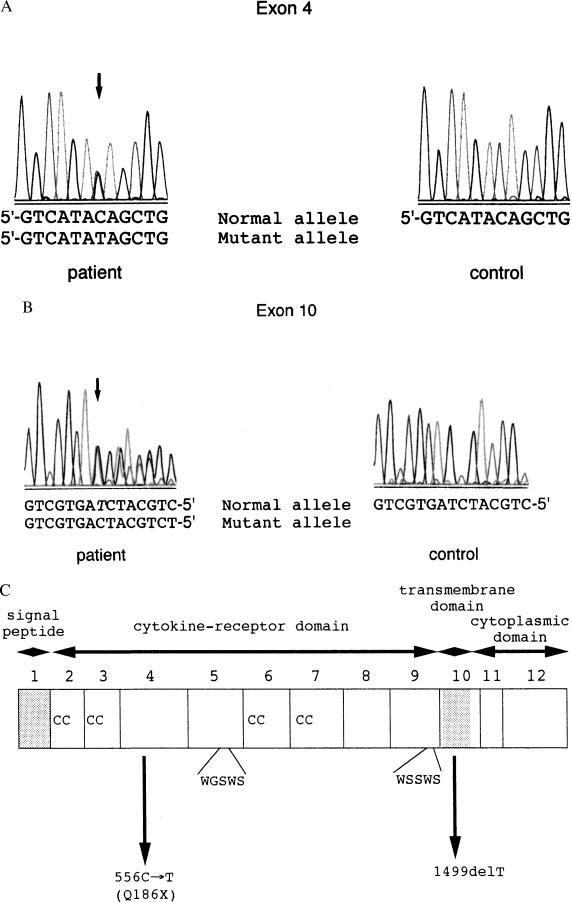
The coding sequence containing 12 exons of the c-mpl gene was analyzed by direct sequencing of PCR-amplified genomic DNA, and two heterozygous mutations were detected in exons 4 and 10. The mutation in exon 4 was a cytosine-to-thymine (C-to-T) transition at cDNA nucleotide position 556, resulting in the substitution of a glutamine (Q) by a stop codon (X) at amino acid position 186 (Q186X) (Fig. 2A). The single nucleotide deletion of thymine at position 1,499 (1,499 delT) in exon 10 creates a frameshift and leads to a premature stop codon (TGA) at nucleotides 1,537–1,539 (Fig. 2B).
Figure 2.
The two truncating c-mpl mutations in the CAMT patient. (A) Sequences of c-mpl exon 4 in the CAMT patient and a control. A heterozygous C-to-T transition at cDNA position 556 (arrow), which is the first nucleotide of codon 156, changes a glutamine residue for a premature stop codon in the patient. (B) Sequences of c-mpl exon 10 in the patient and a control. A heterozygous deletion of nucleotide thymine at position 1,499 in the patient (arrow) results in a frameshift, leading to a premature truncation. For A and B, the nucleotide numbers are based on the predicted cDNA sequence (GenBank accession no. M90102). (C) The schematic structure of human c-Mpl and two point mutations found in the CAMT patient.
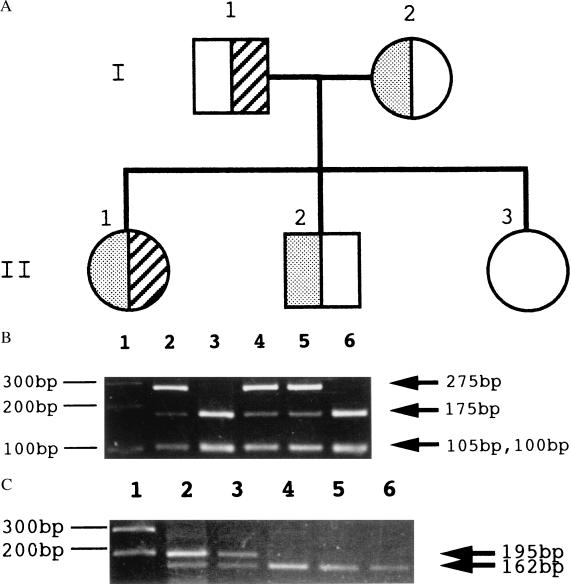
The segregation of the mutations was also analyzed in the patient’s family by the direct sequencing of exons 4 and 10 of the c-mpl gene (data not shown). The analysis indicated that the Q186X and 1,499 delT mutations were from maternal and paternal origins, respectively. Her brother was a heterozygous carrier of Q186X. Her sister was free from the mutations (Fig. 3A). These mutations in the patient and her family were confirmed by restriction-enzyme digestion. The Q186X mutation eliminates a restriction site for PvuII, and the 1,499 delT abolishes a restriction site for Bfa I (Fig. 3 B and C). The heterozygous carriers (father, mother, and brother) of either of the mutations were asymptomatic.
Figure 3.
c-mpl gene mutations in the family with CAMT. (A) A pedigree of the family. Circles represent females and squares males. Half-shaded (left side of the symbol) symbols represent carriers of the Q186X mutation, and half-hatched (right side of the symbol) symbols represent carriers of the 1,499 delT mutation. The proband is II-1. (B) Verification of the C-to-T mutation at cDNA position 556 by PCR amplification and restriction-enzyme analysis. PCR products of exon 4 were digested with PvuII and checked by agarose gel electrophoresis. The 380-bp PCR product of exon 4 is cleaved to 175-bp, 105-bp, and 100-bp fragments by PvuII. The Q186X mutation abolishes one of two PvuII sites, resulting in 275-bp and 105-bp fragments. The patient (lane 2), her mother (lane 4), and her brother (lane 5) possess this mutation in one allele, whereas her father (lane 3) and her sister (lane 6) do not have this mutation. Lane 1 shows the molecular size marker. (C) Restriction-enzyme analysis of the 1,499 delT. The 265-bp PCR product of exon 10 is cleaved to 162-bp, 70-bp, and 33-bp fragments by Bfa I. The 1,499 delT abolishes one of two Bfa I sites, resulting in 195-bp and 70-bp fragments (70-bp and 33-bp bands are not shown). Although the patient (lane 2) and her father (lane 3) have this mutation in one allele, her mother (lane 4), her brother (lane 5), and her sister (lane 6) do not have this mutation. Lane 1 shows the molecular size marker.
DISCUSSION
CAMT is a rare disorder expressed in infancy and characterized by isolated thrombocytopenia and megakaryocytopenia with no physical anomalies. Our previous hematological analysis showed similarities between human CAMT and murine c-mpl (TPO receptor) deficiency. Because the c-mpl gene was considered as one of the candidate genes for this disorder, we analyzed the genomic sequence of the c-mpl gene of a 10-year-old Japanese girl with CAMT, and two heterozygous point mutations were detected: Q186X in exon 4 and 1,499 delT in exon 10. The Q186X mutation of the c-mpl gene, if translated, results in a truncated protein with 71% C-terminal deletion, and the 1,499 delT causes 21% deletion with 13 aberrant amino acids. Because the truncated polypeptide by the mutation Q186X lacks one of two cytokine receptor domains as well as transmembrane and cytoplasmic domains, this mutational product would have no function as a TPO receptor (12). The abnormal protein by the mutation 1,499 delT would be defective as a receptor, because the mutation causes the interruption of the coding region within the transmembrane domain and lacks a cytoplasmic domain (Fig. 2C). An alternative splicing variant, named c-Mpl type S, lacks transmembrane domain and is speculated to be a secreted form of c-Mpl that might function as an inhibitor of TPO (13, 14). This soluble form may be expressed and released from the 1,499 delT allele and blocks TPO effects. Thus, both mutant alleles are likely to be null alleles for TPO signal transduction and to be responsible for CAMT in the patient.
The expression of the c-mpl gene was not detected by reverse transcription–PCR in the marrow mononuclear cells of this patient (7). It is known that mutant mRNAs carrying a premature stop codon have a reduced half-life in the cells because of a possible selective degradation pathway for such mRNAs (15). Therefore, it is possible that c-mpl mRNAs having premature stop codons in this case resulted in rapid degradation. An alternative explanation for the absence of c-mpl mRNAs might be a decrease in the numbers of megakaryocytes and hematopoietic progenitor cells that express the c-mpl gene product. Each of the mutations was segregated from her parents. The present study indicates that at least a certain type of CAMT in humans is caused by a c-mpl mutation that disrupts the function of the TPO receptor.
CAMT is a heterogeneous disorder in the genetic background, because there are several types of CAMT with or without a variety of congenital anomalies in addition to skeletal ones (2). CAMT is distinct from congenital amegakaryocytic thrombocytopenia with absent radii (TAR syndrome) in that, with TAR syndrome, a spontaneous increase in megakaryocytopoiesis usually is observed after 1 year of age, and skeletal anomalies and, at times, cardiac defects are also present (16). A defect of signal transduction has been suggested in TAR syndrome because of normal c-mpl expression and c-Mpl synthesis (17), but the genetic bases remain unresolved (18).
Abnormalities of the central nervous system, such as cerebral and cerebellar hypoplasia, were noted in two brothers from another family who also had CAMT (19). Cerebellar vermis hypoplasia in our patient was mild and also asymptomatic. As c-Mpl is expressed in cells of the megakaryocytic lineage progenitor, early hematopoietic progenitor, brain, and fetal liver (20), it is suggested that deficiency of the c-mpl gene may affect the development of the brain during the fetal period. Thus, these findings warrant further study of the brain in c-mpl-deficient mice, even if they are asymptomatic. Accordingly, the present study suggests that at least a certain type of CAMT associated with abnormalities of the central nervous system in the absence of physical anomalies is caused by c-mpl mutations that disrupt the function of the TPO receptor. Analysis of the TPO receptor gene c-mpl in more patients with CAMT will be useful for understanding the pathophysiology of CAMT, as well as providing the basis for its diagnosis, genetic counseling, and therapeutic judgement concerning application of recombinant TPO.
Acknowledgments
We extend special thanks to Yoko Katafuchi and Tamami Tanaka for technical assistance. We also thank Tazim Verjee for help with preparing the manuscript. This work was supported by grants from the Ministry of Health and Welfare of Japan and from the Ministry of Education, Science, Sports, and Culture of Japan and by the Public Health Service, National Institutes of Health, Institute on Aging Grant AG05628-14 (to R.A.G.).
ABBREVIATIONS
- CAMT
congenital amegakaryocytic thrombocytopenia
- nF
primer for exon n forward
- nR
primer for exon n reverse
- TPO
thrombopoietin
References
- 1.Freedman M H, Estov Z. Am J Pediatr Hematol Oncol. 1990;12:225–230. [PubMed] [Google Scholar]
- 2.Alter B P. In: Hematology of Infancy and Childhood. Nathan D G, Oski F A, editors. Philadelphia: Saunders; 1987. pp. 159–241. [Google Scholar]
- 3.Vigon I, Mornon J-P, Cocault L, Mitjavila M-T, Tambourin P, Gisselbrecht S, Souyri M. Proc Natl Acad Sci USA. 1992;89:5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartley T D, Bogenberger J, Hunt P, Li Y-S, Lu H S, Martin F, Chang M-S, Samal B, Nichol J L, Swift S, et al. Cell. 1994;77:1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 5.de Sauvage F J, Hass P E, Spencer S D, Malloy B E, Gurney A L, Spencer S A, Darbonne W C, Henzel W J, Wong S C, Kuang W J, et al. Nature (London) 1994;369:533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]
- 6.Lok S, Kaushansky K, Holly R D, Kuijper J L, Lofton-Day C E, Oort P J, Grant F J, Heipel M D, Burkhead S K, Kramer J M, et al. Nature (London) 1994;369:565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- 7.Muraoka K, Ishii E, Tsuji K, Yamamoto S, Yamaguchi H, Hara T, Koga H, Nakahata T, Miyazaki S. Br J Haematol. 1997;96:287–292. doi: 10.1046/j.1365-2141.1997.d01-2028.x. [DOI] [PubMed] [Google Scholar]
- 8.Gurney A L, Carver-Moore K, de Sauvage F J, Moore M W. Science. 1994;265:1445–1447. doi: 10.1126/science.8073287. [DOI] [PubMed] [Google Scholar]
- 9.Alexander W S, Roberts A W, Nicola N A, Li R, Metcalf D. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]
- 10.Carver-Moore K, Broxmeyer H E, Luoh S M, Cooper S, Peng J, Burstein S A, Moore M W, de Sauvage F J. Blood. 1996;88:803–808. [PubMed] [Google Scholar]
- 11.Kimura S, Roberts A W, Metcalf D, Alexander W S. Proc Natl Acad Sci USA. 1998;95:1195–1200. doi: 10.1073/pnas.95.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takatoku M, Kametaka M, Shimizu R, Miura Y, Komatsu N. J Biol Chem. 1997;272:7259–7263. doi: 10.1074/jbc.272.11.7259. [DOI] [PubMed] [Google Scholar]
- 13.Mignotte V, Vigon I, de Crèvecoeur E B, Roméo P-H, Lemarchandel V, Chrétien S. Genomics. 1994;20:5–12. doi: 10.1006/geno.1994.1120. [DOI] [PubMed] [Google Scholar]
- 14.Kaushansky K, Broudy V C, Lin N, Jorgensen M, McCarty J, Fox N, Zucker-Franklin D, Lofton-Day C. Proc Natl Acad Sci USA. 1995;92:3234–3238. doi: 10.1073/pnas.92.8.3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sachs A. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 16.Hedberg V A, Lipton J M. Am J Pediatr Hematol Oncol. 1988;10:51–64. doi: 10.1097/00043426-198821000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Ballmaier M, Schulze H, Strauss G, Cherkaoui K, Wittner N, Lynen S, Wolters S, Bogenberger J, Welte K. Blood. 1997;90:612–619. [PubMed] [Google Scholar]
- 18.Strippoli P, Savola A, Iolascon A, Tonelli R, Savino M, Giordano P, D’Avanzo M, Massolo F, Locatelli F, Bornga C, et al. Br J Haematol. 1998;103:311–314. doi: 10.1046/j.1365-2141.1998.00991.x. [DOI] [PubMed] [Google Scholar]
- 19.Hoyeraal H M, Lamvik J, Moe P J. Acta Paediatr Scand. 1970;59:185–191. doi: 10.1111/j.1651-2227.1970.tb08986.x. [DOI] [PubMed] [Google Scholar]
- 20.Columbyova L, Loda M, Scadden D T. Cancer Res. 1995;55:3509–3512. [PubMed] [Google Scholar]