Abstract
The continuing decline of ocean fisheries and rise of global fish consumption has driven aquaculture growth by 10% annually over the last decade. The association of fish farms with disease emergence in sympatric wild fish stocks remains one of the most controversial and unresolved threats aquaculture poses to coastal ecosystems and fisheries. We report a comprehensive analysis of the spread and impact of farm-origin parasites on the survival of wild fish populations. We mathematically coupled extensive data sets of native parasitic sea lice (Lepeophtheirus salmonis) transmission and pathogenicity on migratory wild juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon. Farm-origin lice induced 9–95% mortality in several sympatric wild juvenile pink and chum salmon populations. The epizootics arise through a mechanism that is new to our understanding of emerging infectious diseases: fish farms undermine a functional role of host migration in protecting juvenile hosts from parasites associated with adult hosts. Although the migratory life cycles of Pacific salmon naturally separate adults from juveniles, fish farms provide L. salmonis novel access to juvenile hosts, in this case raising infection rates for at least the first ≈2.5 months of the salmon's marine life (≈80 km of the migration route). Spatial segregation between juveniles and adults is common among temperate marine fishes, and as aquaculture continues its rapid growth, this disease mechanism may challenge the sustainability of coastal ecosystems and economies.
Keywords: aquaculture, emerging infectious disease, migration, salmon, sea lice
Ocean fisheries and ecosystems are in decline (1, 2). The collapse and low resilience of exploited fish stocks (3) and declines in the abundance of global fishery landings (4) illustrate the challenges facing the sustainability of ocean fisheries (4, 5). These effects are partly mitigated by the rise of aquaculture, in which the farming of herbivorous fishes promises to improve global fish supplies (6). However, the decline of ocean fisheries and ecosystems can also be exacerbated by the industrial farming of carnivorous fishes such as salmon (6–8). By feeding farm salmon proteins and oils extracted from wild fish, there is a net loss of fish supply (6). Escaped farm salmon spread domesticated genes into wild populations (9) and have the potential to invade and displace native wild stocks (10–12). Finally, the spread of infectious pathogens from farm to wild salmon may also threaten wild stocks (13). Despite decades of work, the extent and impacts of parasite transmission from farm to wild salmon have remained contentious and unresolved (14, 15).
Most emerging infectious diseases in wildlife arise through complex interactions among humans, wildlife, and domesticated animals (16). The spread of nonnative parasites from livestock to wildlife has reduced the abundance (16–18) and resilience (19) of some wildlife populations and has challenged the conservation of other threatened or endangered species (16, 17, 20). For many marine fishes, aquaculture can change the dynamics of normally benign native parasites by providing parasites novel access to juvenile hosts. For Pacific salmon, juveniles are not sympatric with adults (and their parasites) for their first several months of marine life (21). However, salmon farms can undermine this temporal refuge from lice early in the salmon's life cycle. During their first months at sea, wild salmon are sympatric with large abundances of domesticated salmon (and their parasites). This change in the timing and magnitude of parasite transmission in a host's life history may undermine a functional role of migration in protecting juvenile fish from parasites associated with adult fish.
The rise of salmon farming has coincided with the emergence of native sea lice (Lepeophtheirus salmonis) infestations of sympatric wild juvenile salmonids. Afflicted areas include Norway (22), Scotland (23), Ireland (24), and Canada (25). The infestations are concurrent with declines in affected populations, but the causal linkages are obfuscated by the myriad factors affecting wild fish populations, such as density dependence, climate, fishing, and habitat degradation. Here, we present extensive data sets and mathematical models that couple louse transmission and pathogenicity to estimate the impact of farms on the survival of wild juvenile pink (Oncorhynchus gorbuscha) and chum (Oncorhynchus keta) salmon migrating through an archipelago off the west coast of Canada. The analysis yields quantitative insights into the mechanisms and extent of impacts of aquaculture on disease dynamics in wild fish populations. More generally, the results inform the development of marine conservation and disease theory and its application in fisheries and aquaculture management.
Results
The transmission dynamics data set totaled 14,255 juvenile salmon nonlethally assayed for copepodid, chalimus, and motile stage lice at 1- to 3-km intervals along 40–80 km of three different migration routes containing two to three farms each (Fig. 1 and Fig. 4, which is published as supporting information on the PNAS web site). From among three candidate models, the data best supported a model that had a uniformly distributed ambient population of infectious planktonic larvae and point sources of planktonic larvae situated at the farms (likelihood ratio test, P ≪ 0.0001; Akaike weights ≈1; Tables 1–3, which are published as supporting information on the PNAS web site). Across all data sets, this model fit the data well (Fig. 2 and Figs. 5 and 6, which are published as supporting information on the PNAS web site). The other models contained only farm- or ambient-origin lice. With the parameter estimates from the best-fit model, we reconstructed the spatial distributions of infective larvae originating from each source. Farm salmon were the primary source of lice, raising the density of infective parasite larvae above ambient levels for >80 km of the migration route (Figs. 2, 5, and 6).
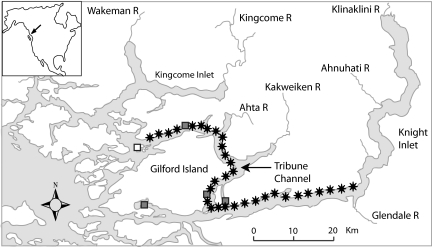
Fig. 1.
Study area and sample sites for one of the data sets (April 28 to May 8, Tribune Channel; Fig. 2). Approximately 50 pink and 50 chum salmon were collected at each sample site (stars) and nonlethally assayed for sea lice. The remaining Tribune Channel data sets had a similar structure. The three active salmon farms under study are identified by filled squares. An additional farm (white square near the western end of Tribune Channel) could have contributed lice but was excluded from the analysis because of its peripheral position relative to the sample sites. Fallow and smolt farms are not shown. Gilford Island is situated east of northern Vancouver Island, BC, Canada.
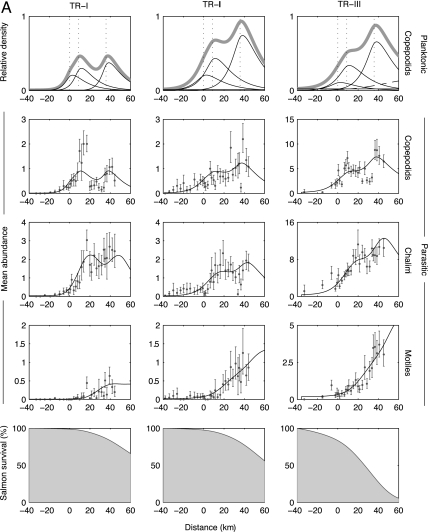
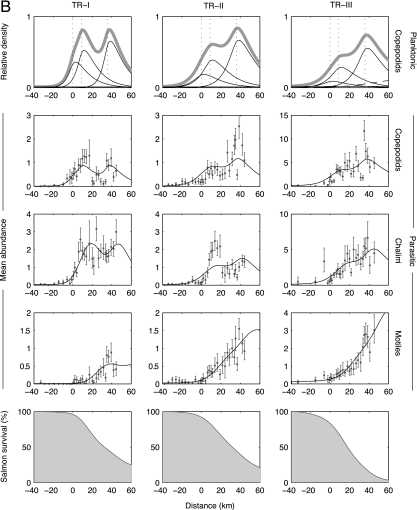
Fig. 2.
Sea lice transmission dynamics and survival of juvenile chum salmon (A) and pink salmon (B) migrating past three active salmon farms. The seaward migration of salmon is from left to right, and the farm locations are shown by vertical dotted lines in the first row. The data were collected along the Tribune Channel migration corridor in 2004 (see Fig. 1). The three columns correspond to three replicate sets of samples taken April 18–28 (TR-I), April 28 to May 8 (TR-II), and May 21–29 (TR-III), 2004 (note the change in scale). The first row shows the estimated spatial distributions of planktonic copepodids originating from all sources (thick gray line), from farm salmon (three thin curves), from ambient sources (horizontal thin line), and the second generation of farm-origin lice (dashed curve, TR-III only). Reproduction of lice parasitizing the juvenile salmon was not considered in TR-I and -II because of the absence of gravid female lice in those data sets. The middle three rows depict the mean abundances of lice (±95% bootstrap confidence interval) and maximum-likelihood model fits (black lines) along the migration route for the developmental progression through parasitic copepodid, chalimus, and motile stages. The bottom row depicts the estimated remaining juvenile salmon population that survived sea lice infestation. Temperature and salinity were measured at each site and averaged 9.0°C and 30.2‰ (TR-I), 10.4°C and 26.1‰ (TR-II), and 12.3°C and 22.2‰ (TR-III).
The data from the survival experiments totaled 3,687 juvenile salmon with initial infection intensities ranging from zero to five chalimus lice. The data best supported a survival model that contained a gamma-distributed random variable for the parasite's developmental stage at which there is a marked increase in pathogenicity (likelihood ratio test; pinks, P = 5 × 10−18 and chums, P = 5 × 10−35; Fig. 3; see also Fig. 7 and Table 4, which are published as supporting information on the PNAS web site). We mapped the survival model onto space via the average juvenile salmon migration speed (≈1 km·day−1; Table 3) and coupled it to the larval distributions and infection rates identified by the transmission dynamics model. By removing ambient lice from the best-fit model, we calculated the proportions of the juvenile salmon populations that survived parasitism from farm-origin lice. These were 5–26% for pink salmon and 10–70% for chum salmon in the Tribune Channel data sets, 49–78% for pink salmon and 69–91% for chum salmon in the Knight Inlet data sets, and 11–35% for pink salmon in the Kingcome Inlet data sets. Detailed results are available in Supporting Text, which is published as supporting information on the PNAS web site.
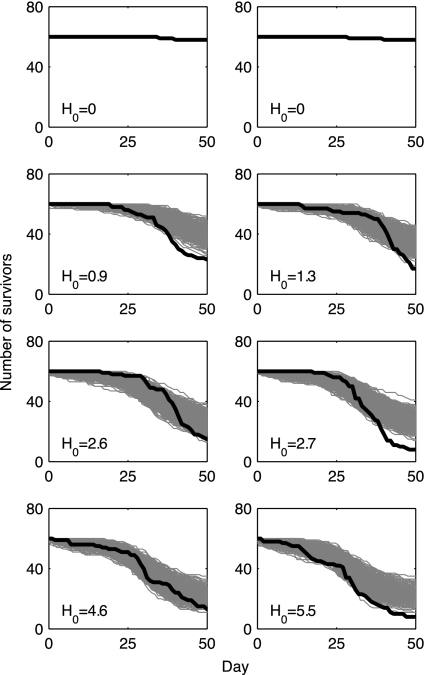
Fig. 3.
Survival of juvenile chum salmon over a range of sea lice abundances. Sixty juvenile chum salmon initially infested with H0 lice (all copepodids or chalimus I/II) were introduced into flow-through ocean enclosures and provisioned with salmon feed. Each image corresponds to an individual enclosure. The black line shows the trajectory for the daily number of survivors. The light-gray lines are the trajectories of 1,000 simulations of the best-fit model. The model was simulated as a Markov chain tracking the number of survivors in time. Each day, the number of mortalities was drawn from the number of survivors on the previous day using a binomial distribution with mortality probability calculated from the best-fit survival model. For all treatment replicates, the model has the same parameter values, except for H0, which is specific to each enclosure.
Discussion
The ecological mechanism underlying this emerging infectious disease is that farm fish undermine a functional role of host migration in protecting wild juvenile hosts from parasites associated with adult hosts. Under natural conditions, L. salmonis is common on adult Pacific salmon (26, 27) but rare on juvenile pink and chum salmon during their first months at sea (25). This is because juvenile salmon enter the sea without lice several months before the return of wild adult salmon (21). However, in areas containing salmon farms, wild juvenile salmon are sympatric with large abundances of domesticated salmon (and their parasites) during their early marine life. Farms provide parasites novel access to these juvenile hosts, resulting in measurable and sometimes severe impacts on salmon survival.
Although most of the lice observed in this study were farm-origin, there were also ambient-origin lice. This was measured in areas landward of the farms where lice abundances were low and spatially uniform. These data can be thought of as a control and conform well to the null models where lice abundances are spatially uniform in the absence of farms. Low abundances of lice have also been observed on juvenile pink and chum salmon during their first months at sea in areas distant from salmon farms (25). These lice represent the combined contributions from resident alternate hosts, Chinook salmon (Oncorhynchus tshawytscha), sea-run cutthroat trout (Oncorhynchus clarki), and Dolly Varden (Salvelinus malma). These species are orders of magnitude less abundant than both farm salmon and returning adult pink and chum salmon, which are the primary natural hosts for lice (26). The different abundances of these hosts mean that farm salmon provide lice a significant novel transmission route, which in this case operates for at least the first 2.5 months of the salmon's marine life (80 km of migration route).
Usually considered benign on adult salmon, L. salmonis was a severe pathogen of juvenile pink and chum salmon. Generally, an abundance of more than two motile lice was lethal, and survival of hosts with one or two motile lice was poor (survival of uninfected hosts was nearly 100%; see Fig. 8, which is published as supporting information on the PNAS web site). As the lice progressed through their life cycle, they also increased in pathogenicity, but the patterns differed between host species. For pink salmon, the onset of increased pathogenicity occurred abruptly with the emergence of preadult lice, but for chum salmon, it was more widely distributed around adult lice (Table 4). The high pathogenicity and abundance of lice resulted in a farm-induced cumulative epizootic mortality of wild juvenile salmon that ranged from 9% to 95%. These results were consistent across multiple data sets spanning temporal, spatial, and taxonomic replication. The estimated mortality of wild salmon is high but consistent with direct field observations of the epizootics, where schools of infested moribund juvenile salmon were abundant.
We did not consider the possibility that food limitation or predation risk would be more severe for infected hosts. Generally, poor nutrition is thought to reduce the resistance of fish hosts to disease (28, 29), and parasitized prey are known to be more vulnerable to predation (30, 31). These interactions would likely increase mortality estimates. Only one assumption, relatively low motile louse mortality over the timescale of the survival trials, could cause an overestimate of the per-capita impact of lice. However, empirical data suggest motile lice are long-lived (32), at least as long as their occurrence in the survival trials (16–36 days). It is unlikely that an alternate problem may have predisposed salmon to the epizootics; research programs by universities, conservation organizations, provincial and federal governments, and industry have not identified a prevalent viral or bacterial pathogen or other physical stressor.
The life cycles of most temperate marine fishes involve a period of spatial segregation between juveniles and adults. This segregation may protect juveniles from parasites associated with adults. For salmon, migration provides a temporal refuge from lice in early marine life. Farm salmon can eliminate this refuge, resulting in high epizootic mortality in wild salmon populations. These effects may not be limited to salmon but may extend to other species coming under culture around the world. Those that have migratory or highly dispersed life histories would be most at risk. As aquaculture continues its rapid growth into new regions, habitats, and species, this disease mechanism may challenge the sustainability of coastal ecosystems and economies.
Methods
To assess the impact of sea lice from salmon farms on the survival of wild juvenile salmon, we collected two types of data sets, one that tracks the infection dynamics of juvenile salmon migrating past salmon farms and another that tracks the survival of infested juvenile salmon collected from the field and reared in ocean enclosures. From the data sets, we used maximum-likelihood and model selection statistics to select and parameterize mechanistic models of sea lice transmission and juvenile salmon survival. These models were then coupled to estimate the mortality of wild salmon caused by farm-origin lice. Below we offer a brief overview of the methods. Further details appear in Supporting Text.
Transmission Dynamics.
For 2 years (2004–2005), we studied the infection dynamics of parasitic sea lice on juvenile pink and chum salmon as they migrated past active salmon farms, each containing ≈600,000 Atlantic salmon (Salmo salar). Three migration routes containing two, two, and three farms were surveyed for 40, 60, and 80 km, respectively. At 1- to 3-km intervals, we sampled ≈100 juvenile salmon as they approached and passed the salmon farms (Figs. 1 and 4). We used a nonlethal sea lice enumeration technique (33) to count the number of copepodid, chalimus, and motile lice on each fish, thus capturing the developmental progression of lice.
To analyze these data, we extended an established spatial model of the stage-structured dynamics of sea lice infecting juvenile salmon migrating past salmon farms (13). The model uses advection–diffusion–decay equations to describe the dispersion of planktonic larvae. For parasitic stages, the infection dynamics on migratory juvenile salmon are described by the delay differential equations:
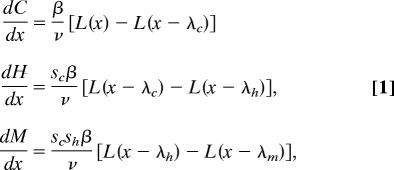 |
which track the mean abundances of copepodid (C), chalimus (H), and motile (M) lice, respectively. Salmon migrate at an average velocity, v, and encounter local densities of infectious planktonic copepodids (L), which then attach to host fish at rate β. The proportions of surviving copepodids and chalimi are sc and sh, respectively. The λ are the cumulative distances salmon travel during successive louse developmental stages (C, H, and M).
We constrained the model by imposing independently estimated parameters for the advection, development, and mortality of planktonic larvae. Further, pink and chum data sets shared four parameters (larval dispersion, louse demographic rates, and ratios of farm and ambient louse production rates) in a composite likelihood function that spanned the data sets of both host species. We modeled the occurrence of infection events and subsequent louse survival as a Poisson-binomial process (ref. 13; Fig. 9, which is published as supporting information on the PNAS web site) and used maximum-likelihood and model selection statistics to fit and compare models. The models consisted of only ambient-origin lice, only farm-origin lice, and both.
Mortality Estimation.
We analyzed survival data of infested and uninfested juvenile salmon collected from the same populations described in Transmission Dynamics. Details of the 2004 data can be found in ref. 34. The 2005 data were collected similarly. The time-series analysis of mortality events consisted of a likelihood-based comparison of survival models that described how lice change in pathogenicity as they mature. The best-fit survival model contained two parasitic stages, a relatively benign pathogen (young chalimus lice) and a severe pathogen (motile stages). The two stages induce mortality in their hosts at rates α1 and α2, respectively. The first stage is divided into a series of n identically and exponentially distributed substages, where n is an estimated constant from the gamma distribution (35).
The survival model was then coupled to the transmission dynamics model by using the chain rule to map time to space. The coupled model tracks changes in the abundance of the two pathogenic stages of lice (P1,i and P2) as juvenile salmon migrate to sea:
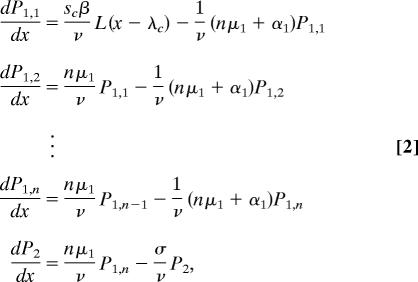 |
where 1/μ1 is the mean duration of the first pathogenic stage, which has variance (nμ1)−1. Having arrived at the second pathogenic stage, lice die at rate σ, which represents the sum of natural parasite mortality and parasite-induced host mortality rates (σ = μ2 + α2), which were not separately identifiable. However, σ could be estimated directly from the transmission dynamics data as σ = v · (λm−λh)−1. The proportion of juvenile salmon at location x surviving sea lice infestation was then determined by:
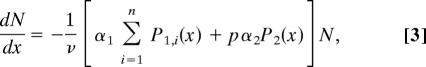 |
where N(x0) = 1, and x0 is the landward extreme of the study area. The quantity p is the proportion of P1 parasites that survive natural parasite mortality to reach the P2 stage. There are four parameters (α1, pα2, n, μ1) that were estimated from the survival data and two parameters (βsc · v−1, σ) estimated from the transmission dynamics data.
Supplementary Material
Acknowledgments
We thank E. Nelson, B. Connors, S. Rogers, and K. Assonitis, as well as the people of the Broughton Archipelago, for help in the field. R. Routledge (Simon Fraser University, Burnaby, BC, Canada) designed the 2004 survival studies. Comments from B. Anholt, M. Wonham, and two reviewers improved the manuscript. This work was supported by a Natural Sciences and Engineering Research Council Canada (NSERC) Canada Graduate Scholarship D and a D. Allen Birdsall Memorial Scholarship (to M.K.); a senior Canada Research Chair and NSERC Collaborative Research Opportunity grant (to M.A.L.); NSERC Discovery grants (to M.A.L. and J.P.V.); an NSERC/Social Sciences and Humanities Research Council of Canada Major Collaborative Research Initiative grant (to J.P.V.); a Tides Canada grant (to A.M.); and matching funds from the National Research Council of Canada Mathematics of Information Technology and Complex Systems program, the David Suzuki Foundation, the Canadian Sablefish Association, and the British Columbia Wilderness Tourism Association.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
See Commentary on page 15277.
References
- 1.Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, et al. Science. 2001;293:629–638. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 2.Pauly D, Watson R, Alder J. Philos Trans R Soc London B. 2005;360:5–12. doi: 10.1098/rstb.2004.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchings JA. Nature. 2000;406:882–885. doi: 10.1038/35022565. [DOI] [PubMed] [Google Scholar]
- 4.Pauly D, Christensen V, Guenette S, Pitcher TJ, Sumalila UR, Walters CJ, Watson R, Zeller D. Nature. 2002;418:689–695. doi: 10.1038/nature01017. [DOI] [PubMed] [Google Scholar]
- 5.Pauly D, Alder J, Bennett E, Christensen V, Tyedmers P, Watson R. Science. 2003;302:1359–1361. doi: 10.1126/science.1088667. [DOI] [PubMed] [Google Scholar]
- 6.Naylor RL, Goldburg RJ, Primavera JH, Kautsky N, Beveridge MCM, Clay J, Folke C, Lubchenco J, Mooney H, Troell M. Nature. 2000;405:1017–1024. doi: 10.1038/35016500. [DOI] [PubMed] [Google Scholar]
- 7.Naylor R, Burke M. Annu Rev Environ Res. 2005;30:185–218. [Google Scholar]
- 8.Naylor RL, Goldburg RJ, Mooney H, Beveridge M, Clay J, Folke C, Kautsky N, Lubchenco J, Primavera J, Williams M. Science. 1998;282:883–884. [Google Scholar]
- 9.Fleming IA, Hindar K, Mjolnerod IB, Jonsson B, Balstad T, Lamberg A. Proc R Soc London; 2000. pp. 1517–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naylor R, Hindar K, Fleming IA, Goldburg R, Williams S, Volpe J, Whoriskey F, Eagle J, Kelso D, Mangel M. Bioscience. 2005;55:427–437. [Google Scholar]
- 11.Volpe JP, Anholt BR, Glickman BW. Can J Fish Aquat Sci. 2001;58:197–207. [Google Scholar]
- 12.Volpe JP, Taylor EB, Rimmer DW, Glickman BW. Conserv Biol. 2000;14:899–933. [Google Scholar]
- 13.Krkošek M, Lewis MA, Volpe JP. Proc R Soc London; 2005. pp. 689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVicar AH. ICES J Mar Sci. 1997;54:1093–1103. [Google Scholar]
- 15.McVicar AH. Aq Res. 2004;35:751–758. [Google Scholar]
- 16.Daszak P, Cunningham AA, Hyatt AD. Science. 2000;287:443–449. doi: 10.1126/science.287.5452.443. [DOI] [PubMed] [Google Scholar]
- 17.Hudson PJ, Rizzoli A, Grenfell BT, Heesterbeek H, Dobson AP. The Ecology of Wildlife Diseases. Oxford: Oxford Univ Press; 2001. [Google Scholar]
- 18.Hochachka WM, Dhondt AA. Proc Natl Acad Sci USA. 2000;97:5303–5306. doi: 10.1073/pnas.080551197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jolles AE, Cooper DV, Levin SA. Ecology. 2005;86:2258–2264. [Google Scholar]
- 20.McCallum H, Dobson AP. Trends Ecol Evol. 1995;10:190–194. doi: 10.1016/s0169-5347(00)89050-3. [DOI] [PubMed] [Google Scholar]
- 21.Groot C, Margolis L. Pacific Salmon Life Histories. Vancouver: UBC Press; 1991. [Google Scholar]
- 22.Bjorn PA, Finstad B. ICES J Mar Sci. 2002;59:131–139. [Google Scholar]
- 23.Mackenzie K, Longshaw M, Begg GS, McVicar AH. ICES J Mar Sci. 1998;55:151–162. [Google Scholar]
- 24.Tully O, Gargan P, Poole WR, Whelan KF. Parasitology. 1999;119:41–51. doi: 10.1017/s003118209900445x. [DOI] [PubMed] [Google Scholar]
- 25.Morton A, Routledge R, Peet C, Ladwig A. Can J Fish Aquat Sci. 2004;61:147–157. [Google Scholar]
- 26.Nagasawa K. Hydrobiologia. 2001;453:411–416. [Google Scholar]
- 27.Beamish RJ, Neville CM, Sweeting RM, Ambers N. Fish Res. 2005;76:198–208. [Google Scholar]
- 28.Blazer VS. Annu Rev Fish Dis. 1992;2:309–323. [Google Scholar]
- 29.MacKinnon BM. ICES J Mar Sci. 1998;55:188–192. [Google Scholar]
- 30.Hudson PJ, Dobson AP, Newborn D. J Anim Ecol. 1992;61:681–692. [Google Scholar]
- 31.Mesa MG, Poe TP, Maule AG, Schreck CB. Can J Fish Aquat Sci. 1998;55:1599–1606. [Google Scholar]
- 32.Pike AW, Wadsworth SL. Adv Parasitol. 2000;44:233–337. doi: 10.1016/s0065-308x(08)60233-x. [DOI] [PubMed] [Google Scholar]
- 33.Krkošek M, Morton A, Volpe JP. Trans Am Fish Soc. 2005;134:711–716. [Google Scholar]
- 34.Morton A, Routledge R. Alask Fish Res Bull. 2005;11:146–152. [Google Scholar]
- 35.Lloyd AL. Proc R Soc London; 2001. pp. 985–993. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.