Abstract
Mammalian nervous system function involves billions of neurons which are interconnected in a multitude of neural circuits. Here we describe a genetic approach to chart neural circuits. By using an olfactory-specific promoter, we selectively expressed barley lectin in sensory neurons in the olfactory epithelium and vomeronasal organ of transgenic mice. The lectin was transported through the axons of those neurons to the olfactory bulb, transferred to the bulb neurons with which they synapse, and transported through the axons of bulb neurons to the olfactory cortex. The lectin also was retrogradely transported from the bulb to neuromodulatory brain areas. No evidence could be obtained for adverse effects of the lectin on odorant receptor gene expression, sensory axon targeting in the bulb, or the generation or transmission of signals by olfactory sensory neurons. Transneuronal transfer was detected prenatally in the odor-sensing pathway, but only postnatally in the pheromone-sensing pathway, suggesting that odors, but not pheromones, may be sensed in utero. Our studies demonstrate that a plant lectin can serve as a transneuronal tracer when its expression is genetically targeted to a subset of neurons. This technology can potentially be applied to a variety of vertebrate and invertebrate neural systems and may be particularly valuable for mapping connections formed by small subsets of neurons and for studying the development of connectivity as it occurs in utero.
Over the past several decades, studies using neuronal “tracers” have provided a wealth of information about the neural circuitry of the mammalian brain. After application or injection, tracers such as horseradish peroxidase (HRP) or wheat germ agglutinin (WGA) are transported through the axons and/or dendrites of neurons, where they can be visualized by histochemical techniques (1). “Transneuronal” tracers, such as WGA, can also be transferred from one neuron to another at or near synapses, thus revealing the locations of connected neurons as well (2, 3).
The usefulness of conventional tracing methods is limited, however. Because the tracers must be delivered to neurons by microinjection or local application, it is difficult or impossible to selectively label small populations of neurons of a given phenotype, or to label neurons in utero to study the embryonic development of connectivity. Furthermore, axons passing through the application region can be damaged and become labeled, confounding the interpretation of results.
In principle, these problems could be circumvented if the neurons of interest produced the tracer themselves. To test this possibility, we asked whether barley lectin (BL) (4), a close relative of WGA, could function as a transneuronal tracer if expressed in olfactory and vomeronasal sensory neurons in transgenic mice. The lectin was transported through the axons of the sensory neurons to the olfactory bulb, transferred to postsynaptic bulb neurons, and then transported through the axons of the bulb neurons to the olfactory cortex. The lectin also was transported retrogradely from the olfactory bulb to neuromodulatory brain areas that provide centrifugal input to the olfactory bulb. Thus a plant lectin can serve as a transneuronal tracer when expressed from a transgene in mice.
MATERIALS AND METHODS
PompBL Transgenic Mice.
A 0.6-kb BL cDNA (gift of N. Raikhel, Michigan State University) was PCR amplified with chimeric primers to create a BspHI site at the start codon and a KpnI site 3′ to a stop codon placed after Gly-197 in the BL protein (4), and then cloned into pBlueScript II (Stratagene) to give pLH8–2. A 0.49-kb HindIII/PstI fragment from pGOMP [11 kb of rat genomic DNA containing the olfactory marker protein (OMP) gene, provided by F. Margolis, University of Maryland (5)] was inserted into pUC 19 (Amersham Pharmacia) to create pLH9–1. The BspHI/KpnI BL cDNA fragment from pLH8–2 was inserted into the NcoI and KpnI sites of pLH9–1. The resulting 0.65-kb fragment was cloned into the AatII and KpnI sites of a modified pGOMP (pLH12–4) that lacked the vector KpnI site to give pPompBL. Transgenic CD-1 mice were generated (6) by using the 9-kb EcoRI insert from pPompBL and genotyped by hybridization of a BL cDNA probe to Southern-blotted tail DNAs.
Immunohistochemistry.
Dissected tissue from embryonic mice or from adult mice perfused with Bouin’s fixative were incubated in Bouin’s fixative, equilibrated with 30% sucrose, and frozen in OCT compound (Sakura Finetek, Torrance, CA). Sections were immunostained with goat anti-WGA (20 μg/ml) (Vector Laboratories), goat anti-OMP (diluted 1:450) (the gift of F. Margolis), or rabbit anti-tyrosine hydroxylase (1:1,000) (Eugentec, Brussels) by using the Elite ABC (peroxidase) system (Vector Laboratories) with minor modifications (secondary antibodies, 1:500 for 2 h; ABC, 1:400 for 1.5 h; 0.1% Triton X-100 in all solutions) and then counterstained with Hoechst 33428, thionin, or hematoxylin. Serial coronal or sagittal sections (16 μm) of nose or brain, analyzed at 200-μm intervals, were examined with anti-WGA and anti-OMP antibodies unless specified otherwise. Alternatively, sections were incubated with anti-synaptophysin (1:15) (Boehringer Mannheim) or anti-SV2 (1:20) (the gift of K. Buckley, Harvard Medical School) overnight at 4°C and then with Cy3 donkey anti-mouse IgG (1:400) (Jackson ImmunoResearch) for 1 h at room temperature. At least three transgenic and two control mice were examined at each age with anti-WGA and anti-OMP. Four L20, three L54, one L63, and four nontransgenic littermates were analyzed at 3–4 wk of age. L20 homozygous (and control) mice also were examined at 1- to 2-day intervals between embryonic day (E) 11 and postnatal day (P) 2, at P4 and P8 (P4 and P8: anterior half of brain only), and at 3 wk, and L54 mice were examined at P0 and P2. Antibodies against tyrosine hydroxylase were used on three L20 homozygous and two control mice at P4, P8, and at 3 wk. Antibodies against synaptic vesicle proteins were tested on homozygous L20 and control mice at 1- to 2-day intervals between embryonic day E12.5 and P2, at P8, and at 3 wk.
In Situ Hybridization.
In situ hybridization (7) was performed with 10-μm paraffin sections from three L20 heterozygotes, two L54 heterozygotes, and three CD-1 controls aged 3–4 wk and from three L20 homozygotes and two controls at embryonic day 17.5. 33P-labeled cRNA probes were generated from the BL-coding region of pLH8-2, a 0.9-kb BamHI fragment of the OMP gene (7), a 2.1-kb M50 odorant receptor cDNA, and a 1.1-kb M5 odorant receptor gene fragment. The M50 and M5 receptor probes were hybridized to nose sections from three L20 homozygotes and two controls between ages E17.5 and 2 months, and the M50 probe was hybridized to olfactory bulb sections from the same 2-month old animals. Brain sections were analyzed at intervals of 400 μm (adults) or 200 μm (E18 mice) by using exposure times of 4–5 days (OE) or 2–4 wk (brain).
Zinc Sulfate Treatment.
Zinc sulfate treatment was essentially as described by Harding et al. (8). Mice (L20 heterozygotes) were anesthetized with ether, and 0.1 ml of 0.17 M zinc sulfate or saline, was injected into the left nostril with a blunted 38 gauge needle. Treatment was begun at 3–4 wk of age and repeated every 10–12 days to minimize regeneration of olfactory neurons. Mice were analyzed with anti-WGA antibodies after they had received seven treatments (two mice with zinc sulfate and two with saline). In preliminary experiments (four mice with zinc sulfate and two with saline) with varied numbers of treatments, the reduction of BL staining in MOB neurons correlated with the number of treatments.
RESULTS
Transneuronal Transfer of BL in Transgenic Mice.
In initial experiments, we constructed a transgene, PompBL, in which the expression of a BL cDNA fragment (4) would be controlled by the promoter of the rat OMP gene (Fig. 1A). OMP, a protein of unknown function, is expressed almost exclusively by two types of olfactory neurons: olfactory sensory neurons in the nasal olfactory epithelion (OE), which detect odors, and sensory neurons in the vomeronasal organ (VNO), which appear to detect pheromones (9, 10). The portion of the OMP gene contained in PompBL was previously shown to drive the expression of another exogenous gene in OE neurons, whereas no expression of that gene was detected in the olfactory bulb or other parts of the brain (5).
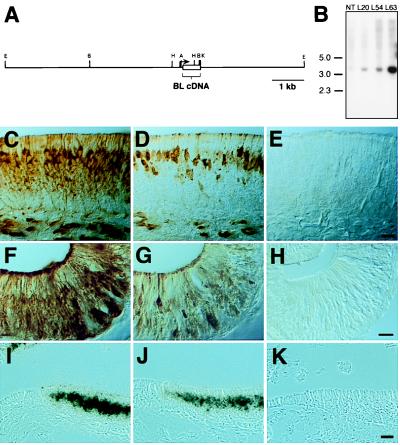
Figure 1.
A BL transgene is expressed in OE and VNO neurons in PompBL transgenic mice. (A) PompBL, in which the coding region of the rat OMP gene was replaced by a BL cDNA fragment (A, AatII; B, BamHI; E, EcoRI; H, HindIII; and K, KpnI). (B) Mice carrying the PompBL transgene were identified by hybridizing tail DNAs digested with BamHI to a BL cDNA probe (NT, nontransgenic; size markers in kb). BL immunoreactivity was seen in the OE (C) and VNO (F) of a neonatal L20 PompBL mouse, but not in the OE (E) or VNO (H) of a nontransgenic mouse. The BL staining patterns resembled OMP staining patterns in the OE (D) and VNO (G), except that immature neurons located in the middle of the OE were BL+, but OMP−. Radioactive BL (I) and OMP (J) cRNA probes both hybridized to OE neurons in a 3-week-old L20 mouse, but not to cells in the adjacent respiratory epithelium. No hybridization to the BL probe was seen in the OE of a nontransgenic mouse (K). [Bar = 17 μm (C–H) and 50 μm (I–K).]
WGA–HRP conjugates placed in the rat nose can be internalized by OE neurons and then transneuronally transferred to their synaptic partners in the olfactory bulb (11–14). WGA is endocytosed by a wide variety of neurons after binding to cell surface GLcNAc residues. BL, which is 98% identical to WGA in amino acid sequence, also recognizes GLcNAc residues (4). The BL protein encoded by PompBL lacked a C-terminal vacuolar targeting sequence which is cleaved in the plant, but retained an N-terminal signal peptide so that it might be secreted, and thus mimic the external application of WGA.
We obtained five transgenic mouse lines carrying the PompBL transgene. By using an anti-WGA antibody that recognizes BL to immunostain tissue sections, we detected BL+ neurons in the OE and VNO in three lines (lines L20, L54, and L63). Southern blots of genomic DNA indicated that the three lines carried 1–2, 2–4, and 8–10 copies of the transgene, respectively (Fig. 1B). All three lines showed the same patterns of olfactory system BL immunoreactivity in preliminary experiments. Sections through the noses and brains of L20 and L54 (or control) mice then were analyzed in greater detail by using antibodies against WGA or OMP. In situ hybridization with 33P-labeled BL or OMP cRNA probes also was performed to determine the locations of BL mRNA and thus identify the sites of transgene expression. No labeling was seen with either anti-WGA antibodies or the BL probe in nontransgenic mice.
The expression of BL in the OE and VNO of PompBL mice closely resembled that of OMP (Fig. 1 C–K). Intense BL staining was seen in the soma and dendrites of OE and VNO sensory neurons and in bundles of sensory axons underlying the sensory epithelia. Supporting cells and stem cells, the other two major cell types present in these epithelia, were unlabeled. The same expression pattern was seen by in situ hybridization with the BL cRNA probe. In neonatal mice, both immature and mature OE neurons were BL-immunoreactive, whereas only mature neurons were OMP+. This minor difference may be because of an earlier onset of expression of the transgene during neurogenesis or to a difference in the half-lives of the two proteins.
In the olfactory bulb, intense BL immunoreactivity was seen in both OE and VNO sensory axons, which synapse with the dendrites of bulb neurons in the glomeruli of the main olfactory bulb (MOB) and accessory olfactory bulb (AOB), respectively (15) (Fig. 2 A–F). The sensory axons also were OMP+, as expected. However, in sharp contrast to OMP, BL was seen in bulb neurons as well. All three types of neurons that synapse with OE axons in the MOB were BL+: periglomerular interneurons encircling the glomeruli, mitral cell output neurons in the deeper, mitral cell layer, and tufted cell output neurons in the external plexiform layer between the glomeruli and mitral cells. Granule cell interneurons, located beneath the mitral cell layer, were unlabeled, except for some faintly stained cells near the mitral cell layer. In the AOB, BL protein was similarly detected in mitral cells, which form a broader layer in the AOB than in the MOB. The pattern of BL labeling in the MOB resembled that previously seen after nasal application of WGA–HRP (11–14). The mechanisms underlying WGA transneuronal transfer have not been defined. The transfer of BL from OE and VNO neurons to bulb neurons may involve the secretion of BL from axon terminals or, alternatively, the sensory neurons may secrete the lectin at other locations, and then endocytose it before axonal transport to axon terminals.
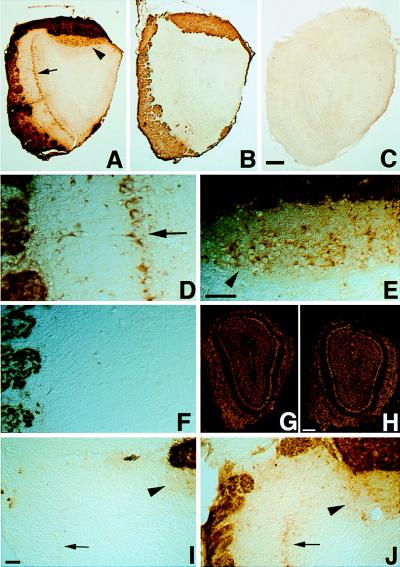
Figure 2.
BL is transneuronally transferred from sensory axons to olfactory bulb neurons. In adjacent coronal olfactory bulb sections from a L20 transgenic mouse, glomeruli containing OE or VNO axons were immunoreactive for BL (A, D, and E) and OMP (B and F), as shown at low (A and B) and high (D–F) magnification. In contrast, mitral cells underlying the MOB (arrow in A and D) and AOB (arrowhead in A and E) were BL+, but OMP− (B and F). No BL labeling was seen in the olfactory bulb of a nontransgenic mouse (C). No difference was seen in olfactory bulb sections from L20 transgenic (G) versus nontransgenic (H) mice that were hybridized to a radioactive BL probe. Nasal irrigation with zinc sulfate (I) to ablate OE neurons caused a drastic reduction in BL immunoreactivity in glomeruli and mitral cells in the MOB (arrow), but not in the AOB (arrowhead), whereas nasal irrigation with saline (J) did not. [Bar = 200 μm (A–C), 25 μm (D–F), 200 μm (G and H), and 100 μm (I and J).]
Our results suggested that BL protein produced by OE and VNO neurons was transported through their axons to the olfactory bulb and then transneuronally transferred to the bulb neurons with which they synapse. The presence of BL in some granule cells, which synapse only with mitral and tufted neurons, further suggested that BL was transneuronally transferred across two consecutive synapses. An alternative possibility was that the bulb neurons themselves expressed the transgene. However, in situ hybridization with a high-specific-activity 33P[BL] probe did not reveal any expression of BL mRNA in the bulb, even after prolonged exposure (Fig. 2 G and H). Furthermore, when we ablated OE neurons by nasal irrigation with zinc sulfate, BL staining in both the glomeruli and neurons of the MOB was drastically reduced, whereas this treatment, which does not damage VNO neurons [or alter the cellular composition of the bulb (8)], did not alter BL staining in the AOB (Fig. 2 I and J). These findings confirmed that the BL protein detected in bulb neurons was acquired by transneuronal transfer from OE neurons.
Transport of Sensory Neuron-Derived Tracer Beyond the Olfactory Bulb.
BL immunoreactivity also was detected in olfactory cortical areas that receive a projection from the MOB. MOB mitral and tufted cells send axons through the lateral olfactory tract to several distinct olfactory cortical areas, including the anterior olfactory nucleus, piriform cortex, and olfactory tubercle. Weak BL staining was seen in the lateral olfactory tract in perinatal animals, and, in adult animals, in the afferent nerve layer of olfactory cortical areas, where mitral and tufted cell axons terminate (Fig. 3 A–D). Some cells in these cortical regions also were weakly BL-immunoreactive, suggesting that BL protein produced in the nose was transneuronally transferred across two sequential synapses. Alternatively, because some cortical neurons send axons to the MOB, these cells might have retrogradely transported BL from the bulb. BL staining in the olfactory cortical areas disappeared after OE neurons were ablated with zinc sulfate (Fig. 3 C and D), confirming that the staining was caused by the transneuronal transfer of BL produced by OE neurons.
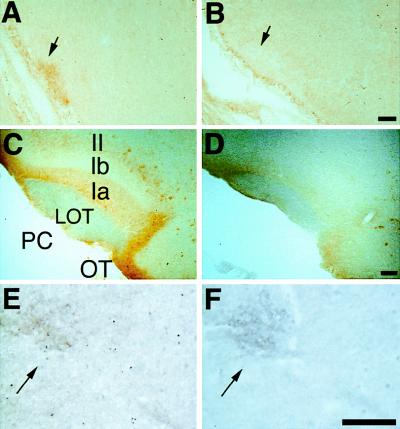
Figure 3.
Transport of BL beyond the olfactory bulb. BL immunoreactivity was detected in the LOT (arrow) in the piriform cortex of E17.5 transgenic (A), but not nontransgenic (B), mice. BL staining was seen in the afferent nerve layer (Ia) and in some cortical neurons in layer II in the piriform cortex (PC) and olfactory tubercle (OT) of adult L20 mice after nasal lavage with saline (C), but not after nasal lavage with zinc sulfate (D), which ablates OE neurons. BL staining in the LOT was weak or absent (as in C) in adult mice. At E17.5, BL labeling (E) was seen in the locus coeruleus (arrow), which was identified by tyrosine hydroxylase immunoreactivity (F) in an adjacent section. [Bar = 100 μm (A and B), 200 μm (C and D), and 50 μm (E and F).
BL immunoreactivity was also evident in the locus coeruleus (Fig. 3 E and F) and the horizontal limb of the diagonal band (data not shown), neuromodulatory brain regions that send axons to the glomerular layer of the bulb (12, 15). This suggested that BL released from sensory axons in the glomeruli also was retrogradely transported from the bulb to those distal areas. These results resembled those previously obtained with the nasal application of WGA–HRP (12, 13), which also was detected in both olfactory cortical and neuromodulatory areas.
In prenatal and perinatal animals, BL protein was detected only in the areas mentioned above, in the ME5 trigeminal nucleus (which sends sensory fibers to the nasal region), and in a small cluster of cells in the hypothalamus (data not shown). However, in older animals, BL immunoreactivity was seen in many brain areas, although others, such as the basal ganglia, neocortex, and thalamus, largely were spared (data not shown). In contrast, no expression of the transgene could be detected in the brain by using in situ hybridization (data not shown). Although the widespread BL staining may be caused by a low level of transgene expression that was not detectable by using in situ hybridization, a more likely explanation is that BL retrogradely transported to neuromodulatory brain regions was, in turn, anterogradely transported from those regions to the many other brain areas that they innervate. Spread of BL from the nose to multiple areas via other neural systems (trigeminal, terminal, and luteinizing hormone-releasing hormone) that innervate the nasal region (16) is yet another possibility.
Effect of the Transneuronal Tracer on Neural Organization and Function.
The above findings indicated that BL can serve as a transneuronal tracer when expressed in a subset of neurons in transgenic mice. One important consideration is whether the prolonged expression of high levels of the lectin has an adverse effect on neural organization or function. We found that an odorant-receptor gene probe hybridized similarly to scattered sensory neurons in the OE, and to a few glomeruli in the olfactory bulb, in PompBL and control mice (Fig. 4 A–D). Thus, BL did not appear to adversely affect either odorant receptor-gene expression in the OE or the targeting of OE axons to specific glomeruli in the bulb (7, 17–20). By immunostaining, tyrosine hydroxylase expression in MOB periglomerular cells, which requires signal transmission through OE sensory axons (21), was comparable in PompBL and control mice, further indicating that BL did not interfere with signal generation or transmission by OE neurons (Fig. 4 E and F). Moreover, neonatal animals could suckle, which requires olfactory function (22, 23), and adult animals appeared normal, except that L63 and homozygous L54 mice did not always mate. These results suggest that even high levels of BL expression may be relatively innocuous, at least in the olfactory system.
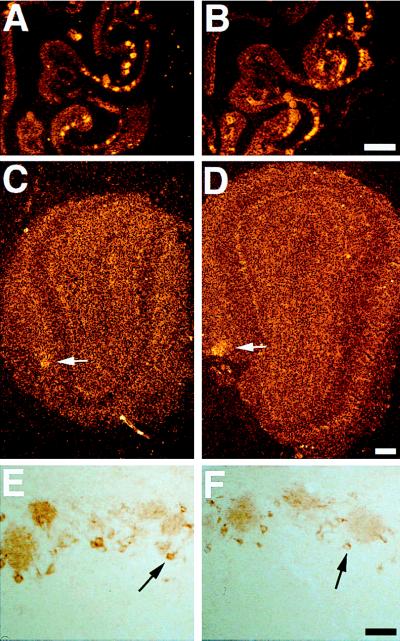
Figure 4.
Olfactory system organization and function in PompBL mice. A radioactive M50 odorant receptor gene probe hybridized similarly to neurons in the lateral zone of the OE of transgenic (A) and nontransgenic (B) mice, and also labeled the convergent axons of those neurons in specific glomeruli (arrow) in both transgenic (C) and nontransgenic (D) olfactory bulbs. Tyrosine hydroxylase immunoreactivity in periglomerular cells (arrow) surrounding glomeruli, and in their dendrites inside glomeruli, was also indistinguishable in P8 transgenic (E) and nontransgenic (F) olfactory bulbs. [Bar = 400 μm (A and B), 200 μm (C and D), 100 μm (E and F).]
The Onset of Transneuronal Transfer in Odor and Pheromone Pathways.
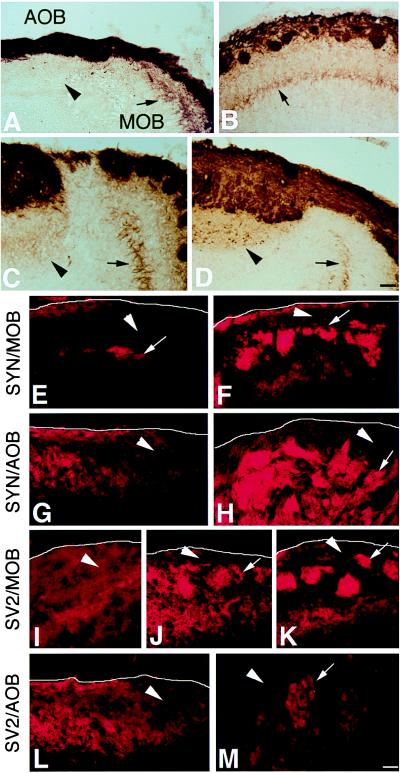
Scattered BL+ neurons were detected in both the OE and VNO at E11.5 (data not shown). However, transneuronal transfer of BL from OE axons to MOB neurons was detected earlier than was transfer from VNO axons to AOB neurons. At E17.5–E18.5, BL was detected in the dendrites of mitral cells in the AOB (data not shown), in their axons in the lateral olfactory tract (Fig. 3A), and in the locus coeruleus (Fig. 3E). By P4, MOB mitral cell soma also were intensely BL+ (Fig. 5A and B). In contrast, mitral cells in the AOB were unstained at P0 and did not show equivalent staining even at P8, although they did at 3 wk of age (Fig. 5 A–D). This suggested that synapse formation, or perhaps synaptic function, may initiate later in the AOB than in the MOB. Interestingly, immunoreactivity for two synaptic vesicle proteins, synaptophysin and SV2 (24, 25), in OE and VNO sensory axons also became localized to glomerular structures later in the AOB than in the MOB (Fig. 5 E–M). Given observations that synaptophysin becomes localized to presynaptic terminals as synapse formation occurs (26, 27), this result is consistent with the idea that connectivity is established later in the AOB than the MOB.
Figure 5.
Transneuronal transfer in the AOB versus MOB during development. At birth (A), BL immunoreactivity was seen in mitral cell dendrites in the MOB (arrow), but not in the AOB (arrowhead), of PompBL mice, even though BL+ sensory axons were present in both areas. By P4, BL+ mitral cell soma (arrow) were clearly visible in the MOB (B). At P8 (C), AOB mitral cells were only faintly BL+, but became strongly BL+ by 3 weeks of age (D) (as shown in Fig. 2) (arrows and arrowheads as in A). Immunostaining of OE and VNO sensory axons in the bulb with antibodies to synaptophysin (syn) or SV2 indicated that the two proteins become localized to glomerular structures later in the AOB than in the MOB: syn in the MOB at E17.5 (E) and P0 (F), and in the AOB at P0 (G) and P8 (H); SV2 in the MOB at E15.5 (I), P0 (J), and P2 (K), and in the AOB at P0 (L) and P8 (M). In E–M, arrowheads indicate incoming sensory axons, arrows indicate axons coalescing in protoglomeruli or glomeruli, and a white line indicates the outer border of the bulb (out of the field in M). [Bar = 50 μm (A–D) and 25 μm (E–M).]
DISCUSSION
The studies presented here demonstrate that BL can serve as a transneuronal tracer when expressed in a subset of neurons in transgenic mice. In mice carrying a BL transgene under the control of the OMP promoter, BL was selectively expressed in odor-sensing neurons in the OE and in pheromone-sensing neurons in the VNO. In the olfactory bulb, BL was transneuronally transferred from the axon terminals of OE and VNO neurons to postsynaptic bulb neurons and then anterogradely transported through the axons of the bulb neurons to the olfactory cortex. BL also appeared to be retrogradely transported to several neuromodulatory brain areas that send axons to olfactory bulb glomeruli, where the axons of OE and VNO neurons terminate. Although BL was expressed in the olfactory system as early as E11.5, no detrimental effect of the lectin on olfactory system organization or function could be discerned.
Our results with BL expressed in transgenic mice resembled those obtained with the nasal application of the transneuronal tracer WGA–HRP (11–14), except that in older transgenic mice BL protein spread to many brain areas. This was most likely because of widespread transport of BL from neuromodulatory areas that had retrogradely transported BL from the bulb. Undesired spread of tracer, as well as potentially adverse effects from the tracer, could presumably be avoided by exerting temporal control over BL transgene expression with one of the antibiotic- or hormone-inducible expression systems currently available (28, 29).
BL expression in transgenic mice allowed us to explore the development of connectivity in the olfactory system. We observed BL transfer from OE neurons to MOB neurons in utero, whereas similar transfer from VNO neurons to AOB neurons did not occur until after birth, even though MOB and AOB neurons and their cortical connections develop at a similar time (16). Although prenatal vomeronasal system function was suggested by 2-deoxyglucose uptake detected in the AOB in utero (30), our studies suggest that it may not be functional until after birth. Our findings are consistent, however, with previous studies that suggest that olfactory learning occurs in utero when odorants are injected into amniotic fluid (31, 32). Interestingly, we detected BL immunoreactivity in the locus coeruleus at E17.5, suggesting that noradrenergic inputs from this area to the MOB, which are required for olfactory learning postnatally (33), are also present prenatally.
The findings described here provide the first evidence for a transneuronal tracer whose expression is genetically targeted to specific neurons. We anticipate numerous potential applications of this approach to a variety of vertebrate and invertebrate neural systems. The expression of BL might be targeted to specific subsets of neurons by using transgenes containing cell-specific promoters, as described here, or by using gene replacement to coexpress BL with genes whose promoters are unknown. With these methods it should be possible to map a variety of neural circuits that could not be discerned by conventional tracing techniques, such as those involving small subsets of neurons, or scattered neurons of the same phenotype. It should also be feasible to study the development of connectivity as it occurs in utero.
Further advantages of the genetic tracing approach over conventional tracing techniques are the reproducibility of labeling between animals, the uniform labeling of cells of a given type, the ability to selectively label neurons of a given type even though they are surrounded by other types of neurons, and the absence of nonspecific labeling caused by damage to neuronal processes. Although we detected robust BL transfer to bulb neurons, the dilution of BL with transneuronal transfer and the strength of the OMP promoter suggests that this approach may not work in all contexts. The success of this approach in various systems will probably depend on a number of factors, including the amount of BL produced and the number (and perhaps activity) of synapses. One useful modification of the BL system would be to use an IRES sequence (34) to coexpress BL with a stationary marker (e.g., β-galactosidase), thus facilitating the discrimination of cells that produce the tracer from cells that have acquired it transneuronally. In the olfactory system, where the few neurons that express a given odorant receptor are scattered in the OE but converge on a few glomeruli in the MOB (7, 20, 21, 35), coexpression of BL with individual odorant receptor genes may reveal how olfactory sensory information is organized in the olfactory cortex.
Acknowledgments
We thank Jennifer Williams and Brian Wilburn for help with the generation and husbandry of transgenic mice, Dan Galin and Emmi Kurosawa-Pelletier for technical assistance, and Buck lab members for helpful discussions. This work was supported by the Howard Hughes Medical Institute (HHMI) and by grants from the National Institutes of Health. L.H. is an HHMI predoctoral fellow. J.-P.M. was a fellow of the Fyssen Foundation.
ABBREVIATIONS
- AOB
accessory olfactory bulb
- BL barley lectin
HRP, horseradish peroxidase
- MOB
main olfactory bulb
- OE
olfactory epithelium
- OMP
olfactory marker protein
- VNO
vomeronasal organ
- WGA
wheat germ agglutinin
- P
postnatal day
- E
embryonic day
References
- 1.Bolam J. Experimental Neuroanatomy: A Practical Approach. Oxford: Oxford Univ. Press; 1992. [Google Scholar]
- 2.Gerfen C R, O’Leary D D, Cowan W M. Exp Brain Res. 1982;48:443–448. doi: 10.1007/BF00238621. [DOI] [PubMed] [Google Scholar]
- 3.Fabian R, Coulter J. Brain Res. 1985;344:41–48. doi: 10.1016/0006-8993(85)91187-4. [DOI] [PubMed] [Google Scholar]
- 4.Raikhel N V, Lerner D R. Dev Genet. 1991;12:255–260. doi: 10.1002/dvg.1020120402. [DOI] [PubMed] [Google Scholar]
- 5.Danciger E, Mettling C, Vidal M, Morris R, Margolis F. Proc Natl Acad Sci. 1989;86:8565–8569. doi: 10.1073/pnas.86.21.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan F, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1986. [Google Scholar]
- 7.Ressler K J, Sullivan S L, Buck L B. Cell. 1994;79:1245–1255. doi: 10.1016/0092-8674(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 8.Harding J W, Getchell T V, Margolis F L. Brain Res. 1978;140:271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- 9.Monti-Graziadei G A, Margolis F L, Harding J W, Graziadei P P. J Histochem Cytochem. 1977;25:1311–1316. doi: 10.1177/25.12.336785. [DOI] [PubMed] [Google Scholar]
- 10.Baker H, Grillo M, Margolis F L. J Comp Neurol. 1989;285:246–261. doi: 10.1002/cne.902850207. [DOI] [PubMed] [Google Scholar]
- 11.Broadwell R D, Balin B J. J Comp Neurol. 1985;242:632–650. doi: 10.1002/cne.902420410. [DOI] [PubMed] [Google Scholar]
- 12.Shipley M T. Brain Res Bull. 1985;15:129–142. doi: 10.1016/0361-9230(85)90129-7. [DOI] [PubMed] [Google Scholar]
- 13.Baker H, Spencer R F. Exp Brain Res. 1986;63:461–473. doi: 10.1007/BF00237470. [DOI] [PubMed] [Google Scholar]
- 14.Itaya S K. Brain Res. 1987;409:205–221. doi: 10.1016/0006-8993(87)90703-7. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd G M, Greer C A. In: The Synaptic Organization of the Brain. 3rd Ed. Shepherd G M, editor. New York: Oxford Univ. Press; 1990. pp. 133–169. [Google Scholar]
- 16.Brunjes P C, Frazier L L. Brain Res. 1986;396:1–45. doi: 10.1016/s0006-8993(86)80188-3. [DOI] [PubMed] [Google Scholar]
- 17.Ressler K J, Sullivan S L, Buck L B. Cell. 1993;73:597–609. doi: 10.1016/0092-8674(93)90145-g. [DOI] [PubMed] [Google Scholar]
- 18.Vassar R, Ngai J, Axel R. Cell. 1993;74:309–318. doi: 10.1016/0092-8674(93)90422-m. [DOI] [PubMed] [Google Scholar]
- 19.Strotmann J, Wanner I, Helfrich T, Beck A, Breer H. Cell Tissue Res. 1994;278:11–20. doi: 10.1007/BF00305773. [DOI] [PubMed] [Google Scholar]
- 20.Vassar R, Chao S K, Sitcheran R, Nunez J M, Vosshall L B, Axel R. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 21.Baker H. Neuroscience. 1990;36:761–771. doi: 10.1016/0306-4522(90)90018-y. [DOI] [PubMed] [Google Scholar]
- 22.Singh P J, Tucker A M, Hofer M A. Physiol Behav. 1976;17:373–382. doi: 10.1016/0031-9384(76)90094-9. [DOI] [PubMed] [Google Scholar]
- 23.Teicher M H, Blass E M. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- 24.Jahn R, Schiebler W, Ouimet C, Greengard P. Proc Natl Acad Sci USA. 1985;82:4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiedenmann B, Franke W W. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 26.Bergmann M, Schuster T, Grabs D, Marqueze-Pouey B, Betz H, Traurig H, Mayerhofer A, Gratzl M. Dev Brain Res. 1993;74:235–244. doi: 10.1016/0165-3806(93)90009-y. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher T L, Cameron P, De Camilli P, Banker G. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baron U, Gossen M, Bujard H. Nucleic Acids Res. 1997;25:2723–2729. doi: 10.1093/nar/25.14.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brocard J, Feil R, Chambon P, Metzger D. Nucleic Acids Res. 1998;26:4086–4090. doi: 10.1093/nar/26.17.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pedersen P E, Stewart W B, Greer C A, Shepherd G M. Science. 1983;221:478–480. doi: 10.1126/science.6867725. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen P E, Blass E M. Dev Psychobiol. 1982;15:349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- 32.Smotherman W P. Physiol Behav. 1982;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- 33.Leon M. J Neurobiol. 1992;23:1557–1573. doi: 10.1002/neu.480231012. [DOI] [PubMed] [Google Scholar]
- 34.Mountford P S, Smith A G. Trends Genet. 1995;11:179–184. doi: 10.1016/S0168-9525(00)89040-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mombaerts P, Wang F, Dulac C, Chao S K, Nemes A, Mendelsohn M, Edmondson J, Axel R. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]