Abstract
It is generally accepted that Ca is essentially involved in regulated secretion, but the role of this cation, as well as others such as Na, is not well understood. An illustrative example occurs in neurohypophysial secretion, where an experimentally induced increase in the cytosolic concentration of Na+ can induce continuous neuropeptide release. In contrast, an increase in cytosolic Ca2+ will have only a transient stimulatory effect. The secretion-promoting targets for Ca2+ are not known; they may be cytosolic, as is usually assumed, but they may also be intravesicular, especially in view of evidence that Ca-rich secretory vesicles are preferentially secreted. In the present work, we have investigated the movements of these cations into and out of secretory vesicles during stimulus–secretion coupling. Isolated rat neurohypophysial nerve endings were stimulated by potassium (55 mM) depolarization, and at 6 min (peak secretion) and 20 min after the onset of stimulation, the elemental content of individual secretory vesicles was measured by quantitative x-ray microanalysis. A depolarization-induced transient increase in intravesicular Na+ concentration was found to coincide with the onset of secretion. Moreover, only a predicted small fraction of peripheral vesicles—presumably the docked ones—were Na+-loaded. The low sulfur concentration of Na+-rich vesicles most likely resulted from vesicle swelling. The results suggest that high intravesicular Na+ concentrations in docked vesicles, occurring by Na+/Ca2+ exchange or by transient fusion pore opening, is a proximal event in exocytosis.
Keywords: exocytosis, neuropeptide secretion, calcium, vesicle swelling, x-ray microanalysis
Ca2+ plays an essential role in several stimulatory, and perhaps also inhibitory, steps of regulated exocytosis but, despite much recent progress, its precise targets are still largely a matter of debate (1–3). In neurohypophysial secretion particularly, it is clear that Ca2+ cannot be the ultimate and sufficient trigger for neurosecretion, because the obligatory depolarization-induced increase in cytosolic Ca2+ concentration stimulates release for only a few minutes (4, 5), even when depolarization is maintained. This behavior contrasts with the known but mechanistically obscure secretagogue effect of Na+ (6–8), wherein an artificially induced increase in cytosolic Na+ concentration can induce a sustained and continuous secretion (8). In previous work on neurohypophysial nerve endings, one of our laboratories reported that the Ca content of the secretory vesicles increased upon secretory stimulation, and that Ca-rich vesicles accumulated when secretion was blocked (9), suggesting a role of intravesicular ion concentration in the secretory mechanism. This hypothesis is further supported by observation that in insulin-secreting cells, Ca2+ depletion from secretory granules inhibits exocytosis (10). Because the swelling of some granule matrices is inhibited by Ca2+ and promoted by Na+ (11, 12), we have investigated in isolated and stimulated rat neurohypophysial nerve endings possible changes in the concentration of these ions inside individual vesicles. The results point to a important role of intravesicular Na in regulated neuropeptide release.
MATERIALS AND METHODS
Preparation of Neurohypophysial Nerve Terminals.
Nerve terminals were prepared from male Sprague–Dawley or Wistar rats (200–300 g) as previously described (4). The animals were anesthetized by CO2 asphyxiation and killed by decapitation, and their pituitaries were dissected out. After removal of the anterior lobe and the pars intermedia, the neural lobe was homogenized at 37°C in 100 μl of solution containing (in mM): sucrose, 270; EGTA, 0.1; and Hepes, 10 (adjusted to pH 7.2 with Tris). The resulting terminals were then suspended in Locke’s saline (in mM: NaCl, 140; KCl, 5; Hepes, 20; glucose, 10; MgCl2, 1; CaCl2, 2.2; pH, 7.4) before centrifugation and, subsequently, rapidly frozen as controls or resuspended in a stimulating solution (in mM: NaCl, 40; KCl, 55; Hepes, 20; glucose, 10; MgCl2, 1; CaCl2, 2.2; N-methyl-d-glucamine, 50), for either 6 min or 20 min before freezing (13).
Cryosectioning and Quantitative X-Ray Microanalysis.
The pellets of isolated terminals, collected on wooden sticks, were quench frozen in liquid ethane, sectioned at ≈−160°C, freeze-dried, and examined by energy-dispersive electron-probe x-ray microanalysis. Cryosectioning was carried out as previously described (14). Sections, nominally 80 nm thick, were mounted on carbon/pioloform-coated grids and cryotransferred into an EM912 Omega electron microscope (LEO Electron Microscopy, Thornwood, NY) or into an HB501 field-emission scanning transmission electron microscope (STEM) (VG Instruments, Beverly, MA). In both instruments, sections were freeze-dried at ≈−100°C and recooled to <−160°C for imaging and analysis. Each energy-dispersive x-ray analysis was 100 s at ≈4-nA probe current rastered over an area well within single granules.
Data Processing.
Data were processed and quantified by established procedures (14–16) using the program DeskTop Spectrum Analyzer (DTSA) for Macintosh (17). Concentrations are given in units of mmol/kg dry weight because the Hall quantification procedure (18) provides such data directly. Concentrations in mmol per liter of original wet volume, which are more intuitively useful in physiology, can be estimated by multiplying dry-weight concentrations by the dry-mass fraction of analyzed organelles; a typical value for secretory granules is ≈50% dry mass (19–21).
Two complete independent experiments were analyzed. Analyses were carried only in presumptive endings (Fig. 1), i.e., profiles of small size (≤3 μm) containing less than 50 large dense-core vesicles; such profiles frequently contain mitochondria and, in the best cases, a cluster of microvesicles. In the larger profiles, called swellings (22), dense-core vesicles are more numerous, microvesicles absent, and mitochondria seldom observed. The Na, Cl, and K contents of mitochondria and/or a cytoplasmic area allowed us to ascertain that the endings were “alive,” i.e., membranes had resealed before resuspension in normal Locke’s saline, and remained intact thereafter (13). The elemental concentrations were processed using Statview software (Abacus Concepts, Berkeley, CA) on a Macintosh PowerPC.
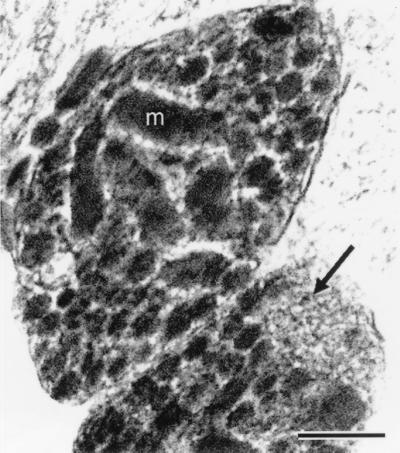
Figure 1.
Electron micrograph (low-dose, dark-field scanning transmission electron microscope (STEM) image) of a freeze-dried ultrathin (ca. 90 nm thick) cryosection prepared from a rapidly frozen pellet of isolated nerve terminals. m, mitochondrion. An arrow points to a cluster of microvesicles; large neurosecretory vesicles fill the rest of the cytoplasmic space. (Bar = 0.2 μm.)
RESULTS
Intravesicular Concentrations of Secretion-Relevant Diffusible Elements.
From the viewpoint of secretion, it is useful to subdivide neurosecretory vesicles into two groups according to their location: (i) “peripheral”, i.e., apposed to the inner side of the plasma membrane and, therefore, potentially secretion-competent; and (ii) “internal”, i.e., all others. The average elemental intravesicular concentrations of Na, S, Cl, and Ca, categorized according to time of depolarization and vesicle location, are reported in Table 1. In 6-min potassium-stimulated endings, the mean value of intravesicular total Ca concentration (Cav) was significantly higher than that of controls (Mann–Whitney, P = 0.025); this Cav increase was even more pronounced after a 20-min potassium challenge (P = 0.004) (Fig. 2). However, in no condition were the mean values and distributions of Cav significantly different in peripheral compared with internal vesicles (Mann–Whitney, control, P = 0.44; 6-min potassium-challenged endings, P = 0.06; 20-min potassium-challenged endings, P = 0.43).
Table 1.
Elemental concentrations in secretory vesicles of isolated rat neurohypophysical nerve endings
| Condition and location | Conc., mmol/kg dry weight
|
Na/S regression R2 (probability) | Na/Cl regression R2(probability) | ||||
|---|---|---|---|---|---|---|---|
| n | Na | S | Cl | Ca | |||
| Control | |||||||
| Peripheral vesicles | 64 | 60 ± 6 | 836 ± 22 | 50 ± 4 | 3.3 ± 0.8 | 0.0002 (P = 0.92) | 0.38 (P = 0.0001) |
| Internal vesicles | 94 | 38 ± 3 | 814 ± 20 | 50 ± 3 | 4.2 ± 0.9 | 0.0005 (P = 0.83) | 0.21 (P = 0.0001) |
| Stimulated 6 min | |||||||
| Peripheral vesicles | 84 | 99 ± 7** | 750 ± 18* | 57 ± 5 | 4.9 ± 0.7* | 0.11 (P = 0.001) | 0.05 (P = 0.04) |
| Internal vesicles | 66 | 50 ± 4* | 814 ± 19 | 46 ± 5 | 4.9 ± 1.1 | 0.008 (P = 0.47) | 0.03 (P = 0.12) |
| Stimulated 20 min | |||||||
| Peripheral vesicles | 67 | 71 ± 6* | 775 ± 18* | 74 ± 5** | 5.6 ± 1.0* | 0.004 (P = 0.61) | 0.09 (P = 0.01) |
| Internal vesicles | 101 | 33 ± 2 | 808 ± 18 | 62 ± 3** | 6.9 ± 1.1* | 0.006 (P = 0.44) | 0.006 (P = 0.42) |
Data are given as mean ± SEM. Column n is the number of vesicles analyzed; the number of endings analyzed ranged from 33 to 35 per experimental condition. Concentrations in mmol/kg dry weight can be converted to mmol/kg wet tissue by dividing by a factor of 2 (see Materials and Methods). Concentrations were also obtained for several other elements as follows (range in parentheses): Mg (36–45); P (126–149); K (98–137). The correlation, when significant, is negative for Na/S and positive for Na/Cl. The R2 probability was obtained by ANOVA. Significantly different from control values by Mann–Whitney test: ∗, P < 0.05; ∗∗, P < 0.001.
Figure 2.
Under conditons of sustained depolarization, vesicle Ca loading is continuous but vesicle Na loading is transient. Average values of Cav and Nav in individual secretory vesicles of rapidly frozen isolated nerve endings. A significant increase in vesicular Ca content is demonstrated for secretory vesicles of potassium-stimulated nerve endings at 6 min as well as at 20 min, but an increase in intravesicular Na is recorded only after 6-min challenge. Significantly different from control values by Mann–Whitney test: ∗, P < 0.05; ∗∗, P < 0.001.
The intravesicular Na concentration (Nav) also increased significantly after a 6-min challenge (P = 0) but, importantly, not after a 20-min one (P = 0.35) (Fig. 2). Another difference is that Nav was always significantly higher in vesicles adjacent to the plasma membrane of the endings—the peripheral, potentially “docked” ones—than in internal vesicles (Mann–Whitney, control, P = 0.001; 6-min potassium-challenged endings, P = 0; and 20-min potassium-challenged endings, P = 0). For peripheral vesicles at 6 min after potassium challenge—the time of peak secretion—the increase in Nav to almost 100 mmol/kg on average (P = 0) was especially striking (Table 1, Fig. 3); Nav was also elevated at 20 min (P = 0.04) but, in this case, significantly less (P = 0.01) than at 6 min. For internal vesicles, the potassium challenge increased Nav marginally at 6 min (P = 0.006), whereas there was no significant increase at 20 min (P = 0.16). The change in distribution was characterized by more frequent occurrence of relatively high Nav, i.e., more than 200 mmol/kg dry weight (equivalent to 200 mM free Na), which is 5-fold higher than average control values (Fig. 3).
Figure 3.
Vesicular Na loading occurs mainly in a subset of peripheral vesicles. Frequency distribution of total Na concentrations in individual secretory vesicles located at the periphery (A) and in the interior (B) after 6-min potassium depolarization. The higher Na content of stimulated samples (averaged in Fig. 2) is due to a subpopulation of peripheral vesicles.
No significant difference in intravesicular total chlorine concentration (Clv) was observed between the means of 6-min potassium-challenged endings and control endings (P = 0.26) (Table 1). Furthermore, the mean values and distributions of Clv were not significantly higher in internal vesicles compared with peripheral vesicles in all cases (Mann–Whitney, control, P = 0.37; 6-min potassium-challenged endings, P = 0.06; and 20-min potassium-challenged endings, P = 0.09).
Correlated Changes in Elemental Concentrations.
In peripheral vesicles of endings stimulated for 6 min, a significant negative correlation (P = 0.001) between Na and S was found; this is the experimental condition where S content is minimal and Na content is maximal (see regression coefficients, R2, Table 1). When looking for discrete and short-lived changes in individual secretory vesicles, averaging populations may not be the best strategy. Therefore, we have focused attention on vesicles with “extreme” contents, i.e., concentrations >2 SDs higher than the mean. The inverse correlation between Na and S is most evident on a graph representing two groups; one group consists of vesicles with the highest Na content and the other consists of vesicles with the highest S content (Fig. 4A). From a total sample of 476 vesicles, no vesicle belonged to both groups. All but two vesicles with the highest Na content—both from the control sample—were peripheral. Among the vesicles with the highest S content, six were peripheral (four of them were in the control sample).
Figure 4.
Depolarization induces swelling of the granule matrix, probably as a consequence of Na/Ca exchange. (A) Scatter plot of S concentration against Na concentration for that subpopulation of vesicles with S or Na contents higher than the mean + 2 SDs reveals a strong anticorrelation; this suggests Na-dependent swelling of the granule matrix. (B) Same type of plot for Ca vs. Na indicates a similar anticorrelation, suggesting Na/Ca exchange.
There was either no correlation or a weakly positive one between Nav and Cav within the subsamples listed in Table 1. However, if we select two groups of vesicles with Na and Ca concentrations higher than the mean + 2 SD, as in Fig. 4A, it again appears that almost no vesicles are common to the two groups (Fig. 4B). The vesicles which have the highest Ca content are relatively poor in Na, whereas the vesicles which have the highest Na content are relatively poor in Ca. Among the group of vesicles with the highest Ca content, 5 are peripheral (2 in the control group, 3 in the sample group stimulated for 20 min). A positive correlation between Nav and Clv was found in control samples, but this disappeared in stimulated endings. The highly variable Clv at peak secretion indicates that the observed increases in Nav are not coupled to Clv changes. For particularly Na-rich peripheral vesicles after 6 min of potassium stimulation, namely, those with Nav higher than the mean of control peripheral vesicles (60 mmol/kg dry weight), there was no correlation with Clv (R2 = 0.03, P = 0.19). These observations provide good evidence that the recorded increases in Nav are not contaminated by measurement of extracellular medium.
DISCUSSION
Intravesicular Ca.
The present data as well as those previously published demonstrate that in neurohypophysial nerve terminals, high cytosolic Ca, which is a known consequence of potassium depolarization, is probably at the origin of high Cav (9, 13, 23). We have shown previously that if the potassium challenge was discontinued after 6 min, Cav returned to control levels (9). If secretion was blocked (by hyperosmotic saline), Cav decrease after the end of depolarization was not observed, suggesting that in isosmotic conditions the Ca-rich vesicles had been preferentially secreted (9). In the present work, we add the observation that after 20 min of continuous potassium challenge, the increase in Cav is even larger than at 6 min. Under such conditions of potassium depolarization, it has been shown by imaging of individual neurohypophysial nerve terminals that free cytosolic Ca concentration ([Ca2+]i), after the initial rise, stabilizes at a plateau around 400–500 nM (24). However, even with continuous depolarization and high [Ca2+]i, neurohypophysial terminals will only briefly release their secretory products (4, 5). Therefore, both high [Ca2+]i and high Cav may be necessary for secretion, but they alone are not sufficient.
Intravesicular Na.
In contrast to Ca, elevations of Na, both cytosolic and intravesicular, correlate with secretion. In neurosecretory nerve endings, as perhaps in other secreting cells, it is known that a sustained, artificially induced increase in cytosolic Na+ leads to continuous secretion (8). Under more physiological conditions of potassium challenge of sealed endings, however, secretion is transient. Here, we have found a strikingly similar and parallel increase in the Nav of endings frozen after a 6-min challenge—i.e., at the peak of secretion—but not after a 20-min challenge. In addition, this Nav increase was only observed in peripheral vesicles adjacent to the plasma membrane (Fig. 3). Peripheral vesicles are obviously more likely to be secreted than internal vesicles. Furthermore, among the five or six peripheral vesicles analyzed in a given ending, there were at least one to three Na-poor vesicles. This last observation is particularly consistent with the parsimonious type of regulated secretion generally found in endocrine tissues; in the neurohypophysis, it is known that whatever the stimulation intensity, large nerve terminals do not release more than a small fraction of their vesicle pool (25).
Na-Associated Swelling.
The S content of resting neurosecretory vesicles was remarkably high and consistent. This is due to the S content of both the neurohormones and their companion protein neurophysin (26). In view of this consistency, the significantly low S concentration in Na-rich peripheral vesicles of stimulated endings was particularly striking. We cannot completely exclude the possibility that this change was caused by a partial loss of the secretory content, but it is more likely that a simple swelling of the vesicular matrix decreased the density of S atoms within the analyzed internal volume of the vesicles. It has been shown in a study of Na+/Ca2+ exchange in isolated bovine neurosecretory vesicles that swelling (monitored by optical density) could be promoted by an excess of Na taken up relative to Ca released (27). If sodium ions exchange for calcium, they will not only double the osmotic activity, thereby tending to induce water intake, but they are also likely to displace charges; it has been proposed that swelling is governed by the fixed charges of the polyionic matrix rather than by osmotic processes (28).
To what extent the swelling observed here would be specific for peptide release is unknown. Neurohypophysial peptide secretion requires dispersion of the vesicular matrix, as shown by the occurrence of numerous “omega figures” following experimentally induced block of secretion (9). In contrast, secretory vesicles that contain both monoamines and peptides, like mast-cell or chromaffin-cell granules, are able to achieve release by the transient opening of an exocytotic pore (as evidenced by capacitance flickers). Flickering allows monoamine release (29, 30) but not dispersion of the matrix, and occurs without swelling (31). Our results do not specify the mechanism of vesicular Na uptake and, in particular, they do not preclude the possibility that some vesicles had transiently fused with the plasma membrane just before the instant of rapid freezing. In any event, the peripheral Na-rich and Ca-poor vesicles that we observed were swollen but not dispersed, which clearly indicates that Na uptake and associated swelling are events just prior to the final step of exocytosis.
How Do Na-Rich Vesicles Take Up Na?
One possible explanation for the observed vesicular Na uptake is Na+ loading via exchange at the level of the vesicular membrane. In particular, this is a plausible mechanism for explaining the experimentally observed anticorrelation between Nav and Cav (Fig. 4B). Na+/Ca2+ exchange has been characterized by biochemical techniques on isolated bovine neurosecretory vesicles (27) and by fluorescent probe imaging on permeabilized rat neurohypophysial terminals (23). Saturation of the intravesicular Ca-binding molecules (32) can raise intravesicular Ca2+ concentration to values that will trigger reverse mode Na+/Ca2+ exchange. Reaching high Nav would necessarily require high perivesicular [Na+]i, which is predicted for cytoplasm near the cell periphery (Fig. 5), due to voltage-sensitive Na channels and/or Na+/Ca2+ exchangers of the plasma membrane.
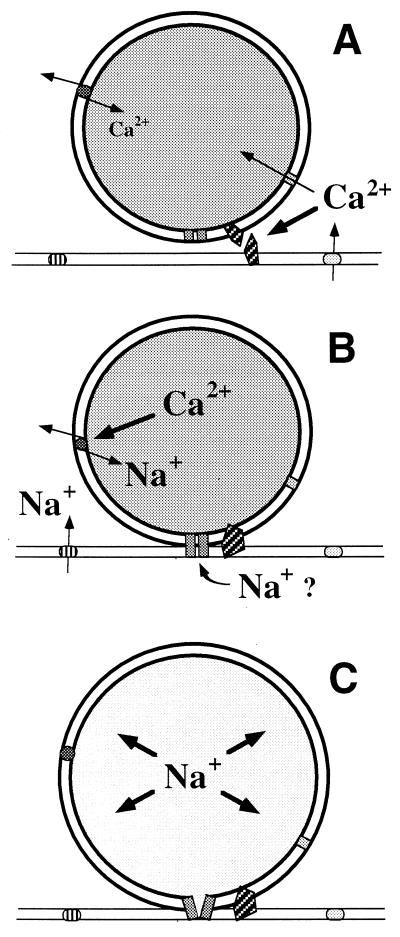
Figure 5.
Schematic interpretation of ion movements inside neurosecretory vesicles in the course of stimulus–secretion coupling. (A) Ca priming. Depolarization-evoked elevation of cytosolic Ca2+ leads to the activation of secretion-related perivesicular molecules, e.g., certain SNAREs (soluble N-ethylmaleimide-sensitive-factor attachment proteins receptors), and also to Ca2+ uptake by the vesicle. Ca2+ uptake would occur by normal mode Na+/Ca2+ exchange and/or by other transporters, including the Ca-sensitive cation channel (35). This Ca acts as a clamp stabilizing the vesicular matrix. (B) Na loading. In some vesicles, possibly when the buffering capacity of the core is saturated, the free Ca2+ content becomes high enough to reverse Na+/Ca2+ exchange. Na+/Ca2+ reversal can only occur in peripheral vesicles because proximity to plasma-membrane Na channels and exchangers is necessary to provide elevated cytosolic Na+. Alternatively or in parallel, transient openings of an exocytotic pore lead to Na+ uptake. (C) Secretion. If these events occurs in a docked vesicle, massive Na+ entry will trigger swelling and decondensation of the matrix, followed by release and dispersal of the secretory product.
Another and not necessarily contradictory way to account for some changes in intravesicular concentrations of Na in peripheral vesicles following stimulation would be the transient opening of a fusion pore (Fig. 5). Such a mechanism could account for the central observation that if Ca-rich vesicles are preferentially secreted (9), they must lose Ca and gain Na prior to secretory dispersion. The conversion from Ca-rich vesicles to Na-rich vesicles can be explained by Donnan equilibrium-driven Na+ uptake followed by competition between these ions for the anionic sites of the vesicular matrix (33, 34).
CONCLUSIONS
The present observation of an intravesicular Na+ transient under conditions of continuous stimulation suggests that the secretagogue effect of Na, as well as several other previous observations on secretory vesicle contents, can readily be explained if this transient is a physiologically critical step in stimulus–secretion coupling. Na+/Ca2+ exchange, primed by an initial intravesicular Ca2+ increase, could serve to direct vesicular Na+ uptake with consequent replacement of Ca2+ by Na+. These events would in turn trigger vesicle swelling and ultimately evoke content dispersion and neuropeptide release. Further investigations are needed to evaluate the relative contributions of Na+/Ca2+ exchange and transient openings of exocytotic pores to the observed intravesicular ionic changes.
Acknowledgments
This work was supported by the Centre National de la Recherche Scientifique (UMR 6548) and by the Intramural Research Program of the National Institutes of Health.
ABBREVIATIONS
- [Ca2+]i
intracellular Ca2+ concentration
- Cav
intravesicular calcium concentration
- Nav
intravesicular sodium concentration
- Clv
intravesicular chlorine concentration
References
- 1.Augustine G J, Burns M E, Debello W M, Pettit D L, Schweizer F E. Annu Rev Pharmacol Toxicol. 1996;36:659–701. doi: 10.1146/annurev.pa.36.040196.003303. [DOI] [PubMed] [Google Scholar]
- 2.Bennett M K. Curr Opin Neurobiol. 1997;7:316–322. doi: 10.1016/s0959-4388(97)80058-x. [DOI] [PubMed] [Google Scholar]
- 3.Neher E. Neuron. 1998;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 4.Cazalis M, Dayanithi G, Nordmann J J. J Physiol (London) 1987;390:55–70. doi: 10.1113/jphysiol.1987.sp016686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fatatis A, Holtzclaw L, Payza K, Russell J T. Brain Res. 1992;574:33–41. doi: 10.1016/0006-8993(92)90796-c. [DOI] [PubMed] [Google Scholar]
- 6.Watson E L, Farnham C J, Friedman J, Farnham W. Am J Physiol. 1981;240:C189–C192. doi: 10.1152/ajpcell.1981.240.5.C189. [DOI] [PubMed] [Google Scholar]
- 7.Suchard S J, Lattanzio F A J, Rubin R W, Pressman B C. J Cell Biol. 1982;94:531–539. doi: 10.1083/jcb.94.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuenkel E L, Nordmann J J. J Physiol (London) 1993;468:357–378. doi: 10.1113/jphysiol.1993.sp019776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thirion S, Stuenkel E L, Nicaise G. Neuroscience. 1995;64:125–137. doi: 10.1016/0306-4522(94)00414-z. [DOI] [PubMed] [Google Scholar]
- 10.Scheenen W J, Wollheim C B, Pozzan T, Fasolato C. J Biol Chem. 1998;273:19002–19008. doi: 10.1074/jbc.273.30.19002. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez J, Villalon M, Verdugo P. Biophys J. 1991;59:1022–1027. doi: 10.1016/S0006-3495(91)82317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curran M J, Brodwick M S. J Gen Physiol. 1991;98:771–790. doi: 10.1085/jgp.98.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thirion S, Troadec J D, Pagnotta S, Andrews S B, Leapman R D, Nicaise G. J Microsc (Oxford) 1997;186:28–34. doi: 10.1046/j.1365-2818.1997.1980760.x. [DOI] [PubMed] [Google Scholar]
- 14.Buchanan R A, Leapman R D, O’Connell M F, Reese T S, Andrews S B. J Struct Biol. 1993;110:244–255. doi: 10.1006/jsbi.1993.1027. [DOI] [PubMed] [Google Scholar]
- 15.Hall T A, Gupta B L. Q Rev Biophys. 1983;16:279–339. doi: 10.1017/s0033583500005114. [DOI] [PubMed] [Google Scholar]
- 16.Shuman H, Somlyo A V, Somlyo A P. Ultramicroscopy. 1976;1:317–339. doi: 10.1016/0304-3991(76)90049-8. [DOI] [PubMed] [Google Scholar]
- 17.Fiori C E, Swyt C R, Myklebust R L. DeskTop Spectrum Analyzer. National Institute of Standards and Technology, Gaithersburg, MD: Office of Standard Reference Data; 1993. [Google Scholar]
- 18.Hall T A, Gupta B L. In: Introduction to Analytical Electron Microscopy. Hren J J, Goldstein J I, Joy D C, editors. New York: Plenum; 1979. pp. 169–198. [Google Scholar]
- 19.Somlyo A V, Broderick H, H, S, Buhle E L J, Somlyo A P. Proc Natl Acad Sci USA. 1988;85:6222–6226. doi: 10.1073/pnas.85.16.6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ornberg R L, Kuijpers G A J, Leapman R D. J Biol Chem. 1988;263:1488–1493. [PubMed] [Google Scholar]
- 21.Brodwick M S, Curran M, Edwards C. J Membr Biol. 1992;126:159–169. doi: 10.1007/BF00231914. [DOI] [PubMed] [Google Scholar]
- 22.Morris J F. J Endocrinol. 1976;68:225–234. doi: 10.1677/joe.0.0680225. [DOI] [PubMed] [Google Scholar]
- 23.Troadec J D, Thirion S, Laugier J P, Nicaise G. Biol Cell. 1998;90:339–347. [PubMed] [Google Scholar]
- 24.Stuenkel E L, Nordmann J J. J Physiol (London) 1993;468:335–355. doi: 10.1113/jphysiol.1993.sp019775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindau M, Rosenboom H, Nordmann J J. In: Molecular and Cellular Mechanisms of Neurotransmitter Release. Stjärne L, Greengard P, Grillner S, Hökfelt T, Ottoson D, editors. New York: Raven; 1994. pp. 173–187. [Google Scholar]
- 26.Castel M, Gainer H, Dellmann H D. Int Rev Cytol. 1984;88:303–459. doi: 10.1016/s0074-7696(08)62760-6. [DOI] [PubMed] [Google Scholar]
- 27.Saermark T, Thorn N A, Gratzl M. Cell Calcium. 1983;4:151–170. doi: 10.1016/0143-4160(83)90031-3. [DOI] [PubMed] [Google Scholar]
- 28.Robinson I M, Fernandez J M. Curr Opin Neurobiol. 1994;4:330–336. doi: 10.1016/0959-4388(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez de Toledo G, Fernandez–Chacon R, Fernandez J M. Nature (London) 1993;363:554–558. doi: 10.1038/363554a0. [DOI] [PubMed] [Google Scholar]
- 30.Albillos A, Dernick G, Horstmann H, Almers W, Alvarez de Toledo G, Lindau M. Nature (London) 1997;389:509–512. doi: 10.1038/39081. [DOI] [PubMed] [Google Scholar]
- 31.Breckenridge L J, Almers W. Proc Natl Acad Sci USA. 1987;84:1945–1949. doi: 10.1073/pnas.84.7.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicaise G, Maggio K, Thirion S, Horoyan M, Keicher E. Biol Cell. 1992;75:89–99. doi: 10.1016/0248-4900(92)90128-n. [DOI] [PubMed] [Google Scholar]
- 33.Verdugo P. Am Rev Respir Dis. 1991;144:S33–S37. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- 34.Bergendorff A, Uvnäs B. Acta Physiol Scand. 1973;87:213–222. doi: 10.1111/j.1748-1716.1973.tb05383.x. [DOI] [PubMed] [Google Scholar]
- 35.Lee C J, Dayanithi G, Nordmann J J, Lemos J R. Neuron. 1992;8:335–342. doi: 10.1016/0896-6273(92)90299-s. [DOI] [PubMed] [Google Scholar]