Abstract
To test the utility of gene therapeutic approaches for the treatment of pain, a recombinant herpes simplex virus, type 1, has been engineered to contain the cDNA for an opioid peptide precursor, human preproenkephalin, under control of the human cytomegalovirus promoter. This virus and a similar recombinant containing the Escherichia coli lacZ gene were applied to the abraded skin of the dorsal hindpaw of mice. After infection, the presence of β-galactosidase in neuronal cell bodies of the relevant spinal ganglia (lacZ-containing virus) and of human proenkephalin (preproenkephalin-encoding virus) in the central terminals of these neurons indicated appropriate gene delivery and expression. Baseline foot withdrawal responses to noxious radiant heat mediated by Aδ and C fibers were similar in animals infected with proenkephalin-encoding and β-galactosidase-encoding viruses. Sensitization of the foot withdrawal response after application of capsaicin (C fibers) or dimethyl sulfoxide (Aδ fibers) observed in control animals was reduced or eliminated in animals infected with the proenkephalin-encoding virus for at least 7 weeks postinfection. Hence, preproenkephalin cDNA delivery selectively blocked hyperalgesia without disrupting baseline sensory neurotransmission. This blockade of sensitization was reversed by administration of the opioid antagonist naloxone, apparently acting in the spinal cord. The results demonstrate that the function of sensory neurons can be selectively altered by viral delivery of a transgene. Because hyperalgesic mechanisms may be important in establishing and maintaining neuropathic and other chronic pain states, this approach may be useful for treatment of chronic pain and hyperalgesia in humans.
Information related to noxious thermal, mechanical, and chemical stimuli is transmitted from the skin and other peripheral sites to the central nervous system by myelinated Aδ and unmyelinated C nociceptors (1). Both types of neurons synapse in the dorsal horn of the spinal cord onto various second-order neurons that either carry information to the thalamus and other brain centers or synapse on other spinal neurons. Transmission of sensory information by both types of primary afferents is mediated predominantly by the excitatory amino acid glutamate. Additionally, C fibers appear to release the amino acid aspartate as well as a variety of neuropeptides, including substance P and calcitonin gene-related peptide (1).
In the dorsal horn, application of exogenous opioids such as morphine or release of endogenous opioid peptides, including the enkephalins and dynorphin, activated either by descending or other sensory inputs that activate local circuit neurons, produces analgesia (2, 3). The actions of these endogenous opioid peptides appear to involve activation of μ, δ or κ opioid receptors, all of which are present in the dorsal horn (3, 4). These receptors are predominantly located on the presynaptic terminals of the primary afferents, although postsynaptic opioid receptors are also present (4, 5). These opioid receptors are coupled to G-proteins that inhibit adenylate cyclase, inhibit calcium channels, and stimulate potassium channels, thus tending to reduce neuronal responsiveness (3, 6, 7).
Preproenkephalin A is one of three genes encoding endogenous opioid peptides (8–10). Its product, proenkephalin, is synthesized in a wide variety of central and peripheral neurons, including substantia gelatinosa interneurons of the dorsal horn and in chromaffin cells of the adrenal medulla (11–15). Mice made deficient in preproenkephalin by knockout procedures display several altered responses to painful or threatening environmental stimuli (16). Although these mice do not display altered spinal nociception by the tail-flick assay, their response to the formalin test was altered significantly, closely resembling that of naloxone-treated mice. These findings suggest that endogenous enkephalins do participate in the response to nociceptive stimuli.
Several of the properties of herpes simplex virus type 1 (HSV-1) make it an obvious candidate for gene delivery to the peripheral nervous system (for reviews, see refs. 17–19). First, the virus is neurotropic. Experimental infections in mice show that not only the trigeminal ganglia but also spinal ganglia neurons can be latently infected with human HSV-1 after inoculation of skin or internal organs. Second, in latently infected neurons, the viral DNA is maintained for the life of the host in an episomal form without replication and with little or no expression of viral proteins. Only one region of the genome is actively transcribed during this period, the latency-associated transcript. Third, the large size of the viral genome allows addition of 10–15 kilobases of DNA, adequate for introduction of most genes of interest. Fourth, the viruses are easily manipulated in tissue culture, although the time required to construct and purify a recombinant virus is 3–6 months.
Expression of a variety of marker and other genes encoded by recombinant HSV-1 vectors has been demonstrated in vivo (20–22). In cells of the dorsal root and trigeminal ganglia (containing the cell bodies of sensory neurons), expression of transgenes under the control of strong constitutive promoters can persist for months despite little or no viral protein synthesis. This long-term expression has been observed with marker genes under control of the human cytomegalovirus immediate–early enhancer-promoter (22) and the Moloney murine leukemia virus long terminal repeat in combination with one of the two latency-associated transcript promoters, LAP1 (21, 23). The present study uses a recombinant HSV-1 vector to deliver the human preproenkephalin (hPPE) gene to sensory neurons. The results demonstrate that proenkephalin is synthesized and processed to active opioid peptides in sensory neurons and that release of these peptides in the spinal cord is antihyperalgesic.
METHODS
Virus Construction.
A cassette containing the human cytomegalovirus immediate–early promoter/enhancer, SV40 intron, human preproenkephalin cDNA, and SV40 polyadenylation sequence was cloned from plasmid pCMVhPPE (24) into pBluescript II SK(±) (Stratagene) containing an inserted lox P site. This plasmid was reacted with Cre recombinase in the presence of ICP4− viral DNA containing a lox P site in the thymidine kinase locus, as described (25). The virus, designated SHPE, was isolated on a complementing cell line and was purified by three rounds of limiting dilution (26). Virus SHPE was rescued by reintroduction of ICP4 DNA with selection on Vero cells, generating a recombinant vector designated KHPE. Southern blotting confirmed correct insertion of the proenkephalin cassette. KHPE-mediated expression of proenkephalin was verified by infection of primary bovine adrenal chromaffin cells and BSC-40 cells followed by Western blotting with antiproenkephalin mAb PE-25 (27, 28).
Animal Procedures.
Animal procedures were approved by the University of South Carolina and University of Illinois at Chicago Institutional Animal Care and Use Committees. Female Swiss–Webster mice (5–6 weeks old; Harlan Breeders, Indianapolis) were used for all experiments. Animals were anesthetized with 400 mg/kg of tribromoethanol. The hair was removed from the dorsal surface of each hindfoot, and the skin was scarified by using medium-coarse sandpaper. Five microliters of viral suspension (0.5–1.0 × 108 plaque-forming units) or vehicle then was applied to one foot and was spread by using the side of a pipettor tip. After ≈10 min, when all of the liquid was adsorbed or evaporated, the animals were returned to their home cages. For killing, animals again were anesthetized with tribromoethanol (500 mg/kg) and were perfused by cardiac puncture with PBS followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH 7.5). Dissected spinal cords with ganglia attached were incubated further in fixative for 1–2 h at 4°C.
Histochemical Procedures.
After washing three times with 0.1 M sodium phosphate (pH 7.5), β-galactosidase activity was visualized in mouse dorsal root ganglia by incubation in this buffer containing 2 mM MgCl2, 5 mM K4Fe(CN)6, 5 mM K3Fe(CN)6, 0.01% sodium deoxycholate, 0.02% Nonidet P-40, and 1 mg/ml X-Gal (stock 20 mg/ml in dimethylformamide) at 37° for 20–24 h (20). For immunohistochemistry, the lumbar spinal cord was placed in PBS containing 30% sucrose for 24 h and thereafter was blocked in the transverse plane. Cryostat sections (40 μm) were washed three times in PBS containing 4% normal goat serum and 0.3% Triton X-100, then were incubated for 12 h with gentle agitation at 4°C with a 1:500 dilution of mouse mAb PE-24. Antibody PE-24 binds to amino acids 175–185 of the human preproenkephalin sequence (28) and does not cross-react with either rat or bovine preproenkephalin as bacterially expressed products (B. A. Spruce, personal communication). As a control, other sections were processed in parallel without primary antibody. Sections were incubated further in a 1:50 solution of Texas Red-conjugated goat anti-mouse (The Jackson Laboratory) in PBS for 1 h in the dark (22°C) with constant agitation. The sections then were washed three times with PBS and were incubated with a Met-enkephalin antibody (Peninsula Laboratories; 1:250 solution prepared as for PE-24). After 12 h, the sections were washed three times in PBS and were transferred to a 1:50 solution of sheep anti-rabbit FITC (The Jackson Laboratory) for 1 h in the dark. The sections were washed three times in PBS, were mounted on gelatin-coated slides, were air dried, were coverslipped with Fluoromount, and were examined under epifluorescence.
Behavioral Testing.
The foot withdrawal response to noxious radiant heat previously used for rats (29) was adapted for use in mice. Mice were anesthetized lightly with 600 mg/kg urethane and were examined for the time required for foot withdrawal after thermal stimulation of the dorsum of the hindpaw. Animals were tested both at low rates of heating, stimulating predominantly C nociceptors, and high heating rates, mainly stimulating Aδ nociceptors (30, 31). Where indicated, sensitization of C-fiber, low heating-rate responses was achieved by topical application of 20 μl of 0.1% capsaicin (in H2O/ethanol; 50/50 vol/vol) to the infected foot (30). In other experiments, Aδ nociceptors were sensitized with topical dimethyl sulfoxide (100%), which produces a decrease in response latency for the high but not low rate of foot heating (31). In most experiments, after determining response latencies, either 50 mg/kg naloxone (i.p.), 1.0 mg/kg naloxone (intrathecally), or a comparably delivered vehicle was administered to determine whether endogenous release of opioid peptides mediated any difference observed in response latencies between different groups of animals. Analysis of variance demonstrated that responses of animals not infected with virus were identical to those infected with the KHZ virus in all experiments. Analyses of variance also were performed to determine whether responses of animals infected with the KPE virus were different from those that had been infected with the KHZ virus. Follow-up analyses (Tukey) were performed to determine whether latencies at various time points were significantly different between groups, including after sensitization and after application of naloxone.
RESULTS
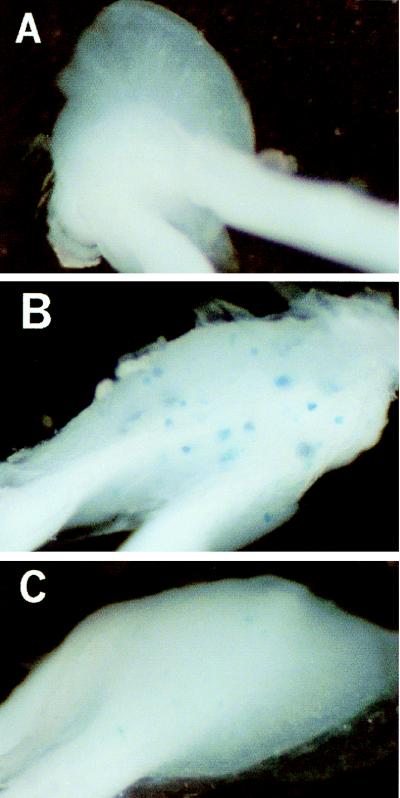
A recombinant HSV-1 vector was constructed to express the human preproenkephalin cDNA under the control of the human cytomegalovirus immediate–early promoter/enhancer (Fig. 1). This virus, designated KHPE, contains the promoter-transgene construct inserted into the viral thymidine kinase gene. This insertion of transgenes into the thymidine kinase gene inactivates the viral gene (32) and disables viral replication in nondividing cells such as neurons (33). A similar recombinant virus, designated KHZ, containing the E. coli lacZ gene in place of the human preproenkephalin cDNA also was used (34, 35). Expression of β-galactosidase in sensory neurons of mouse dorsal root ganglia was examined after infecting animals on the dorsal skin of the hindfoot with KHZ. At 4 days postinfection, KHZ-infected animals exhibited histochemical reactions for β-galactosidase in 20–100 cell bodies in the L3-L5 ganglia ipsilateral to the infection (Fig. 2B), with occasional staining in the L2 ganglion. Similar levels of expression were observed at 2 weeks postinfection (results not shown). At 5–6 weeks postinfection, both the number and intensity of staining for β-galactosidase was reduced in KHZ-infected animals (Fig. 2C), indicative of reduced enzyme expression, between 10 and 25% of that observed at 4 days. Expression of β-galactosidase at 10 weeks postinfection was similar to that at 5–6 weeks (results not shown).
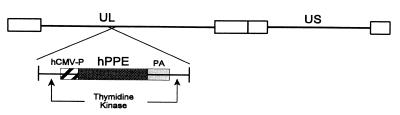
Figure 1.
Schematic representation of recombinant herpes virus KHPE. See Methods for virus construction. UL, unique long segment; US, unique short segment; hCMV-P, human cytomegalovirus immediate–early promoter/enhancer; hPPE, human preproenkephalin cDNA; PA, simian virus 40 polyadenylation sequence.
Figure 2.
Histochemical staining for HSV-1 vector-mediated β-galactosidase expression in mouse dorsal root ganglia. Swiss–Webster mice (6 weeks old) were infected, where indicated, with 5 × 107 plaque-forming units of virus. Staining in the ipsilateral L3 ganglia is shown. (A) Animal with no virus infection. (B) Animal infected with virus KHZ and killed 4 days postinfection. (C) Animal infected with virus KHZ and killed 40 days postinfection.
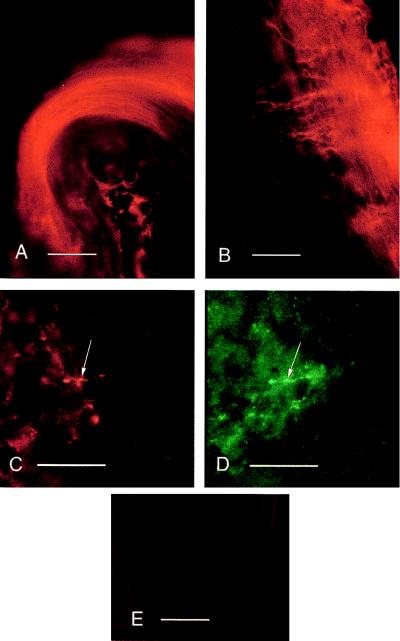
Expression of human proenkephalin in the mouse spinal dorsal horn and axons of sensory neurons in nerve roots after infection with virus KHPE was detected by using a human-specific mAb directed against proenkephalin (Fig. 3 A–C). Human proenkephalin immunoreactivity was observed in primary afferent axons in dorsal roots (Fig. 3A), in axons invading the dorsal horn (Fig. 3B), and in primary afferent terminals in the substantia gelatinosa of the dorsal horn (Fig. 3C). In all cases, human proenkephalin immunoreactivity was restricted to the side ipsilateral to the infection. Some of the proenkephalin terminals were also immunoreactive for the fully processed peptide neurotransmitter Met-enkephalin (Fig. 3D).
Figure 3.
Immunohistochemistry of HSV-1 vector-mediated expression of human preproenkephalin in mouse dorsal roots and spinal cord. (A) Human proenkephalin (PE-24) immunoreactivity in dorsal root axons of a left dorsal rootlet visualized with Texas Red. Dorsal is down, medial (toward cord) is to the right. (Bar = 100 μm.) (B) Human proenkephalin immunofluorescence in primary afferent axons at the dorsal root entry zone of the dorsal horn ipsilateral to the original infection. Dorsal is up, medial is to the left, and the edge of spinal cord is to the right. (Bar = 100 μm.) (C) Human proenkephalin immunofluorescence in primary afferent terminals (arrow) in the inner substantial gelatinosa. Dorsal is up, and medial is to the right. (Bar = 20 μm.) (D) Met-enkephalin immunoreactivity in the same afferent terminals as in C (arrow) visualized with fluorescein. Area is chosen for relative paucity of Met-enkephalinergic terminals. (Bar = 20 μm.) (E) Lack of human proenkephalin (PE-24) immunoreactivity in dorsal horn at right dorsal root entry zone contralateral to the original infection visualized with Texas Red. Dorsal is up, and the outside of the cord is to the right. (Bar = 100 μm.)
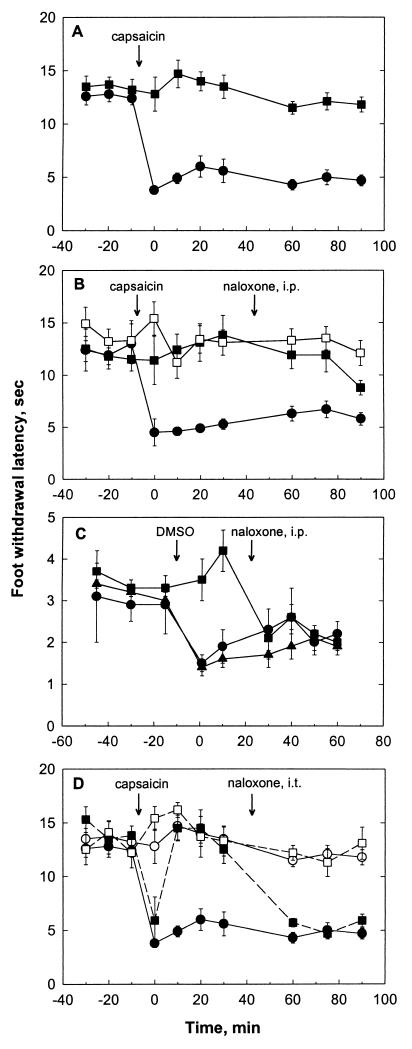
Animals infected with recombinant viruses KHZ and KHPE also were evaluated for effects on foot withdrawal responses evoked by noxious radiant heat in normal and hyperalgesic mice. This method uses different rates of noxious skin heating, which allows separate assessment of behavioral responses produced by the activation and sensitization of myelinated (Aδ) or unmyelinated (C) nociceptors. Specifically, high-rate heating (6.5°C/min) and topical dimethyl sulfoxide preferentially activate and selectively sensitize Aδ nociceptors whereas low-rate heating (0.9°C/min) and topical capsaicin selectively activate and sensitize C fiber nociceptors (29–31, 36). Animals were infected with virus on the depilated and scarified dorsal skin of the hindfoot and were tested for foot withdrawal latency to both high and low rates of heating between 1 and 6 weeks postinfection. Results from this testing are shown in Fig. 4. Baseline latencies for both rates of heating were similar for animals infected with KHPE or KHZ and were similar to latencies recorded for uninfected animals. In uninfected mice and in mice infected with KHZ, application of capsaicin sensitized C nociceptors (Fig. 4 A and B) but not Aδ nociceptors (results not shown), as evidenced by significantly (P < 0.05, ANOVA) reduced foot withdrawal latencies (30). In contrast, mice infected with KHPE exhibited no such sensitization (P > 0.05, ANOVA). In a similar manner, the selective reduction of Aδ nociceptor-mediated responses induced by application of dimethyl sulfoxide was blocked by infection with KHPE (Fig. 4C), as evidenced by the significant difference (P < 0.05, ANOVA) between KHPE-infected and KHZ-infected animals. This blockade of both Aδ and C fiber-mediated hyperalgesia in KHPE-infected animals was observed at all times examined, although it appeared more robust at 4 and 6 weeks than at 1–2 weeks after infection (results not shown). In some cases, a reversal of sensitization appeared only after an initial hyperalgesic response (Fig. 4D).
Figure 4.
Foot withdrawal responses to noxious radiant heat. Animals were infected unilaterally with either KHZ or KHPE. Topical capsaicin (A, B, and C) or DMSO (C) was applied to the infected foot as indicated. Data are presented as mean ± SEM, n = 6–8. (A) Response to a low rate of skin heating, preferentially activating C nociceptors. ●, KHZ-infected animal, ipsilateral foot; ■, KHPE-infected animal, ipsilateral foot. (B) Response to a low rate of skin heating. Naloxone (50 mg/kg i.p.) was administered where indicated. ●, KHZ-infected animal, ipsilateral foot; ■, KHPE-infected animal, ipsilateral foot; □, KHPE-infected animal, contralateral foot (no capsaicin applied). (C) Response to a high rate of skin heating, preferentially activating Aδ nociceptors. ●, KZ-infected animal, ipsilateral foot; ■, KHPE-infected animal, ipsilateral foot; ▴, uninfected animal. (D) Response to a low rate of skin heating. Naloxone (1 mg/kg intrathecally) was administered where indicated. KHZ-infected animal (solid line): ●, ipsilateral foot; ○, contralateral foot (no capsaicin applied). KPE-infected animal (broken line): ■], ipsilateral foot; □, contralateral foot (no capsaicin applied).
Systemic administration of the opioid antagonist naloxone (50 mg/kg, i.p.) partially (C-fiber response) or completely (Aδ-fiber response) restored sensitization in KHPE-infected animals but had no effect on KHZ-infected or mock-infected animals (Fig. 4 B and C), indicating at least partial opioid mediation of the observed antihyperalgesic effects. Intrathecal administration of naloxone (1.0 mg/kg) also completely reversed the KHPE-induced blockade of sensitization for C fiber-mediated responses (Fig. 4D), indicating that at least part of the antihyperalgesic effect of the virus was mediated by spinal release of opioids. Naloxone administration in KHPE-infected or KHZ-infected animals without prior sensitization was without effect (not shown).
DISCUSSION
The present results demonstrate prolonged, herpes-mediated expression of transgenes in mouse sensory neurons. Although expression of β-galactosidase at 5–6 weeks postinfection was reduced to levels 10–25% of those observed at 4 days, expression was observed without further diminution up to 10 weeks, the longest time tested. A similar time course of transgene expression has been reported by others (22). The finding that the human cytomegalovirus immediate–early promoter/enhancer drives expression of transgenes, both acutely and after establishment of latency, confirms previous studies (22) on the utility of this promoter for expression in sensory neurons. Although a number of other promoters have been tested for acute and prolonged expression in the murine sensory neurons, the only other promoter demonstrated to maintain transgene expression during viral latency in vivo is the Moloney murine leukemia virus long terminal repeat (20, 21, 23).
The results also demonstrate that infection of peripheral endings of sensory neurons with recombinant HSV-1 encoding a neuropeptide precursor, human proenkephalin, results in synthesis and processing of this precursor in mouse sensory neurons. A similar viral construct that results in opioid peptide synthesis in primary afferents was reported while this work was in progress (37). The presence of both the precursor and processed enkephalins in central terminals of the primary afferents (Fig. 3) shows production of biologically active transgene products (8–15). The results further demonstrate that infection of cutaneous nociceptive afferents with a proenkephalin-encoding HSV-1 produces opioid-mediated antihyperalgesia without affecting basal nociceptive responses. This finding has been confirmed with two additional viral recombinants encoding proenkephalin (S.P.W. and D.C.Y., unpublished work). In contrast, animals that received infections of a lacZ-containing HSV-1 did not show any changes in their nociceptive responsiveness. These data clearly suggest that viral introduction of the human proenkephalin cDNA into nociceptive primary afferents alters the responsiveness of both C and Aδ afferents to stimuli which would normally produce hyperalgesia.
The mechanisms by which the observed antihyperalgesia occurs are not clear however. Blockade of the effects of the proenkephalin-encoding virus by intrathecal naloxone suggests that observed antihyperalgesic effect is mediated by the release of opioid peptides from the central terminals of the primary afferents, although involvement of peripheral opioid receptors cannot be excluded (38). In this way, the observed opioid-mediated antihyperalgesia mimics the well known effects of endogenous or intrathecally administered enkephalins (2–4). However, the lack of effect on basal foot withdrawal responses suggests that the opioids effecting the antihyperalgesia may not be tonically released in the infected animals but, rather, may be released only when there is a substantial activation of the afferents. In support of this is the finding that, in KHPE-infected animals that were not exposed to capsaicin or DMSO, naloxone did not alter response latencies as would be expected if opioids were being released tonically. It is also interesting to note that the dosages of naloxone given were quite high, possibly indicating a nonspecific effect. Preliminary experiments indicated that such high doses were necessary to reverse the antihyperalgesia. If enkephalins were being released very near to presynaptic terminals, high doses of antagonists might be necessary to block the locally high concentrations of the peptides.
Several issues raised by these studies will require further study. For example, the specific site, presynaptic versus postsynaptic, at which the opioid peptides act is unclear. Presynaptic opioid effects have been demonstrated for both Aδ and C fiber-mediated responses (see refs. 5 and 6). Taken together with these previous findings of presynaptic opioid effects, the results are consistent with at least part of the antihyperalgesic effects resulting from autoinhibition of nociceptive afferents at the presynaptic terminals. A second question raised is that of the development of opioid receptor tolerance. Although our results suggest that enkephalin release depends on afferent activity, development of tolerance could be problematic during long periods of nociception. We are currently investigating this possibility.
An issue raised by these results is the apparent lack of a direct correlation between marker transgene expression and the behavioral effects. Expression of β-galactosidase in the dorsal root ganglia of KHZ-infected rats decreases by at least 75% from 4 days to 6 weeks postinfection. Although the antihyperalgesic effects of KHPE were observed as soon as 4–5 days postinfection, the reproducibility and magnitude of these effects appeared to increase over at least the first 2 weeks and remained robust until at least 7 weeks postinfection (the longest time tested). Several factors could account for this apparent discrepancy including (i) the time required for complete healing of the viral inoculation site, (ii) the time required to synthesize and transport an adequate number of vesicles containing proenkephalin products to the nerve terminals, (iii) modulation of sensory function by very low levels of proenkephalin products, or (iv) proenkephalin-induced plastic changes in the sensory neurons. Further studies will be required to examine possible plastic changes. Even the lower levels of transgene expression observed (Figs. 2 and 3) at 4–6 weeks may be adequate to maintain peptide stores in these neurons in which turnover may be low in the absence of robust stimuli. If future work demonstrates that the antinociceptive effect also diminishes over greater time periods than that tested, it will be of interest to determine whether reinfection will demonstrate effects similar to the original infection.
The results of the present study are exciting because they imply that, by viral delivery of a transgene, the function of sensory neurons can be selectively altered. In this case, hyperalgesia was blocked without altering baseline nociception. If the neuropeptide precursor proenkephalin is being synthesized, processed to active peptides, and stored in secretory vesicles in the infected sensory neurons, as the present studies seem to suggest, a pool of releasable enkephalins will exist in the terminals of these neurons, possibly stored with other nociceptive neuropeptides, including substance P and calcitonin gene-related peptide. Basal release of the transgene products should be minimal; vesicular storage, probably in large dense-core vesicles, would limit enkephalin release to those instances of high-level or persistent stimulation necessary to evoke peptide release.
These observations further suggest that KHPE or a similar recombinant herpes virus may be useful for treatment of chronic pain in humans. Hyperalgesia, which may be important in establishing and maintaining neuropathic and other chronic pain states, was selectively blocked by infection with this proenkephalin-encoding virus. Advantages of this type of gene therapy would include precise anatomical targeting of the specific nociceptors transmitting pain impulses, the lack of systemic opioid adverse effects, and a long (weeks to months) duration of action. In addition, it appears that normal sensory transmission would not be disrupted when persistent and robust pain sensitization are absent.
Acknowledgments
The authors gratefully acknowledge technical support by L. Annette Smith. Antiproenkephalin antibodies were generously supplied by Dr. Barbara Spruce (University of Dundee). This work was supported by National Science Foundation Grant IBN 94-09201 (S.P.W.), by the University of South Carolina Research and Productive Scholarship Fund (S.P.W.) and by Public Health Service Grant DA08256 (D.C.Y.).
ABBREVIATION
- HSV-1
herpes simplex virus type 1
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Bonica J J. The Management of Pain. Philadelphia: Lea and Febiger; 1990. [Google Scholar]
- 2.Yaksh T L, Noueihed R. Annu Rev Pharmacol Toxicol. 1985;25:433–462. doi: 10.1146/annurev.pa.25.040185.002245. [DOI] [PubMed] [Google Scholar]
- 3.Mansour A, Fox C A, Akil H, Watson S J. Trends Neurosci. 1994;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- 4.Ji R R, Zhang Q, Law P Y, Low H H, Elde R, Hökfelt T. J Neurosci. 1995;15:8156–8166. doi: 10.1523/JNEUROSCI.15-12-08156.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Y, Pirec V, Yeomans D C. Br J Pharmacol. 1997;121:1210–1216. doi: 10.1038/sj.bjp.0701239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glaum S R, Miller R J, Hammond D L. J Neurosci. 1994;14:4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Werz M A, MacDonald R L. Brain Res. 1982;239:315–321. doi: 10.1016/0006-8993(82)90859-9. [DOI] [PubMed] [Google Scholar]
- 8.Comb M, Seeburg P H, Adelman J, Eiden L, Herbert E. Nature (London) 1982;295:663–666. doi: 10.1038/295663a0. [DOI] [PubMed] [Google Scholar]
- 9.Gubler U, Seeburg P H, Hoffman B J, Gage P, Udenfriend S. Nature (London) 1982;295:206–208. doi: 10.1038/295206a0. [DOI] [PubMed] [Google Scholar]
- 10.Noda M, Furutani Y, Takahashi H, Toyosato M, Hirose T, Inayama S, Nakanishi S, Numa S. Nature (London) 1982;295:202–206. doi: 10.1038/295202a0. [DOI] [PubMed] [Google Scholar]
- 11.Yang H-Y T, DiGiulio A M, Fratta W, Hong J S, Majane E A, Costa E. Neuropharmacology. 1980;19:209–215. doi: 10.1016/0028-3908(80)90140-9. [DOI] [PubMed] [Google Scholar]
- 12.Mocchetti I, Guidotti A, Schwartz J P, Costa E. J Neurosci. 1985;5:3379–3385. doi: 10.1523/JNEUROSCI.05-12-03379.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H Y, Hong J S, Costa E. Neuropharmacology. 1977;16:303–307. doi: 10.1016/0028-3908(77)90112-5. [DOI] [PubMed] [Google Scholar]
- 14.Hökfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Proc Natl Acad Sci USA. 1977;74:3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sar M, Stumpf W E, Miller R J, Chang K J, Cuatrecasas P. J Comp Neurol. 1978;182:17–37. doi: 10.1002/cne.901820103. [DOI] [PubMed] [Google Scholar]
- 16.König M, Zimmer A M, Steiner H, Holmes P V, Crawley J N, Brownstein M J, Zimmer A. Nature (London) 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 17.Fink D J, DeLuca N A, Goins W F, Glorioso J C. Annu Rev Neurosci. 1996;19:265–287. doi: 10.1146/annurev.ne.19.030196.001405. [DOI] [PubMed] [Google Scholar]
- 18.Smith F, Jacoby D, Breakfield X O. Restor Neurol Neurosci. 1995;8:21–34. doi: 10.3233/RNN-1995-81207. [DOI] [PubMed] [Google Scholar]
- 19.Glorioso J C, DeLuca N A, Fink D J. Annu Rev Microbiol. 1995;49:675–710. doi: 10.1146/annurev.mi.49.100195.003331. [DOI] [PubMed] [Google Scholar]
- 20.Dobson A T, Margolis T P, Sedarati F, Stevens J G, Feldman L T. Neuron. 1990;5:353–360. doi: 10.1016/0896-6273(90)90171-b. [DOI] [PubMed] [Google Scholar]
- 21.Lokensgard J R, Bloom D C, Dobson A T, Feldman L T. J Virol. 1994;68:7148–7158. doi: 10.1128/jvi.68.11.7148-7158.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ecob-Prince M S, Hassan K, Denheen M T, Preston C M. J Gen Virol. 1995;76:1527–1532. doi: 10.1099/0022-1317-76-6-1527. [DOI] [PubMed] [Google Scholar]
- 23.Davar G, Kramer M F, Garber D, Roca A L, Andersen J K, Bebrin W, Coen D M, Kosz-Vnenchak M, Knipe D M, Breakfield X O, et al. J Comp Neurol. 1994;339:3–11. doi: 10.1002/cne.903390103. [DOI] [PubMed] [Google Scholar]
- 24.Liu F, Housley P R, Wilson S P. J Neurochem. 1996;67:1457–1462. doi: 10.1046/j.1471-4159.1996.67041457.x. [DOI] [PubMed] [Google Scholar]
- 25.Gage P J, Sauer B, Levine M, Glorioso J C. J Virol. 1992;66:5509–5515. doi: 10.1128/jvi.66.9.5509-5515.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kang W, Wilson M A, Bender M A, Glorioso J C, Wilson S P. Brain Res. 1998;792:133–135. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- 27.Spruce B A, Curtis R, Wilkin G P, Glover D M. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Böttger V, Böttger A, Lane E B, Spruce B A. J Mol Biol. 1995;247:932–946. doi: 10.1006/jmbi.1995.0191. [DOI] [PubMed] [Google Scholar]
- 29.Yeomans D C, Proudfit H K. Pain. 1994;59:85–94. doi: 10.1016/0304-3959(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 30.Yeomans D C, Proudfit H K. Pain. 1996;68:141–150. doi: 10.1016/S0304-3959(96)03177-6. [DOI] [PubMed] [Google Scholar]
- 31.Yeomans D C, Pirec V, Proudfit H K. Pain. 1996;68:133–140. doi: 10.1016/S0304-3959(96)03176-4. [DOI] [PubMed] [Google Scholar]
- 32.Krisky D M, Wolfe D, Marconi P C, Ramakrishnan R, Mata M, Rouse R J D, Fink D J, Glorioso J C. Gene Ther. 1998;5:1593–1603. doi: 10.1038/sj.gt.3300766. [DOI] [PubMed] [Google Scholar]
- 33.Tenser R B, Hay K A, Erdis W A. J Virol. 1989;63:2861–2865. doi: 10.1128/jvi.63.6.2861-2865.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mester J C, Pitha P M, Glorioso J C. Gene Ther. 1995;2:187–196. [PubMed] [Google Scholar]
- 35.Rasty S, Goins W F, Glorioso J C. Methods Mol Genet. 1995;7:114–129. [Google Scholar]
- 36.Baumann T K, Simone D A, Shain C N, LaMotte R H. J Neurophysiol. 1991;66:212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- 37.Bras J M A, Epstein A L, Bourgoin S, Hamon M, Cesselin F, Pohl M. J Neurochem. 1998;70:1299–1303. doi: 10.1046/j.1471-4159.1998.70031299.x. [DOI] [PubMed] [Google Scholar]
- 38.Kinnman E, Nygards E B, Hansson P. Anesth Analg. 1997;84:595–599. doi: 10.1097/00000539-199703000-00024. [DOI] [PubMed] [Google Scholar]