To the Editor,
Pseudomonas aeruginosa produces and secretes rhamnose-containing glycolipid biosurfactants called rhamnolipids. The advantages of biosurfactants are low irritancy and even anti-irritating effects, as well as compatibility with human skin [1].
The focus of this study is di-rhamnolipid, α-L-rhamnopyranosyl-(1–2)α-L- rhamnopyranosyl-3-hydroxydecanoyl-3–hydroxydecanoic acid, also referred to as BAC-3. BAC-3 is also known as the heat-stable hemolysin or glycolipid secreted into the environment by Pseudomonas aeruginosa. It has a molecular weight of 650 daltons, is amphiphilic and aggregates into micelles [2]. In vivo data relates specifically to the treatment of autoimmune diseases and includes the effects of BAC-3 on cellular immunosuppression, oxazolone-induced delayed type hypersensitivity, immunomodulation and immunorestoration [3]. Lastly, the clinical trials with BAC-3 conducted prior to this study on the treatment of psoriasis, lichen ruber planus, neurodermatitis and human wound healing have confirmed excellent ameliorative effects of di-rhamnolipid when compared to conventional therapy using corticosteroids [4].
Cultivation and propagation of neonatal human fibroblasts and keratinocytes was performed as we have described previously [5].
Keratinocytes were grown in serum free medium, consisting of the Medium 154 (Cascade Biologics Inc, Portland, OR) with 0.2 mM Ca 2+, supplemented with human keratinocyte growth supplement (Cascade Biologics Inc, Portland, OR) or serum containing medium consisting of DMEM (Invitrogen, Grand Island, NY) with 1.2 mM Ca 2+, supplemented with 10% FCS (Gemini Bio-products Inc., Calabasas, CA), 400 ng/ml hydrocortisone (Sigma-aldrich, St. Louis, MO), 10 ng/ml epidermal growth factor (Upstate Biotechnology). Tests with fibroblasts were performed in standard medium consisting of DMEM with 1.2 mM Ca2+ supplemented with 10% FCS.
Di-rhamnolipid BAC-3 used in the following study was in a powder form and provided by TajCo Inc. (TajCo Inc., Davis, CA) [6].
Proliferation tests were performed on fibroblasts and keratinocytes grown over the period of 2 weeks and counted using trypan blue exclusion test. Proliferative potential of keratinocytes treated with BAC-3 and grown in serum containing medium was evaluated according to the method first described by Rheinwald and Green [7]. For measuring viability, cells were incubated with AlamarBlue solution (Biosource International, Camarillo, CA) and the absorbance was read at 600 nm in spectrophotometer. Degree of apoptosis was assayed by measuring all active caspases using CaspaTag Fluorescein Caspase Activity Kit (Intergen Company, NY, USA) and accompanying protocol. For positive apoptosis control, cells were treated with 1 μg/ml FAS antibody (Chemicon International) in a combination with UVB light (fibroblasts 396 J/m2, keratinocytes 792 J/m2 ).
Data is expressed as the mean±S.D. of the triplicates and is representative of three independent experiments. Statistical analysis was performed using ANOVA and accepting p<0.05 as significant.
At 1 mg/ml and 500 μg/ml BAC-3 showed a cytotoxic effect on both keratinocytes and fibroblasts since shortly after the treatment with di-rhamnolipid the cell membranes were disrupted and the cytoplasmic structures appeared to dissolve, suggesting the process of cell necrosis. At 200, 100 and 50 μg/ml, BAC-3 significantly inhibited proliferation of fibroblasts grown in standard culture medium containing 1.2 mM Ca2+ and 10% fetal calf serum. In accordance with proliferation results at 1 mg/ml and 500 μg/ml concentrations, BAC-3 killed cells. Di-rhamnolipid concentration of 200 μg/ml showed a 61% decrease (p<0.01) in cell viability resembling the apoptotic effect of 396 J/m2 UVB irradiation and 1 μg/ml FAS antibody treatment. At concentration of 100μg/ml and lower, BAC-3 decreased the cell viability dose dependently. At 50 μg/ml, programmed cell death (apoptosis) reached its peak (91% increase in caspase activity as compared to control, p<0.01) and higher or lower concentrations were less efficient.
At 100, 50 and 10 μg/ml, BAC-3 significantly inhibited the proliferation of keratinocytes in the absence of serum and at low calcium concentrations (0.2 mM), i.e. under culture conditions that promote keratinocyte proliferation but not differentiation [8]. Interestingly, 50 μg/ml concentration appeared to be a "threshold" inhibitory concentration. At 10 μg/ml, after two week treatment, the cells appeared healthy but stopped dividing, i.e. cell proliferation was inhibited by 35% (p<0.01). BAC-3 at 10 and 5 μg/ml decreased the keratinocyte viability by 13% (p<0.05) and 9% (p<0.05), respectively as compared to control and concentrations of 1 μg/ml and lower had almost no effect. The inhibition is consistant with the rise in the level of active caspases. At 1 μg/ml, the induction of keratinocyte apoptosis reached its peak (70% increase in caspase activity as compared to control, p<0.01) and higher or lower concentrations were less effective.
Quite different results were obtained when keratinocytes were cultivated in the serum-containing medium. At 100, 50 and 10 μg/ml BAC-3 significantly stimulates proliferation of keratinocytes in the presence of serum and at high calcium concentrations (1.2 mM Ca2+), i.e. under culture conditions favoring keratinocyte differentiation [9]. At 50 μg/ml BAC-3 has maximum effect in stimulating cell proliferation (34%) and viability (25%) as compared to control (Fig 1) with 49% decrease in caspase activity as compared to control (p<0.01). Figure 2 represents summary of BAC-3 effects.
Figure 1.
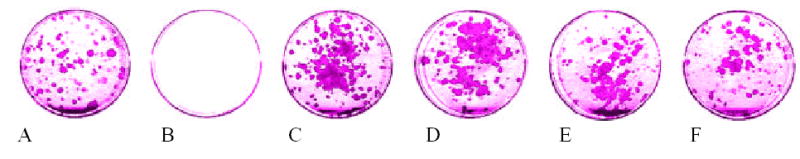
The effect of di/rhamnolipid on the viability of neonatal human keratinocytes grown in medium containing 10% fetal bovine serum. 500 keratinocytes were initially seeded with 1.5 × 105 3T3 mouse fibroblasts in 60mm tissue culture dishes. Starting from the first day in culture, cells were treated with 500μg/ml, 100μg/ml, 50μg/ml, 10μg/ml and 1 μg/ml concentrations of di-rhamnolipid BAC-3. After two week treatment cells were fixed with 10% formaline/PBS and stained with Rhodanile blue solution (1% Rhodamine, 0.5% NileBlue; Sigma-aldrich, St.Louis, MO, USA) and the dishes were scanned and the scanned images analyzed using program based on histogram values construed with Adobe Photoshop 4.0 software (Adobe System Incorporated, San Jose, CA, USA) to determine the area of the keratinocytes colonies.
Figure 2.
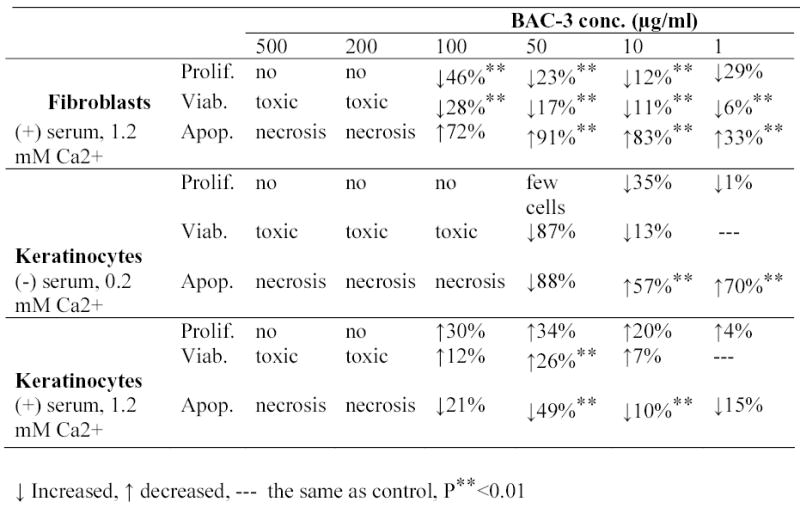
Effect of di-rhamnolipid BAC-3 on fibroblast and keratinocytes cell cultures.
Our studies demonstrate that it is possible to have a BAC-3 concentration (50 μg/ml) which, in the presence of serum and under the conditions favoring keratinocytes differentiation, inhibits the proliferation of fibroblasts yet stimulates the proliferation of keratinocytes. In full thickness wounds, blood vessels are disrupted and epidermal cells are exposed to serum components, as opposed to the intact skin [10]. BAC-3 results on keratinocytes grown in the presence of serum are in support of efficacy of BAC-3 shown in skin treatment and wound healing. On the other hand, the results obtained with keratinocytes grown in serum free medium relate to the clinical use of BAC in conditions when it is desirable to achieve the inhibition of keratinocyte proliferation (for example, in psoriasis). Additional measurements on the process of apoptosis have confirmed that the effect of BAC-3 is specific and not simply due to its cytotoxicity.
Di-rhamnolipid mode of action could involve a direct cell-receptor binding, creating complexes with serum components, binding to the membrane proteins, passing through the phospholipid bilayer of the cell membrane and interacting with DNA transcriptional and translational machinery.
Acknowledgments
This work was supported by NIH SBIR grant A1 R43 AR44443-01A1 to TajCo Inc.
References
- 1.Maier RM, Soberon-Chavez G. Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Appl Microbiol Biot. 2000;54:625–633. doi: 10.1007/s002530000443. [DOI] [PubMed] [Google Scholar]
- 2.Johnson MK, Boese-Marazzo D. Production and properties of heat-stable extracellular hemolysin from Pseudomonas aeruginosa. Infect Immunol. 1980;29:1028–1033. doi: 10.1128/iai.29.3.1028-1033.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piljac G, Piljac V. Immunological activity of rhamnolipids. U.S. Patent. 1995a;5:466, 675. [Google Scholar]
- 4.Piljac G, Piljac V. Pharmaceutical preparation based on rhamnolipid. U.S. Patent. 1995b;5:455, 232. [Google Scholar]
- 5.Dans MJ, Isseroff RR. Inhibition of collagen lattice contraction by pentoxifylline and interferon-alpha, -beta, and –gamma. J Invest Dermatol. 1994;102:118–121. doi: 10.1111/1523-1747.ep12371743. [DOI] [PubMed] [Google Scholar]
- 6.Rheinwald JG, Green H. Epidermal growth factor and multiplication of cultured human epidermal keratinocytes. Nature. 1977;265:421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- 7.Syldatk C, Wagner F. Production of biosurfactants. In: Kosaric N, Cairns WL, Gray NC, editors. Biosurfactants and biotechnology. Surfactant Science Series 25. New York Basel; Dekker: 1987. pp. 89–120. [Google Scholar]
- 8.Rheinwald JG, Green H. Serial cultivation of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975;6:331–334. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 9.Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culure. J Invest Dermatol. 1983;81:33–40. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- 10.Waldorf H, Fewkes J. Wound healing. Adv Dermatol. 1995;1:77. [PubMed] [Google Scholar]


