Abstract
Elucidating the molecular mechanisms leading to the induction and specification of thyroid follicular cells is important for our understanding of thyroid development. To characterize the key events in this process, we previously established an experimental embryonic stem (ES) cell model system, which shows that wild-type mouse CCE ES cells can give rise to thyrocyte-like cells in vitro. We extend our analysis in this report by using a genetically manipulated ES cell line in which green fluorescent protein (GFP) cDNA is targeted to the TSH receptor (TSHR) gene, linking GFP expression to the transcription of the endogenous TSHR gene. The appearance of GFP-positive cells was dependent on the formation of embryoid bodies from undifferentiated ES cells and was greatly enhanced by TSH treatment during the first 2–4 d of differentiation. With the support of Matrigel, highly enriched ES cell-derived GFP-positive cells formed thyroid follicle-like clusters in a serum-free medium supplemented with TSH. Importantly, these clusters display the characteristics of thyroid follicular cells. Immunofluorescent studies confirmed the colocalization of TSHR with the Na+/I− symporter in the clusters and indicated that Na+/I− symporter was expressed exclusively in the plasma membrane. In addition, I− uptake activity was observed in these cells. Our results indicate that ES cells can be induced to differentiate into thyroid follicular cells, providing a powerful tool to study embryonic thyroid development and function.
Abbreviations: DAPI, 4′, 6-Diamidino-2-phenylindole; EB, embryoid body; EBDM, embryoid body differentiation medium; ES, embryonic stem; GFP, green fluorescent protein; h, human; IMDM, Iscove’s modified Dulbecco’s medium; LIF, leukemia inhibitory factor; MDCK, Madin-Darby canine kidney; MTG, monothioglycerol; NIS, Na+/I− symporter; Tg, thyroglobulin; TPO, thyroperoxidase; TSHR, TSH receptor
THE THYROID GLAND produces the thyroid hormones T3 and T4, which are needed for metabolic homeostasis, growth, and development. Although defects in any of the multiple steps required for thyroid development and thyroid hormone synthesis can cause thyroid dysfunction, the molecular mechanisms by which many of these diseases develop are unclear. This lack of information stems from the lack of a suitable model to study early human thyroid development. To date, most of our understanding of thyroid cell biology has been dependent upon the ability to generate primary cultures from fetal or adult thyroids and from immortalized thyroid cell lines (1–5). Despite their experimental utility, primary and immortalized thyroid cells have limited value in the study of the molecular basis of thyroid development. Clearly, a new model of thyroid development is sorely needed.
Over the last few years, the use of embryonic stem (ES) cells has attracted attention due to their potential contributions to basic biology and regenerative medicine. ES cells, isolated from the inner cell mass of blastocyst-stage embryos, are master cells that retain the capacity to generate any cell type in vitro and in vivo (6, 7). Mouse ES cells, when cultured with an irradiated embryonic fibroblast feeder layer or with leukemia inhibitory factor (LIF), could be maintained in the undifferentiated state for prolonged periods (7). Upon withdrawal of LIF or depletion of the feeder layer, ES cells spontaneously differentiate to form three-dimensional cellular aggregates or embryoid bodies (EBs) that contain elements of all three embryonic germ layers: ectoderm, mesoderm, and endoderm (8). The EB system recapitulates stages of early embryogenesis through gastrulation, including the formation of postimplantation embryonic tissues. By manipulating the culture conditions under which ES cells differentiate, EB cells can further differentiate into cells of all lineages. Although protocols are currently available to generate a few specific cell types, primarily neural, hemopoietic, cardiac, and pancreatic β-cells as well as hepatocytes (9–19), from ES cells, directed differentiation into thyroid cells has remained a challenge.
As a first step to assess the differentiation of ES cells into thyroid lineage, we recently established culture conditions for the derivation of thyrocyte-like cells from mouse wild-type CCE ES cells, which included a two-wk period of treatment with TSH (20). In these studies, cultures of EB-derived adherent cell populations contained thyrocyte-like cells, as demonstrated by the appearance of a set of genes traditionally associated with thyroid follicular cells, i.e. the thyroid transcription factor PAX8, the Na+/I− symporter (NIS; which transports iodide into the thyroid cells), thyroglobulin (Tg; the precursor of thyroid hormones), thyroperoxidase (TPO; the enzyme responsible for Tg iodination), and the receptor for TSH (TSHR) (20). Importantly, thyroid-specific function, such as cAMP generation by TSH, was maintained in this model (20). These attributes suggested that differentiating ES cells might provide a useful in vitro model for thyroid cell differentiation and proliferation. In this report we describe our attempts to optimize these culture conditions to support thyroid cell maturation, which is essential for future clinical applications.
In the present study we extended our analysis using ES cells that were genetically modified so as to permit thyroid cell enrichment. An ES cell line carrying a fusion gene comprised of an enhanced green fluorescent protein-neomycin-resistant (GFP-Neor) cassette was generated such that the expression of the fusion gene was under the control of the TSHR promoter (21). This ES cell line was subsequently used to generate a TSHR-null mouse. The TSHR-null mouse showed severe congenital hypothyroidism and thyroid hypoplasia and died at 4 wk of age. In contrast, heterozygous (TSHR+/−) mice were unaffected (21). Of note is that thyroid follicles in TSHR-null mice were poorly developed, with fewer follicular cells and more nonfollicle-associated interstitial cells (21), implying that follicular growth and differentiation are at least in part dependent on the TSHR. By targeting GFP to the TSHR locus, we tracked GFP expression during thyroid development in vivo. As expected, green fluorescence in thyroid follicles was found in both null and heterozygous mice (21). In this study we optimized culture conditions to allow the directed differentiation of these heterozygous (TSHR+/−) ES cells into thyroid follicular cells in vitro. The genetically engineered TSHR+/− ES cell line offers three main advantages in the study of the developmental progression of thyroid follicular cells: 1) it is regulated by the TSHR promoter and is TSH responsive; 2) it uses GFP as a reporter; and 3) TSHR-expressing cells can be selected using G418. Our results indicate that thyroid follicular cells derived from ES cells have properties consistent with the thyroid neofollicle. To our knowledge, our method marks the first successful derivation of thyroid follicular cells from ES cells and highlights the potential of ES cells for studying the genes and factors involved in the specification of thyroid lineage.
Materials and Methods
Growth and maintenance of ES cells
The development of the TSHR+/− ES cell line has been recently reported (21). In brief, a targeting vector carrying a GFP-neor cassette was introduced into position 1 of the mouse TSHR exon 1 coding sequence and was then linearized and electroporated into wild-type W9.5 ES cells. Subsequently, independent clones heterozygous and homozygous for the TSHR mutation were selected by G418 and confirmed by Southern blotting (21). ES cells passaged fewer than five times were maintained on irradiated embryonic fibroblast feeder cells as previously described in DMEM (Invitrogen Life Technologies, Inc., Grand Island, NY) supplemented with 15% fetal calf serum, penicillin-streptomycin (100 U/ml; Invitrogen Life Technologies, Inc.), 10 ng/ml LIF (StemCell Technologies, Inc., Vancouver, Canada), and 1.5 × 10−4 M monothioglycerol (MTG; Sigma-Aldrich Corp., St. Louis, MO). Cultures were maintained in a humidified chamber in a 5% CO2/air mixture at 37 C. ES cells cultures were monitored daily, and cells were passaged at 1:3 ratios every 2 d.
Differentiation of ES cells
Before initiation of EB differentiation, cells were transferred to Iscove’s modified Dulbecco’s medium (IMDM; Invitrogen Life Technologies, Inc.) containing the above components on 0.1% gelatin-coated dishes. To induce formation of EBs, cells were trypsinized into a single-cell suspension and plated at varying densities (103 to 8 × 104 cells/ml) in 60-mm petri-grade dishes. EB differentiation medium (EBDM) contained IMDM supplemented with penicillin/streptomycin, 15% fetal calf serum, 2 mM L-glutamine, 5% protein-free hybridoma medium (PFHM-II, Invitrogen Life Technologies, Inc.), 0.5 mM ascorbic acid (Sigma-Aldrich Corp.), transferrin (200 μg/ml; Roche, Ridgefield, CT), and 1.5 × 10−4 MMTG. The EBs were cultured for 6 d. In some experiments, serum-stimulated EBs were harvested at d 2 of differentiation, allowed to settle by gravity for 10 min, and then re-plated in 60-mm dishes of IMDM supplemented with 15% KnockOut serum replacement medium (Invitrogen Life Technologies, Inc.), penicillin/streptomycin, 0.5 M ascorbic acid, and 1.5 × 10−4 M MTG [serum replacement (SR) medium]. The commercially available KnockOut SR medium is a serum-free formulation optimized for mouse ES cells. The components of KnockOut SR have been described previously (22).
TSH induction was carried out using a two-step protocol. In the first step, EB differentiation was initiated in EBDM for 2 d. In the second step, serum-stimulated EBs were allowed to settle by gravity, transferred to new dishes, and cultured in IMDM supplemented 100 μU/ml human recombinant TSH (hTSH; Fitzgerald Industries, Concord, MA). Cells were harvested for gene expression analysis on culture d 0, 2, 4, and 6. For analysis of the TSHR-expressing subpopulations, cells were sorted on d 4 for GFP expression and reaggregated in EBDM overnight. The reaggregated EBs were transferred to six-well dishes coated with Matrigel (BD Biosciences, Bedford, MA) in serum-containing medium for 6 d, then additionally cultured in SR medium supplemented with 100 μU/ml hTSH. Cells from replated cultures were harvested on d 21 (total time for differentiation), when differentiation was complete for RT-PCR analysis and immunostaining.
FACS analysis and cell sorting
EBs were trypsinized and harvested. The resulting single-cell suspension was either analyzed on a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA) or sorted on a MoFlo cell sorter (DakoCytomation, Carpinteria, CA). FACS data were generated on CellQuest software (BD Biosciences).
Gene expression analysis
For gene expression analysis, total RNA was isolated using an RNeasy kit (Qiagen, Valencia, CA) and was treated with ribonuclease-free deoxyribonuclease (Qiagen). Two micrograms of total RNA were reverse transcribed into cDNA using the Thermoscript First Strand Synthesis System (Invitrogen Life Technologies, Inc.). PCR was performed using standard protocols with 2.5 U platinum Taq polymerase (Invitrogen Life Technologies, Carlsbad, CA). Amplification conditions were as follows: initial denaturation at 94 C for 2 min, followed by 35–40 cycles of denaturation at 94 C for 30 sec, annealing at 50–61 C for 45 sec, extension at 72 C for 45 sec, and final extension at 72 C for 7 min. Annealing temperature in all cases was set at 2 C below the calculated denaturation temperature. The amount of cDNA in each sample was normalized using β-actin as a control. RNA controls were included to monitor genomic contamination. The amplified PCR products were separated on 2% agarose gels and visualized by ethidium bromide staining. The identities of related PCR products were confirmed by direct sequencing. The primers used in this study were as follows: Wnt1 (forward), 5′-GATTGCGAAGATGAACGCTGTTTC-3′; Wnt1 (reverse), 5′-TCCTCCACGAACCTGTTGACGG-3′; NeuroD (forward), 5′-CTTGGCCAAGAACTACATCTGG-3′; NeuroD (reverse), 5′-GGAGTAGGGATGCACCGGGAA-3′; Rex1 (forward), 5′- CGTGTAACATACACCATCCG-3′; Rex1 (reverse), 5′-GAAATCCTCTTCCAGAATGG-3′; Gata1 (forward), 5′-CATTGGCCCCTTGTGAGGCCAGAGA-3′; Gata1 (reverse), 5′-ACCTGATGGAGCTTGAAATAGAGGC-3′; c-fms (forward), 5′-GCGATGTGTGAGCAATGGCAGT-3′; c-fms (reverse), 5′-AGACCGTTTTGCGTAAGACCTG-3′; Oct4 (forward), 5′-GGCGTTCTCTTTGGAAAGGTGTTC-3′; Oct4 (reverse), 5′-CTCGAACCACATCCTTCTCT-3′; β-actin (forward), 5′-ATGAAGATCCTGACCGAGCG-3′; β-actin (reverse), 5′-TACTTGCGCTCAGGAGGAGC-3′; GFP (forward), 5′-CTCGTGACCACCCTGACC-3′; GFP (reverse), 5′-GGTCACGAACTCCAGCAGG-3′; TSHR (forward), 5′-GAGTGTGCGTCTCCACCCTGTGA-3′; TSHR (reverse), 5′-TTCCAGCCGCTGCAGAGTTGCAT-3′; Pax8 (forward), 5′-TGCCTTTCCCCATGCTGCCTCCGTGTA-3′; Pax8 (reverse), 5′-GGTGGGTGGTGCGCTTGGCCTTGATGTAG-3′; NIS (forward), 5′-GCTCTCATCAGCTACCTAACTG-3′; NIS (reverse), 5′-CTCAGAGGTTGGTCTCAACATC-3′; Tg (forward), 5′-TGGGACGTGAAAGGGGAATGGTGC-3′; Tg (reverse), 5′-GTGAGCTTTTGGAATGGCAGGCGA-3′; TPO (forward), 5′-TGCCAACAGAAGCATGGGCAAC-3′; and TPO (reverse), 5′-GCACAAAGTTCCCATTGTCCAC-3′.
Immunofluorescent staining
Cells were fixed in 4% paraformaldehyde in PBS [10 mM phosphate and 150 mM NaCl (pH 7.4)]. After fixation, the cells were washed twice with PBS, permeabilized in PBS containing 0.1% Triton X-100 for 10 min, washed with 0.1% Tween 20 in PBS for 10 min, then preblocked with 3% BSA or 2–5% serum from the same species in PBS. The following primary antibodies were used: rabbit antirat NIS (1:100) and rabbit antihuman Tg (1:4000; DakoCytomation). For detection of primary antibodies, the cells were washed, then incubated with Alexa Fluro 954 chicken antirabbit IgG (1:2000; Molecular Probes, Eugene, OR) for 30 min at room temperature. The stained cells were washed before mounting with 10 μl Vectashield mounting medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA). Images were captured using an Axioskop fluorescent microscope (Zeiss, Thornwood, NJ). NIS antibody was provided by Dr. Nancy Carrasco (Albert Einstein College of Medicine, Bronx, NY).
Radioactive iodide uptake
Cells were washed with modified Hanks’ balanced salt solution, then incubated in the same buffer containing 20 μM sodium iodide supplemented with 10 μCi carrier-free Na125I to give a specific activity of 100 mCi/mmol for 45 min at 37 C in a humidified atmosphere. Parallel wells of cells were incubated in the absence or presence of perchlorate, respectively. Aspirating the radioactive solution and washing twice with ice-cold Hanks’ balanced salt solution terminated uptake reactions. To determine the amount of 125I− accumulated in the cells, 95% ethanol was added to each well for 20 min at 4 C, then quantitated in a γ-counter, as previously described (23). The DNA content of each well was measured on the material not extracted by ethanol after trichloroacetic acid precipitation. 125I− uptake was expressed as picomoles per microgram of DNA per 45 min. 125I− uptake in Madin-Darby canine kidney (MDCK) cells stably transfected with wild-type NIS (NIS/MDCK) was measured as a positive control. The values represent the mean ± SE of two independent experiments performed in triplicate. The NIS/MDCK clone was provided by Dr. Nancy Carrasco.
Results
TSHR heterozygosity does not affect the in vitro differentiation potential of ES cells
In the present study we used heterozygous TSHR+/− ES cells to study the induction and specification of the thyrocyte lineage in ES cell differentiation cultures. For this heterozygous ES cell line to be a reliable model to study the development of thyroid follicular cells in vitro, it was important to confirm that the inactivation of one allele did not interfere with these developmental processes. To address this question and validate whether TSHR+/− ES cells can direct pluripotent ES cells toward a thyroid fate, we first compared their developmental potential with that of wild-type parental W9.5 ES cells. We promoted the differentiation of ES cells by modifying a previously described protocol (20). In brief, ES cells were grown in culture medium on irradiated embryonic fibroblast feeder cells. They were then harvested and transferred to plastic petri dishes and cultured in suspension for 6 d to induce their spontaneous differentiation into EBs. We found that undifferentiated ES cells and floating EBs generated from the TSHR+/− ES cells were indistinguishable in morphology and size from those developing from wild-type W9.5 ES cells (Fig. 1A).
Fig. 1.
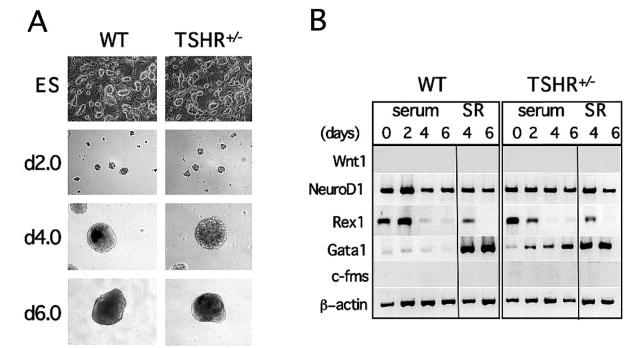
Characterization of TSHR+/− ES cells. A, Phase contrast micrographs of undifferentiated ES cells and differentiated EBs show that TSHR+/− ES cells and EBs were indistinguishable from their wild-type (WT) counterparts with respect to morphology and size. Numbers on the left indicate days of differentiation. Images were taken with an ECLIPSE TE200-S camera (Nikon Corp., Melville, NY) at × 10 magnification. B, RT-PCR expression analysis of EBs differentiated in serum-containing (serum) medium or in serum-containing medium followed by SR medium for various markers characteristics of primary germ layers, including Wnt1, NeuroD, Gata1, and c-fms. Rex1 is a stem cell marker. Numbers on top of the figure indicate days of differentiation.
We next analyzed mRNA transcripts from the cells at various stages for the presence of stem cell marker Rex1, a zinc finger transcription factor found in ES cells but not in their differentiated progeny (24). Although both TSHR+/− and wild-type ES cells expressed Rex1 before the onset of differentiation (Fig. 1B), EBs from both cultures down-regulated the gene within 4 d of differentiation (Fig 1B), consistent with previous findings (24). As a comparison, differentiation during ES/EB development was additionally examined by determining the expression of a panel of markers that cover all embryonic germ layers: Wnt1 (an early marker of neural crest cells), NeuroD (a neuroectoderm developmental marker), Gata1 (a zinc finger transcription factor that is essential at multiple stages of hemopoiesis), and c-fms (the receptor for macrophage colony-stimulating factor and a mesoderm developmental marker) (25–27). Wnt1 and c-fms expression was not detected at any time in either TSHR+/− or wild-type ES cells (Fig 1B), but NeuroD and Gata1 were broadly expressed at every time point in both TSHR+/− and wild-type cultures (Fig 1B). In addition, Gata1 was up-regulated between d 2–6 in TSHR+/− ES cells, defining the onset of hemopoiesis within the EBs. In contrast to TSHR+/− ES cells, Gata1 expression in wild-type ES cells was not significantly different from that on d 0–6 of differentiation. The findings reported in this study indicate that the gene expression patterns of most germ layer markers were similar in wild-type and TSHR+/− ES cells throughout the 6-d EB time course.
Restricted exposure of EBs to serum
To determine whether EB development could continue in the absence of serum, EBs were stimulated with serum for 2 d, then transferred to serum-free cultures for an additional 4 d. After testing several serum-free culture conditions, we found that medium supplemented with KnockOut SR medium effectively supported the growth of floating EBs. As shown in Fig. 1B, Gata1 expression significantly increased in both wild-type and TSHR+/− cultures after 2 d of differentiation under SR conditions. In contrast, removing serum after 2 d did not affect NeuroD expression in either wild-type or TSHR+/− cultures (Fig. 1B). We found no significant difference in cell numbers on d 4 and 6 between TSHR+/− and wild-type cultures (data not shown). These observations demonstrated that the gene expression pattern of TSHR+/− EBs was similar to that of wild-type EBs in both the presence and absence of serum. Together, these findings suggest that inactivation of one allele of the TSHR gene does not alter the developmental program during EB differentiation and validates the use of TSHR+/− ES cells as a model system to both identify genes crucial in ES cell development and direct differentiation into the thyroid lineage.
Kinetics of GFP and TSHR expression in early TSHR+/− EB cells
We explored whether GFP levels could be used to monitor TSHR expression. We used flow cytometry to analyze the temporal expression patterns of GFP after in vitro differentiation of TSHR+/− ES cells. GFP first appeared in 5.5% of the cells after 2 d of differentiation (Fig. 2A). By d 4, about 7.8% of EB cells were expressing GFP (Fig. 2A). The population of GFP-positive cells then declined, and positive cells were rare by d 6. Wild-type ES cells did not express GFP under these conditions (Fig. 2A). To be a reliable marker of TSHR expression, GFP expression must reflect the expression pattern of the endogenous gene. To test this hypothesis, we harvested EBs from TSHR+/− ES cells every 2 d over a 6-d differentiation period and analyzed TSHR transcription levels using RT-PCR. As shown in Fig. 2B, TSHR expression peaked between d 2 and 4 of differentiation and dropped sharply by d 6, consistent with the previously described pattern for GFP (Fig. 2A). These data suggest that GFP expression faithfully mimics endogenous TSHR expression in differentiating EBs and confirm that TSHR expression can be easily measured and quantified in this ES cell line by monitoring GFP expression with FACS analysis.
Fig. 2.
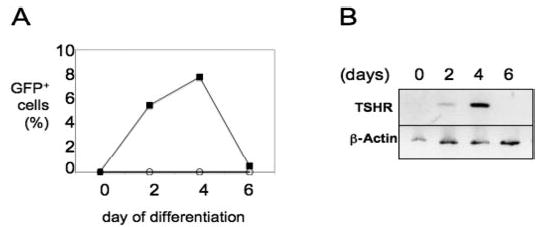
Kinetics of GFP and TSHR expression in early TSHR+/− EBs. A, Percentage of GFP-positive cells during the first 6 d of culture in EBDM. ○, Wild-type EBs; ▪, TSHR+/− EBs. B, Kinetics of TSHR gene expression patterns during the first 6 d of EB development. Total RNA was isolated from differentiated EBs cultured as described in Materials and Methods. cDNA was analyzed from total RNA using oligo(deoxythymidine) primers. RT-PCR was performed to detect the expression of TSHR. β-Actin served as an internal control. Numbers on top of the figure indicate days of differentiation.
Effects of TSH on GFP and TSHR expression
Previous studies have demonstrated that TSH stimulation of TSHR activates a cAMP-dependent pathway that leads to the transcriptional regulation of genes necessary for thyroid hormone synthesis and secretion (28). Therefore, we investigated whether TSH plays a role in early EB differentiation in this ES cell model. We exposed both TSHR+/− and wild-type EBs to human recombinant TSH on d 2 of differentiation and cultured them in SR medium for 2 additional days (Fig. 3A). Notably, GFP expression increased from 7% to 20% after 2 d of culture in the presence of TSH and the absence of serum (Fig. 3B, upper panel). In addition, both TSHR and GFP transcripts were significantly up-regulated in TSH-treated EBs (Fig. 3B, lower panel). These data demonstrate that at the dose (100 μU/ml) used, TSH can stimulate TSHR expression in EBs in the absence of serum.
Fig. 3.
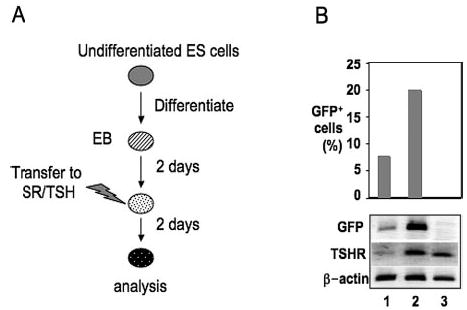
Effects of TSH on GFP and TSHR expression. A, General outline of the differentiation protocol. B, Upper panel, Flow cytometric analysis of GFP expression in TSHR+/− EBs differentiated in SR medium (lane 1) or SR medium supplemented with hTSH (lane 2). Note that GFP expression was substantially induced in TSH-supplemented cells cultured in SR medium. Wild-type EBs were used as negative controls (lane 3). Lower panel, RT-PCR expression analysis demonstrating the effect of TSH on TSHR and GFP gene expression in d 4 EBs.
Thyroid potential of GFP-expressing cells
We used flow cytometry to enrich d 4 GFP-positive cells to near homogeneity to assess their viability and differentiation capacity (Fig. 4, A and B). After sorting, the cells were reaggregated to form green EB-like spheres in suspension (Fig. 4C), transferred to serum-containing medium for 6 d, and then additionally cultured in medium containing serum or SR medium supplemented with TSH. Cells were plated onto growth factor-reduced Matrigel-coated dishes (BD Biosciences, Bedford, MA) to offer anchors for attachment and cell to cell contact. Histological examination of 21-d cultures found numerous putative follicle-like clusters in TSH-treated cells (Fig. 5), but not in cells cultured in the absence of TSH or with serum alone (data not shown).
Fig. 4.
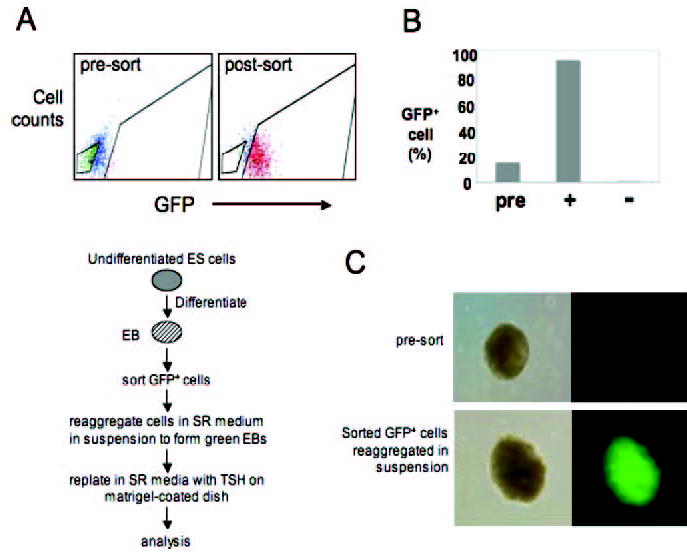
Selective enrichment of GFP-expressing cells. A, FACS analysis of d 4 EBs and culture protocol for differentiation of sorted GFP+ cells. B, FACS analysis of cells from the presorted (pre), GFP-positive (+), and GFP-negative (−) populations. C, Immunofluorescent analysis demonstrates the appearance of green EBs in reaggregated, sorted GFP+ cells in suspension. Note that presorted EBs do not show detectable levels of fluorescence. Left panels, Phase contrast images; right panels, fluorescent microscopic images.
Fig. 5.
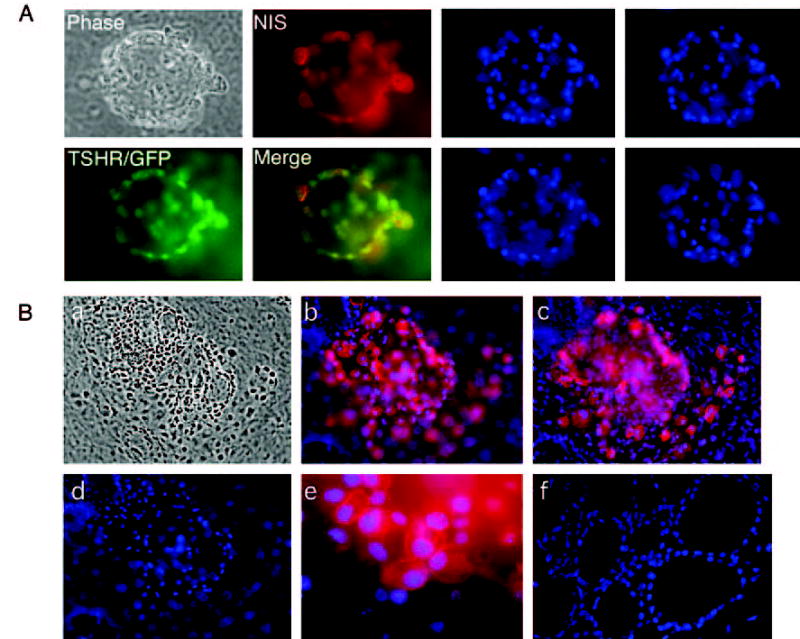
GFP+NIS+ cells are organized into one or more follicle-like cell clusters. Left panel, A follicle-like cluster derived from TSHR+/− ES cells after 21 d of differentiation visualized microscopically (phase) or after staining with an antibody to NIS (red). TSHR expression is indicated by the green GFP signal. An overlaid image shows the colocalization of NIS with TSHR (yellow). Right panel, The same field of cells after nuclear staining with DAPI (blue). Images were taken from different optical sections. B, Immunofluorescent images of several follicle-like clusters derived from TSHR+/− ES cells after 21 d of differentiation. a, Phase contrast exposure. b and c, Immunofluorescent staining, demonstrating the expression of NIS protein. Note that a rim of cells several layers thick adhered closely to the NIS-positive cells. d, Nuclear DAPI staining. e, High magnification of anti-NIS immunofluorescence, demonstrating the expression of NIS in the plasma membrane. Immunofluorescence was not detected when the experiment was performed with the second antibody alone (data not shown). f, Nuclear DAPI staining of native mouse thyroid tissue.
To determine whether these cells could acquire thyroid phenotypes, we analyzed the cell clusters for the presence and spatial orientation of TSHR (via GFP) and the thyroid differentiation marker NIS (via immunofluorescent analysis). We stained these cells with anti-NIS peptide antibody, followed by staining with Alexa Fluro 954 chicken antirabbit IgG and observation with fluorescent microscopy. Cells were fixed and permeablized for indirect immunofluorescence analysis to detect the anti-NIS antibody, which is directed against the intracellular carboxyl terminus. Morphologically, the most unique characteristic of the differentiated cells was the development of follicle-like clusters similar to those found in the thyroid gland in vivo. Follicle formation proceeded with several large central lumina, as shown in Fig. 5. Several layers of cells, whose identities remain to be determined, surrounded these follicular spaces. In many instances, GFP (green) and NIS (red) were localized to either the cytoplasm or surface of these cells, respectively, they also colocalized (Fig. 5A, left panel, yellow). Nuclear DAPI staining of various optical sections of the same follicle-like cluster revealed a unique three-dimensional structure (Fig. 5A, right panel). An NIS-positive rim of cells several layers thick adhered closely to the follicle-like clusters. Because we confirmed that NIS was exclusively expressed in the plasma membrane (Fig. 5B), and because coexpression of NIS and TSHR is a hallmark of thyroid follicular cells, this observation suggests that the current culture conditions can support the growth of mature thyroid follicular cells.
Figure 6A shows an example of extensive anti-NIS staining in large GFP+ cellular aggregates. However, in this case, these cells had not been organized into the recognizable follicle structures shown in Fig. 5. There were also more cells in the aggregates than in the follicle-like structures. Nevertheless, with or without follicle formation, multiple layers of GFP− NIS− cells were in close contact with cells coexpressing TSHR and NIS. Immunofluorescent analysis indicated that TSH-treated cultures generated approximately 7–10 follicle-like structures/1.7 cm2 (growth surface per well of a four-well chamber slide). In contrast, d 4 EBs grown directly onto Matrigel without cell sorting could not spontaneously differentiate into these follicle-like clusters (data not shown). Overall, about 1% of the cells in TSH-treated cultures were GFP+NIS+ (with or without follicle formation).
Fig. 6.
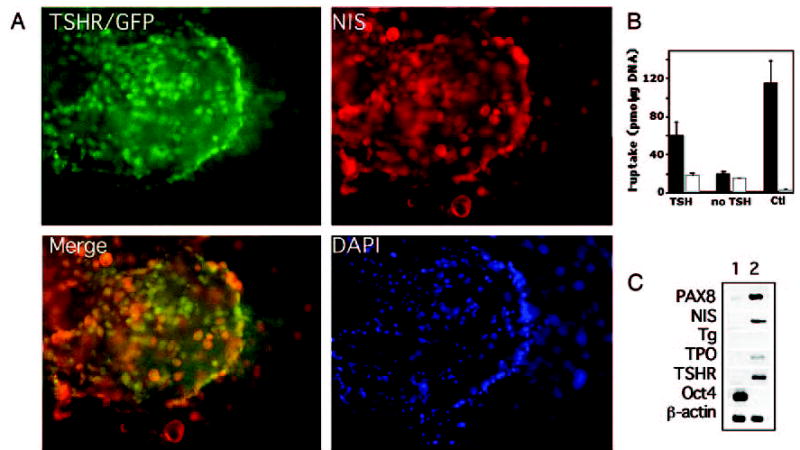
Thyroid potential of GFP+NIS+ cells. A, Several clusters of GFP+NIS+ cells derived from TSHR+/− ES cells after staining with an antibody to NIS (red). In merging, some areas showed overlay (yellow). Blue indicates nuclear DAPI staining. B, I− uptake activity at 20 μM external sodium iodide in TSHR+/− ES cells after 21 d of differentiation. ▪, Absence of 40 μM NaClO4; □, presence of 40 μM NaClO4. Note that cells treated with TSH showed I− uptake activity, whereas cells maintained in the absence of TSH did not. I− uptake in NIS/MDCK cells (Ctl) was measured as a positive control for functional NIS activity. The values represent the mean ± SE of two independent experiments performed in triplicate. C, Gene expression analysis by RT-PCR shows differentiation of thyroid follicular cells from ES cells. RNA was isolated from undifferentiated ES cells (lane 1) and thyroid follicular cells grown for 21 d (lane 2) and was analyzed for the expression of thyroid marker genes PAX8, NIS, Tg, TPO, and TSHR. Oct4 is an undifferentiated ES cell marker. β-Actin served as an internal control standard.
We next measured I− uptake (20 μM) under steady-state conditions, as described in Materials and Methods. After 21 d of differentiation (which included 10 d of exposure to TSH), the cells treated with TSH showed I− uptake activity (59.6 ± 14.3 vs. 17.7 ± 2.2 pmol/μg DNA·45 min, I− vs. ClO4−), in contrast to cells maintained without TSH (19.4 ± 2.6 vs. 15.2 ± 0 pmol/μg DNA·45 min, I− vs. ClO4−; Fig. 6B). To further characterize the differentiation of ES cells into the thyroid lineage, we used RT-PCR to examine the expression patterns of thyroid lineage-associated genes. We found that undifferentiated ES cells expressed the undifferentiated stem cell marker Oct4, but none of the thyroid cell markers (Fig. 6C). After 21 d of differentiation, mature thyroid cells expressed thyroid genes, including PAX8, NIS, TPO, and TSHR (Fig. 6C). Although Tg was not expressed in the TSH-stimulated cell populations (see Discussion), these observations support the idea that subpopulations at this stage of development represent differentiated cells committed to the thyroid lineage. In addition, our findings confirm and extend those of previous experiments by demonstrating that TSH induces the thyroid developmental program in vitro.
Discussion
Pluripotent ES cells, which have an extraordinary capacity for differentiation, provide a unique cellular system to study the commitment and differentiation of embryonic thyroid cells. In this report we provide the first comprehensive analysis of thyroid follicular cell development from mouse ES cells. We showed that in an ES cell line with GFP targeted to the TSHR locus, GFP is expressed in early EBs. We also demonstrated that TSH induces these cells to develop into the thyroid lineage. Analysis of TSHR-expressing populations subsequently led to the identification of clusters of cells with structural and functional properties consistent with thyroid neofollicles. This study demonstrates that ES cells can adopt a thyroid fate when induced to differentiate in culture.
The current approach relies on a genetically manipulated TSHR+/− ES cell line. One of the initial goals of this targeting approach was to generate a TSHR-null mouse to study TSHR function in vivo (21). Using a previously established ES cell differentiation protocol (20), the present study demonstrates that mouse TSHR+/− ES cells can give rise to putative thyroid neofollicles in vitro and confirms that GFP can be used as a reporter to reliably identify thyroid precursors in a mixed population of differentiated ES cells. The experimental approach described in this report emphasizes the power of appropriate stimulating factors in the generation of differentiated thyroid cells from ES cells. Within this system, the level of GFP protein expression was significantly higher in serum-free/TSH-containing cultures than in cultures containing serum alone. TSH also induced the expression of TSHR, suggesting the dependence of TSHR on TSH itself. This finding is consistent with our previous studies of the wild-type CCE ES cell line (20).
However, we found that thyroid gene expression patterns varied between TSHR+/− and CCE ES cells induced to differentiate into the thyrocyte lineage in vitro. For example, after 6 d of differentiation, EBs derived from CCE ES cells expressed the TSHR gene when cultured in the presence of serum (20). In contrast, 6-d-old EBs arising from TSHR+/− ES cells did not express the TSHR gene. One possible interpretation of these data is that differentiation into the thyrocyte lineage varies among different mouse ES cell lines; TSHR+/− ES cells and their wild-type counterpart W9.5 ES cells were derived from a substrain of 129/Sv-+p+Tyr-cMgfSl-J/+ mice (21), but CCE ES cells were derived from 129/Sv mice (29). Although various ES cells are regarded as equally pluripotent, a number of recent studies have indicated that individual mouse and human ES cell lines exhibit varying degrees of efficiency when called upon to differentiate into specific cell types. For example, Kramer et al. (30) reported that the ES cell lines CCE, BLC6, D3, E14, and R1, all of which express the stem cell marker Oct4, exhibit varying degrees of spontaneous chondrogenic differentiation. D3, R1, E14, and CCE were poorly able to differentiate into mature chondrocytes, but the BLC6 ES cell line forms chondrogenic nodules very efficiently (30). Likewise, Wobus et al. (31) reported differences in the ability of various ES cell lines to differentiate into cardiomyocytes and skeletal muscle cells. The differentiation capacity of an ES cell line might be dependent upon the mouse strain from which it was established, or it could be due to their origins from different blastocystic precursors (32). Our results suggest that mouse ES cell lines of different origins vary in their ability to differentiate into thyrocytes. They also suggest that within a specific ES cell line, it is possible to select for the ability to differentiate into the thyrocyte lineage.
We found that GFP and TSHR were expressed in a dynamic and temporal fashion in early EBs. The temporally restricted expression patterns of GFP and TSHR were first seen after 2 d of differentiation, but may not be maintained in SR medium supplemented with TSH until after an additional 4 days. These findings indicate that TSHR-expressing cells require serum and cannot be maintained with continuous TSH stimulation alone. It is conceivable that the presence of other growth factors may also be important to maintain TSHR expression. However, interpretation of these studies is complicated by the fact that the analysis was carried out in mixed lineage populations. Nevertheless, the ability to separate TSHR-expressing populations (via GFP) has enabled us to further characterize these populations and ultimately follow their differentiation to a thyroid fate.
Our immunofluorescent findings showed that thyroid follicle-like clusters coexpress TSHR and NIS, and NIS is properly targeted to plasma membranes in these clusters. After 21 d of differentiation, the cells treated with TSH showed I− uptake activity, whereas cells maintained in the absence of TSH did not. This suggests that the presence or absence of TSH in the growth medium significantly influences NIS function. Because coexpression of NIS and TSHR is a unique characteristic of thyroid cells, we believe that it is highly likely that these structures represent thyroid follicles. This observation is of particular interest because immortalized thyroid cell lines, such as FRTL-5 cells, cannot form follicles when grown as monolayers in culture (33, 34). Characterization of this newly described capability of ES cell-derived thyrocytes to form putative neofollicles will allow detailed analyses of the signals involved in thyroid development and function. For example, the fact that some GFP+NIS+ cell clusters do not form follicles suggests that culture conditions are not yet optimal. Exploration of the differences between various thyroid cell structures may help us to learn how to develop proliferation and differentiation protocols to efficiently generate functional thyrocytes.
In the present study putative thyroid follicular cells accounted for only a small percentage (~1%) of differentiated ES cells. A comparison of cell clusters in sorted and non-sorted cultures highlighted the importance of providing appropriate cell populations for the generation of differentiated thyrocyte progeny from ES cells. In addition, our sorting experiments demonstrated that the application of Matrigel to GFP-positive cells was essential for cell survival and differentiation after reaggregation. Matrigel matrix is a continuous sheet of specialized extracellular matrix that has been shown to play a crucial role in cell differentiation through rearrangement of the cytoskeletal network. It has been used for the attachment and differentiation of thyroid follicular cells (35). Because cell to cell communication is necessary for the formation of thyroid follicles, it is possible that the intrinsic properties of ES cells, in association with Matrigel and exogenous soluble factors from neighboring cells, provide the necessary framework for mature thyroid follicular cell differentiation. Previously, Mulcahy et al. (36) reported that FRTL-5 cells cultured as three-dimensional multicellular spheroids in the presence of TSH developed either one or more large central lumina (similar to thyroid follicles in vivo) or multiple small follicular cavities. The spheroids also had ultrastructural features characteristic of high cellular activity: numerous microvillous projections, cytoplasmic vacuoles, and vesicles containing Tg-like materials (36). With respect to the present study, it is not clear whether NIS was correctly polarized at the basolateral surface or whether microvillous projections and junctions mimicking those found in the thyroid gland in vivo were formed. To further characterize these populations, we are attempting to isolate and enrich the presumed thyroid follicular cells from the mixed populations of differentiated ES cells. Additional ultrastructural analyses might help us understand how cell to cell and cell to matrix interactions influence stem cells during thyroid development. Ultimately, functional analysis of the ES cell-derived thyrocytes will require transplantation into animal models.
Using RT-PCR analysis, we demonstrated that ES cell-derived thyroid follicular cells expressed a number of thyroid-specific genes, including PAX8, NIS, TPO, and TSHR. However, the expression of Tg was not detectable, supporting the idea that Tg synthesis is independent of TSH and suggesting that additional factors must control Tg expression and thyroid hormone synthesis. This observation was not unexpected; we previously observed that although human Tg secretion was under TSH control in human thyroid monolayer cells in vitro, thyroid follicle formation and Tg expression could proceed in the absence of TSH (37–41). In studies of CCE ES cells, basal Tg expression in the absence of serum was also minimal, indicating that in addition to TSH, a variety of trophic factors may be required for full Tg expression (20). Finally, Postiglione et al. (42) used the TSH mutant (pitdw/pitdw) mouse to demonstrate that TSH is not required for Tg synthesis in vivo. The molecular trigger for Tg production remains to be determined, but ES cells clearly provide a useful new model for the investigation of pertinent signaling pathways.
In summary, we have established a new method for generating thyroid follicular cells from mouse ES cells. Establishment of this system provides an attractive model for future experiments aimed at delineating important parameters and conditions required for thyroid gland development. Thyroid stem cells may also provide models for understanding thyroid cancer development, because both papillary and follicular thyroid cancers are frequently accompanied by distinct oncogenic rearrangements. In view of the fact that the differentiation of ES cells varies in some respects between mice and humans, findings in mice will need to be confirmed in human thyroid stem cells. Overall, these studies may have important implications for our understanding, not only of normal thyroid development, but also of the mechanisms underlying human disease.
Acknowledgments
We thank Dr. Nancy Carrasco (Albert Einstein College of Medicine) for the gift of anti-NIS antibody and the NIS/MDCK cell line. We also thank Drs. Takao Ando, Rauf Latif, and Samira Daniel for helpful suggestions.
Footnotes
M.C.A., M.L., A.K., G.K., T.F.D., and R.Y.L. have nothing to declare.
This work was supported by National Institutes of Health Grant R01-DK-068057-01A1 (to R.Y.L.).
References
- 1.Ambesi-Impiombato FS, Coon HG. Thyroid cells in culture. Int Rev Cytol. 1979;(Suppl):163–172. doi: 10.1016/s0074-7696(08)60619-1. [DOI] [PubMed] [Google Scholar]
- 2.Fayet G, Hovsepian S. Isolation of a normal human thyroid cell line: hormonal requirement for thyroglobulin regulation. Thyroid. 2002;12:539–546. doi: 10.1089/105072502320288375. [DOI] [PubMed] [Google Scholar]
- 3.Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Prog Nucleic Acids Res Mol Biol. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 4.Tasevski V, Benn D, Peters G, Luttrell B, Simpson A. The Fischer rat thyroid cell line FRTL-5 exhibits a nondiploid karyotype. Thyroid. 1998;8:623–626. doi: 10.1089/thy.1998.8.623. [DOI] [PubMed] [Google Scholar]
- 5.Ealey PA, Emmerson JM, Bidey SP, Marshall NJ. Thyrotrophin stimulation of mitogenesis of the rat thyroid cell strain FRTL-5: a metaphase index assay for the detection of thyroid growth stimulators. J Endocrinol. 1985;106:203–210. doi: 10.1677/joe.0.1060203. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 7.Keller GM. In vitro differentiation of embryonic stem cells. Curr Opin Cell Biol. 1995;7:862–869. doi: 10.1016/0955-0674(95)80071-9. [DOI] [PubMed] [Google Scholar]
- 8.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, Soreq H, Benvenisty N. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng X, Cai J, Chen J, Luo Y, You ZB, Fotter E, Wang Y, Harvey B, Miura T, Backman C, Chen GJ, Rao MS, Freed WJ. Dopaminergic differentiation of human embryonic stem cells. Stem Cells. 2004;22:925–940. doi: 10.1634/stemcells.22-6-925. [DOI] [PubMed] [Google Scholar]
- 10.Li T, Zheng J, Xie Y, Wang S, Zhang X, Li J, Jin L, Ma Y, Wolf DP, Zhou Q, Ji W. Transplantable neural progenitor populations derived from rhesus monkey embryonic stem cells. Stem Cells. 2005;23:1295–1303. doi: 10.1634/stemcells.2005-0026. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura F, Yoshikawa M, Kanda S, Nonaka M, Yokota H, Shiroi A, Nakase H, Hirabayashi H, Ouji Y, Birumachi J, Ishizaka S, Sakaki T. Potential use of embryonic stem cells for the treatment of mouse Parkinsonian models: improved behavior by transplantation of in vitro differentiated dopaminergic neurons from embryonic stem cells. Stem Cells. 2003;21:171–180. doi: 10.1634/stemcells.21-2-171. [DOI] [PubMed] [Google Scholar]
- 12.Meyer JS, Katz ML, Maruniak JA, Kirk MD. Embryonic stem cell-derived neural progenitors incorporate into degenerating retina and enhance survival of host photoreceptors. Stem Cells. 2006;24:274–283. doi: 10.1634/stemcells.2005-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brustle O, Jones KN, Learish RD, Karram K, Choudhary K, Wiestler OD, Duncan ID, McKay RD. Embryonic stem cell-derived glial precursors: a source of myelinating transplants. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]
- 14.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 15.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 16.Kouskoff V, Lacaud G, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc Natl Acad Sci USA. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacaud G, Gore L, Kennedy M, Kouskoff V, Kingsley P, Hogan C, Carlsson L, Speck N, Palis J, Keller G. Runx1 is essential for hematopoietic commitment at the hemangioblast stage of development in vitro. Blood. 2002;100:458–466. doi: 10.1182/blood-2001-12-0321. [DOI] [PubMed] [Google Scholar]
- 18.Yamada T, Yoshikawa M, Kanda S, Kato Y, Nakajima Y, Ishizaka S, Tsunoda Y. In vitro differentiation of embryonic stem cells into hepatocyte-like cells identified by cellular uptake of indocyanine green. Stem Cells. 2002;20:146–154. doi: 10.1634/stemcells.20-2-146. [DOI] [PubMed] [Google Scholar]
- 19.Shirahashi H, Wu J, Yamamoto N, Catana A, Wege H, Wager B, Okita K, Zern MA. Differentiation of human and mouse embryonic stem cells along a hepatocyte lineage. Cell Transplant. 2004;13:197–211. doi: 10.3727/000000004783984016. [DOI] [PubMed] [Google Scholar]
- 20.Lin RY, Kubo A, Keller GM, Davies TF. Committing embryonic stem cells to differentiate into thyrocyte-like cells in vitro. Endocrinology. 2003;144:2644–2649. doi: 10.1210/en.2002-0122. [DOI] [PubMed] [Google Scholar]
- 21.Marians RC, Ng L, Blair HC, Unger P, Graves PN, Davies TF. Defining thyrotropin-dependent and -independent steps of thyroid hormone synthesis by using thyrotropin receptor-null mice. Proc Natl Acad Sci USA. 2002;99:15776–15781. doi: 10.1073/pnas.242322099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price PJ, Goldsborough MD, Tilkins ML. Embryonic stem cell serum replacement. International Patent Application WO98/30679 1998 [Google Scholar]
- 23.Dohan O, Gavrielides MV, Ginter C, Amzel LM, Carrasco N. Na+/I− symporter activity requires a small and uncharged amino acid residue at position 395. Mol Endocrinol. 2002;16:1893–1902. doi: 10.1210/me.2002-0071. [DOI] [PubMed] [Google Scholar]
- 24.Rogers MB, Hosler BA, Gudas LJ. Specific expression of a retinoic acid-regulated, zinc-finger gene, Rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development. 1991;113:815–824. doi: 10.1242/dev.113.3.815. [DOI] [PubMed] [Google Scholar]
- 25.Bang AG, Goulding MD. Regulation of vertebrate neural cell fate by transcription factors. Curr Opin Neurobiol. 1996;6:25–32. doi: 10.1016/s0959-4388(96)80005-5. [DOI] [PubMed] [Google Scholar]
- 26.Simon MC. Transcription factor GATA-1 and erythroid development. Proc Soc Exp Biol Med. 1993;202:115–121. doi: 10.3181/00379727-202-43519a. [DOI] [PubMed] [Google Scholar]
- 27.Yamane T, Kunisada T, Yamazaki H, Era T, Nakano T, Hayashi SI. Development of osteoclasts from embryonic stem cells through a pathway that is c-fms but not c-kit dependent. Blood. 1997;90:3516–3523. [PubMed] [Google Scholar]
- 28.Parmentier M, Libert F, Maenhaut C, Lefort A, Gerard C, Perret J, Van Sande J, Dumont JE, Vassart G. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 29.Robertson E, Bradley A, Kuehn M, Evans M. Germ-line transmission of genes introduced into cultured pluripotential cells by retroviral vector. Nature. 1986;323:445–448. doi: 10.1038/323445a0. [DOI] [PubMed] [Google Scholar]
- 30.Kramer J, Hegert C, Hargus G, Rohwedel J. Mouse ES cell lines show a variable degree of chondrogenic differentiation in vitro. Cell Biol Int. 2005;29:139–146. doi: 10.1016/j.cellbi.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Wobus A, Rohwedel J, Strubing C, Shan J, Adler K, Maltsev V. In vitro differentiation of embryonic stem cells. In: Klug S, Thiel R, editors. Methods in developmental toxicology. Oxford: Blackwell; 1997. pp. 1–17. [Google Scholar]
- 32.Gardner RL, Brook FA. Reflections on the biology of embryonic stem (ES) cells. Int J Dev Biol. 1997;41:235–243. [PubMed] [Google Scholar]
- 33.Burgi-Saville ME, Reut B, Gerber H, Peter HJ, Paulsson M, Kaempf J, Simon F, Marti U, Gerber H, Burgi U. Alginate gel culture allows the retention of extracellular matrix and follicular structure of rat thyroid tissue but does not lead to the formation of follicles by FRTL-5 cells. Thyroid. 1998;8:1147–1155. doi: 10.1089/thy.1998.8.1147. [DOI] [PubMed] [Google Scholar]
- 34.Fusco A, Berlingieri MT, Di Fiore PP, Portella G, Grieco M, Vecchio G. One- and two-step transformations of rat thyroid epithelial cells by retroviral oncogenes. Mol Cell Biol. 1987;7:3365–3370. doi: 10.1128/mcb.7.9.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bechtner G, Schopohl D, Rafferzeder M, Gartner R, Welsch U. Stimulation of thyroid cell proliferation by epidermal growth factor is different from cell growth induced by thyrotropin or insulin-like growth factor I. Eur J Endocrinol. 1996;134:639–648. doi: 10.1530/eje.0.1340639. [DOI] [PubMed] [Google Scholar]
- 36.Mulcahy RT, Rosenkrans WA, Jr, Penney DP, Cooper RA. The growth and morphology of FRTL-5 thyroid epithelial cells grown as multicellular spheroids in vitro. In Vitro Cell Dev Biol. 1985;21:513–520. doi: 10.1007/BF02620844. [DOI] [PubMed] [Google Scholar]
- 37.Feldman A, Singh A, Diamond EJ, Schwartz AE, Friedman EW, Davies TF. Variability in production and immunoreactivity of in-vitro secreted human thyroglobulin. Clin Endocrinol (Oxf) 1987;27:691–701. doi: 10.1111/j.1365-2265.1987.tb02953.x. [DOI] [PubMed] [Google Scholar]
- 38.Huber GK, Davies TF. Human fetal thyroid cell growth in vitro: system characterization and cytokine inhibition. Endocrinology. 1990;126:869–875. doi: 10.1210/endo-126-2-869. [DOI] [PubMed] [Google Scholar]
- 39.Martin A, Valentine M, Unger P, Lichtenstein C, Schwartz AE, Friedman EW, Shultz LD, Davies TF. Preservation of functioning human thyroid organoids in the scid mouse. I. System characterization. J Clin Endocrinol Metab. 1993;77:305–310. doi: 10.1210/jcem.77.2.8345031. [DOI] [PubMed] [Google Scholar]
- 40.Graves PN, Davies TF. Thyrotropin regulates thyroglobulin mRNA splicing and differential processing. Mol Cell Endocrinol. 1993;93:213–218. doi: 10.1016/0303-7207(93)90126-5. [DOI] [PubMed] [Google Scholar]
- 41.Valentine M, Martin A, Unger P, Katz N, Shultz LD, Davies TF. Preservation of functioning human thyroid “organoids” in the severe combined immunodeficient mouse. III. Thyrotropin independence of thyroid follicle formation. Endocrinology. 1994;134:1225–1230. doi: 10.1210/endo.134.3.8119163. [DOI] [PubMed] [Google Scholar]
- 42.Postiglione MP, Parlato R, Rodriguez-Mallon A, Rosica A, Mithbaokar P, Maresca M, Marians RC, Davies TF, Zannini MS, De Felice M, Di Lauro R. Role of the thyroid-stimulating hormone receptor signaling in development and differentiation of the thyroid gland. Proc Natl Acad Sci USA. 2002;99:15462–15467. doi: 10.1073/pnas.242328999. [DOI] [PMC free article] [PubMed] [Google Scholar]


