Abstract
The dendritic and axonal morphology of rat subicular neurons was studied in single cells labeled with Neurobiotin. Electrophysiological classification of cells as intrinsic burst firing or regular spiking neurons was correlated with morphologic patterns and cell locations. Every cell had dendritic branches that reached the outer molecular layer, with most cells having branches that reached the hippocampal fissure. All but two pyramidal cells had axon collaterals that entered the deep white matter (alveus). Branching patterns of apical dendrites varied as a function of the cell’s soma location along the fissure–alveus axis of the cell layer. The first major dendritic branch point for most cells occurred at the superficial edge of the cell layer giving deep cells long primary apical dendrites and superficial cells short or absent primary apical dendrites. In contrast, basal dendritic arbors were similar across cells regardless of cell position. Apical and basal dendrites of all cells had numerous spines. Superficial and deep cells also differed in axonal collateralization. Deep cells (mostly intrinsically bursting [IB] class) had one or more ascending axon collaterals that typically remained within the region circumscribed by their apical dendrites. Superficial cells (mostly regular spiking [RS] class) tended to have axon collaterals that reached longer distances in the cell layer. Numerous varicosities and axonal extensions were present on axon collaterals in the cell layer and in the apical dendritic region, suggesting intrinsic connectivity. Axonal varicosities and extensions were found on axons that entered presubiculum, entorhinal cortex or CA1, supporting the notion that these were projection cells. Local collaterals were distinctly thinner than collaterals that would leave the subiculum, suggesting little or no myelin on local collaterals and some myelin on efferent fibers. We conclude that both IB and RS classes of subicular principal cells make synaptic contacts in and apical to the cell layer. Based on the patterns of axonal arborization, we suggest that subiculum has at least a crude columnar and laminar architecture, with ascending collaterals of deep cells forming columns and broader axonal arbors of superficial cells serving to distribute activity across multiple columns.
Indexing terms: parahippocampal region, hippocampus, limbic cortex, excitatory synapse
The subiculum has received a growing amount of experimental attention for numerous reasons. Among these reasons are its position as a major relay at the end of the trisynaptic pathway, a principal circuit component connecting the entorhinal cortex with the hippocampus (for reviews, see Witter et al., 1989; Naber et al., 2000). As anatomic details continue to emerge, it has become clear that subiculum is a component of many different circuits involving parahippocampal and hippocampal structures (reviewed in Naber et al., 2000). As this complex connectivity is examined, efforts have been made to elucidate patterns in the organization of inputs and outputs that would help define subiculum’s role as a relay, an integrator, or a distributor of activity involving hippocampal and parahippocampal cortices.
Complicating this effort have been reports that the population of subicular pyramidal cells is functionally separable into at least two groups. In recordings from single neurons, some cells are capable of firing a burst of action potentials that includes a calcium spike (Mason, 1993; Mattia et al., 1993; Stewart and Wong, 1993; Taube, 1993), whereas other cells fire only regular sodium spikes (Mason, 1993; Stewart and Wong, 1993; Taube, 1993). The firing patterns are used to define intrinsically bursting (IB) and regular spiking (RS) cell classes. Attempts to identify morphologic differences between cell classes have only showed that both classes consist of spiny pyramidal cells (Mason, 1993; Taube, 1993; Greene and Totterdell, 1997). Without a clear morphologic difference, classic anatomic studies of subicular circuitry may not reveal patterns of connections that are specific for one or another functional class of cells. On the other hand, the functional division is not without some controversy. This uncertainty arises because the subthreshold properties of the cells are only subtly different (Mason, 1993; Stewart and Wong, 1993; Taube, 1993; Behr et al., 1996; Greene and Totterdell, 1997) and, more importantly, because it is possible to convert the firing patterns of some cells. This behavior has led some to suggest that the firing of all subicular cells can be interconverted with appropriate membrane polarization or drug treatment (Jung et al., 1999).
Our position has been that the classes are separate. Any cells that can be made to burst have been called intrinsically bursting by us (e.g., Stewart and Wong, 1993; Stewart, 1997). Regular spiking cells, in our hands, cannot be converted to burst firing cells. They can fire at high rates with appropriately strong stimuli (Stewart, 1997), and their outputs appear to be completely separate from the outputs of burst firing cells (Stewart, 1997).
Although Greene and Totterdell (1997) did not find a morphologic feature to correlate with electrophysiological cell types, they did find that the two types of cells were distributed differently within the cell layer. The two functional cell classes were also differentially responsive to somatostatin (Greene and Mason, 1996) and NADPH reactivity (Valtschanoff et al., 1993; Greene et al., 1997). Most recently, differences in nitric oxide synthase immunoreactivity further support at least a division of the subicular cell population (Lin and Totterdell, 1998).
We sought to re-examine the morphologic properties of electrophysiologically classified subicular pyramidal cells with several goals in mind. First, there are good reasons to suspect that some morphologic differences will be found. Lorente de Nó (1934) described several different kinds of pyramidal cells in his seminal piece on hippocampal cell morphology. It is possible that the data are so few on subicular cell morphology and electrophysiology that patterns simply cannot be substantiated. Second, with evidence that the outputs of different pyramidal cell classes may be different, we wanted to examine the axonal arbors of identified cells. Not only would this potentially serve as an anatomical correlate of electrophysiological cell classes, but there are no data on the local connectivity of subicular pyramidal neurons. An understanding of the local connectivity is critical for understanding the subiculum as a seizure focus (Behr and Heinemann, 1996) and, more importantly, how subiculum may function as a relay, an integrator, and a distributor of activity in the multitude of hippocampal formation circuits. Lastly, electrophysiological properties, dendritic morphology, or patterns of axonal arborization may clarify boundaries between subregions of the subiculum (Köhler, 1985; Witter et al., 1989; Naber and Witter, 1998; Naber et al., 2000).
MATERIALS AND METHODS
Slice techniques
Male Sprague-Dawley albino rats (120–450 g; mean, 275 g; median, 265 g; age range, 1–3 months) were anesthetized with halothane (inhalation, sufficient to cause unconsciousness) and decapitated. Each brain was removed from the skull, bisected, and placed in ice cold artificial CSF. Horizontal, coronal, parasagittal, or angled transverse slices (400 μm) were cut by using a Vibroslice system and perfused with artificial CSF (in mM: NaCl 124, KCl 5, CaCl2 2, MgCl2 1.6, NaHCO3 26, and glucose 10; pH = 7.4 in an atmosphere of 5% CO2 in O2). Slices were maintained in an interface chamber at 35–36°C. Some cells were recorded and labeled in subsections of full slices. These subsections typically were prepared to isolate the subiculum from surrounding regions. The procedures are described in the Materials and Methods section of the companion paper.
Intracellular recording techniques
Intracellular recording electrodes were pulled from 1-mm-diameter capillary glass and filled with 3 M potassium acetate (tip resistances = 85–120 MΩ) or a solution containing 2% Neurobiotin in 2 M KAc (tip resistances = 95–180 MΩ). Intracellularly recorded signals were amplified by a high input impedance amplifier with facilities for current injection and capacitance compensation (Neurodata IR-283, Cygnus Technology, Delaware Water Gap, PA; Axoclamp 2A, Axon Instruments, Foster City, CA). Somatic intracellular recordings were considered suitable for analysis if they had stable resting potentials of at least −60 mV, input resistances >20 MΩ, and overshooting action potentials. These criteria yield impalements that have been shown to be adequate for assessing the electrophysiological classification of neurons as intrinsic bursting or regular spiking and for labeling with Neurobiotin (e.g., Mason, 1993; Mattia et al., 1993; Stewart and Wong, 1993; Greene and Totterdell, 1997). Dendritic recordings from subicular bursting neurons were presumed on the basis of the cells’ responses to current injection and confirmed for cells labeled with Neurobiotin by locating the cell body relative to the recording location (e.g., Funahashi et al., 1999). All electrodes were placed under direct visual guidance with the dissecting microscope. Recording locations were measured in relation to readily observable landmarks (deep white matter, pial surface, layer II pre-subiculum) for later measurements on histologic specimens.
Depolarizing and hyperpolarizing current pulses were used to elicit firing and subthreshold membrane potential changes in each recorded cell. Cells were classified as intrinsically bursting or regular spiking, depending on their subthreshold properties and, most importantly, their firing properties. However, our classification scheme is simple. If a burst, as we defined it above, can be evoked by current injection, irrespective of the other conditions such as the membrane potential, that cell is considered a bursting neuron. For a cell to be a regular spiking cell, it can never be seen to fire a burst. Even if the cell fires repetitively at 200 Hz for several spikes during a massive synaptic excitation, we expect to be able to suppress individual spikes with hyperpolarizing current injection to demonstrate that the series of spikes is not a unitary event as in the bursting cells (Stewart, 1997; Harris and Stewart, companion paper).
Neurobiotin labeling
After recording, Neurobiotin was injected into some cells with 2–4 nA depolarizing current pulses (150-msec duration at 3.3 Hz for 20–30 minutes). The time that slices were maintained in the interface chamber, between application of Neurobiotin tracer and placement of the brain slice in fixative, ranged from 30 to 120 minutes. Slices were fixed in 2.5% phosphate buffered glutaraldehyde (pH 7.4) or 2% paraformaldehyde and 0.2% picric acid in 0.1 M phosphate buffer (pH 7.4) from 1 to 30 days at 4°C. Before resectioning, slices were rinsed in phosphate buffer and cryoprotected in 30% sucrose overnight. Frozen sections (40–50 μm thick) were cut from the fixed tissue and kept in phosphate buffered saline (pH 7.4). Sections were treated with 1% H2O2 for 20–30 minutes and Triton X-100 (0.4–0.5% in buffer) for 2 to 3 hours. Sections were then incubated in the Vectastain ABC Reagent (PK-6100, Vector Labs, Burlingame, CA) overnight, and reacted 5–15 minutes with diaminobenzidine (0.5 mg/ml working concentration, nickel-intensified; SK-4100, Vector Labs, Burlingame, CA) and H2O2 (0.003%). Sections were mounted from 0.05 M phosphate buffer (pH 7.4) onto gelatin-coated slides and counterstained with thionin (0.25–1%). After dehydration through a graded series of water/ethanol, tissue was cleared with xylene and cover-slipped with Permount. Tissue shrinkage from fixation was estimated to be less than or equal to 15% based on measurements of the fresh and fixed slice diameters.
Extracellular biocytin
In some experiments, multiple neurons were labeled with biocytin applied extracellularly. Crystals of biocytin-HCl (Sigma, St. Louis, MO) or a broken capillary glass microelectrode (tip diameter <10 μm) containing 8.4% biocytin in water were used to label cells in 400-μm brain slices. Biocytin was applied from the pipette by diffusion from the pipette tip or iontophoretically by using 3 nA current pulses (50% duty cycle) for 1–3 minutes. The time between tracer application and fixation of the tissue specimen ranged from 1 to 3 hours. Tissue was processed in the same manner as slices containing individually labeled neurons.
Imaging and reconstruction of labeled neurons
Digital photomicrographs of labeled cells were obtained with a high-resolution 3-chip CCD camera (Sony DKC5000, Sony Electronics; resolution: 1,544 × 1,120 × 3 pixels) connected to a Macintosh OS–based computer for image storage. This system has been described previously (Martínez-Marcos et al., 1999).
Drawings and reconstructions of labeled axons and dendrites were made with a standard drawing tube or by using a Neurolucida system (Zeiss microscope, precision motorized microscope stage, Lucivid, Neurolucida, and Neuroexplorer software, Microbrightfield, Colchester, VT). Neurolucida tracings were made at 63× (glycerine immersion, NA = 1.25). Reconstructions of single neurons were made from three to six sections. Dendritic spines and axonal extensions or varicosities (e.g., Shepherd and Harris, 1998) were not drawn. These are illustrated only in the photomicrographs.
Measurements of soma, dendrite, or axon diameter, or extent were made from reconstructed digital images taken at 40× or 100× by using the measurement tools in Canvas 7 software (Deneba Systems, Miami, FL). Some measurements were made by using Neurolucida software for those cells drawn with the Neurolucida system.
RESULTS
Our morphologic data set consists of 79 subicular neurons that were labeled with Neurobiotin after intracellular recording and at least partially recovered after processing the tissue sections. Cells were primarily collected from ventral horizontal slices (n = 56 neurons from 56 slices), but cells were also taken from coronal (n = 4 cells), parasagittal (n = 2 cells), or transverse slices angled 15–35 degrees off the sagittal plane (n = 17 cells: 15 degrees, 5 cells; 20 degrees, 2; 25 degrees, 8; 30 degrees, 1; 35 degrees, 1). Subiculum in thionin-stained horizontal and angled transverse slices is shown in Figure 1.
Fig. 1.
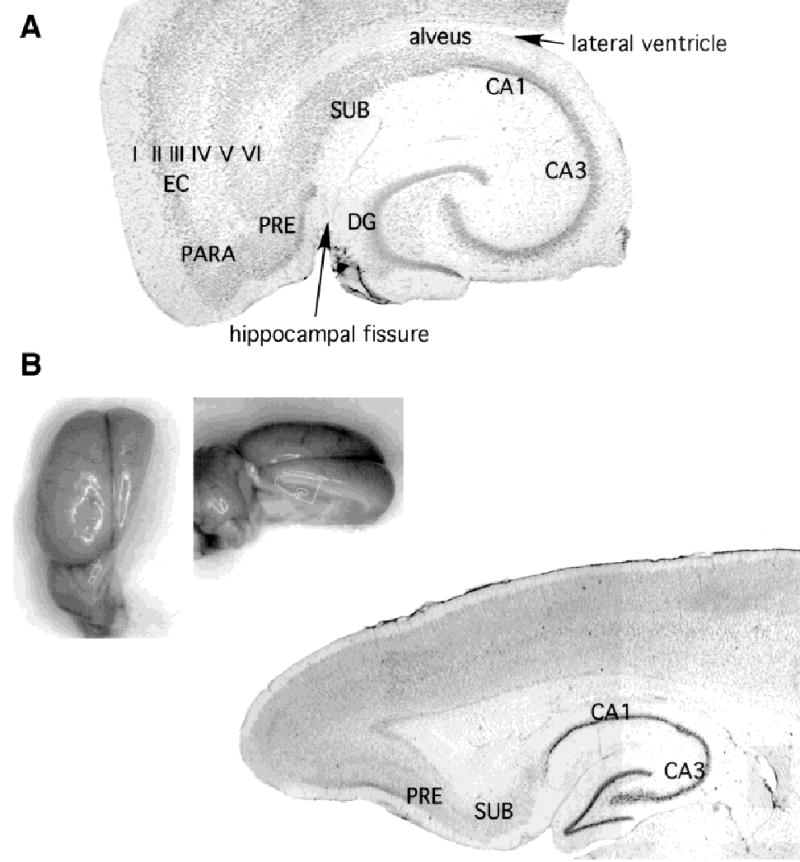
Subiculum in horizontal and near-parasagittal rat brain slices. Thionin-stained sections to illustrate the location of subiculum in relation to other hippocampal formation structures in horizontal (A) and an angled transverse (B) slice. Abbreviations are as follows: EC, entorhinal cortex (the layers of entorhinal cortex are labeled with roman numerals); PARA, parasubiculum; PRE, presubiculum; SUB, subiculum, CA1 and CA3, Ammon’s horn; DG, dentate gyrus. The locations of the lateral ventricle and the hippocampal fissure are labeled in A. Insets for B show plane of section at approximately 20 degrees off the sagittal plane. No differences in the radial architecture of subicular pyramidal cell dendritic arbors or local axon collaterals were detected as a function of the plane of section for most cells, provided the plane of section captured the axis of the deep cell apical dendrites. The apical dendrites of some superficial cells were found to tip off this axis (e.g., Fig. 6).
Cells were recorded and labeled in horizontal sections from ventral subiculum and angled sections from dorsal subiculum between the coronal and parasagittal planes. Cells from dorsal and ventral portions of subiculum were similar electrophysiologically and morphologically. Specifically, there were no differences in the radial architecture of the dendritic arbors or local axonal arbors to suggest an asymmetrical cell morphology. For these reasons, the data from all planes of section are discussed together.
Overall axonal trajectories
A total of 51 of 53 cells with visible axons were found to have at least one axon collateral that entered the deep white matter of the alveus/angular bundle. These cells included 34 intrinsic bursting, 9 regular spiking, 4 putative regular spiking, and 4 unclassified cells. In several of these cells, these collaterals were traced in serial sections for over a millimeter in the white matter under CA1 or into presubiculum. Other cells were found to have axon collaterals that climbed obliquely back across the subicular cell layer to enter the superficial layers of presubiculum or the apical dendritic zone of CA1 (this projection is discussed in more detail in Harris and Stewart, 2001).
Electrophysiological classification
Morphologically identified cells were also classified on the basis of their electrophysiological characteristics determined during the intracellular recording. In particular, cells were classified as intrinsically bursting (IB) or as regular spiking (RS) as we and others have done previously (Mason, 1993; Stewart and Wong, 1993; Taube, 1993; Behr et al., 1996; Greene and Totterdell, 1998).
We were able to electrophysiologically classify most, but not all morphologically identified cells. Forty-seven cells were identified as intrinsically bursting cells, and 17 were regular spiking cells. Previous reports of electrophysiologically classified subicular neurons give the fraction of bursting neurons between approximately 54% (Behr et al., 1996) and 100% (Mattia et al., 1993). The fraction of burst firing neurons reported in this study (47 of 64; 73%) is comparable to previous reports: 74% (rat, Mason, 1993), 69% (rat, Taube, 1993), 79% (rat, Stewart, 1997), 66% (guinea pig, Stewart and Wong, 1993), 69% of morphologically identified cells, and 74% of all recorded cells (rat, Greene and Totterdell, 1997).
Seven other cells from our dataset had less stable recordings, and although six appeared to be regular spiking and one intrinsic bursting, we have not tabulated these cells in the electrophysiological class totals (e.g., Fig. 2). Two reasons prevented us from having both morphologic and electrophysiological identifications of all cells. First, in 12 instances, two cells were labeled with Neurobiotin even though a single cell was recorded. In these cases, we could not be certain which cell was the impaled cell. Interestingly, all 12 recorded neurons were intrinsic bursting neurons (two examples are shown in Fig. 4). Evidence of a membrane defect suggestive of an impalement site (e.g., Funahashi et al., 1999) was not found, and measured positions of electrodes relative to tissue landmarks could not discriminate between cells. In one pair, shown in Figure 4, one cell was clearly darker, suggesting more label in one this cell. Second, eight cells had what we referred to as equivocal recordings. In these cells, we were unable to elicit a burst of action potentials in response to current injection, although subthreshold and spike properties resembled bursting neurons or recordings were particularly noisy, but the recovered morphology was excellent.
Fig. 2.
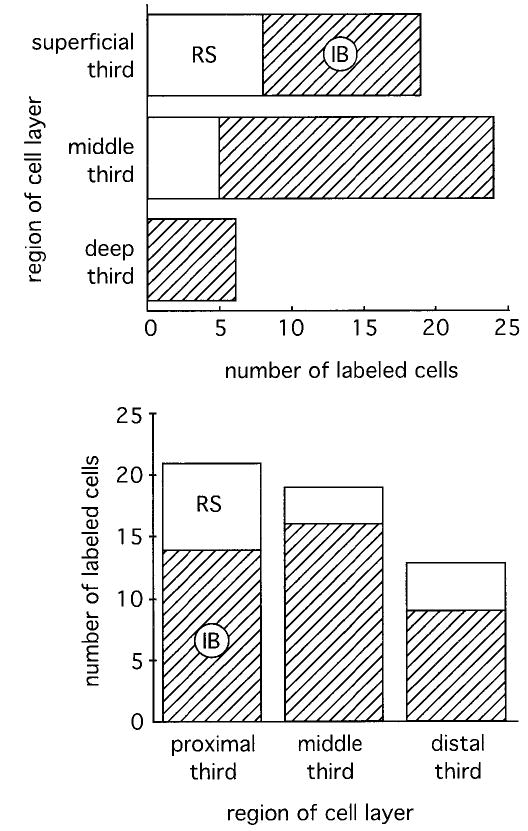
Histograms of the distribution of electrophysiologically identified cell classes within the cell layer along the superficial to deep axis (top) and the CA1 to presubiculum axis (bottom). Solid white is used to represent the regular spiking (RS) cell class, and hatched areas represent the intrinsic bursting (IB) cells. Most of the regular spiking cells were found in the superficial part of the cell layer, but not all superficial cells are regular spiking cells. In terms of their distribution along the CA1–presubiculum axis, we found more regular spiking cells near CA1 (proximal) than at the center or near presubiculum (distal). No statistical differences were found in the distributions of cells along either axis. Top, Fisher’s exact test; bottom, chi-square test.
Fig. 4.
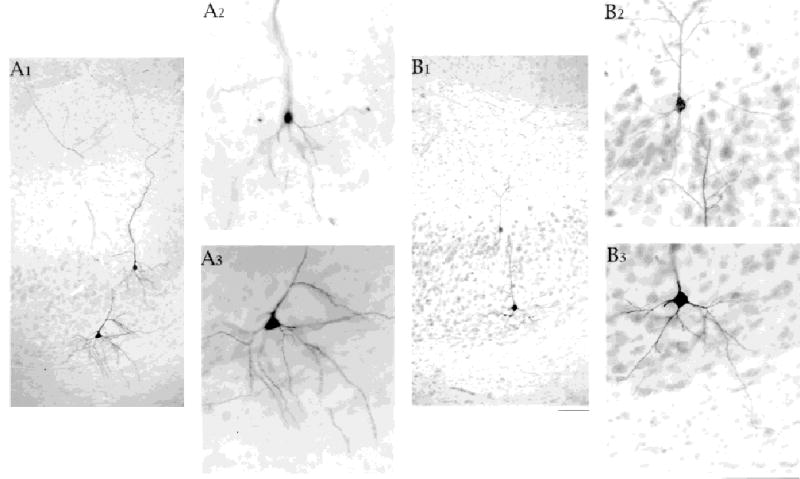
Basal dendrites of superficially and deeply located cells extend similar distances from the cell body. Two cell pairs shown. One cell in each pair was an intrinsically bursting cell. B: We believe the bursting cell is the deep cell because of the difference in staining intensity. A: A1 is a 10× photomicrograph of one cell pair showing the basal dendritic arbor of the superficial cell confined to the cell layer and the basal dendrites of the deep cell reaching into the neuropil below the cell layer. A2 and A3 are 25× photomicrographs of the two cells. B1 is a 10× photomicrograph of a second cell pair illustrating similar properties of the dendrites. In B2, the basal dendrites of the superficial cell and the apical dendrites of the deep cell are in close proximity. Scale bars = 100 μm in B1 (applies to A1,B1), in B3 (applies to A2,A3,B2,B3).
Somata and apical dendrites
All cells were classified as principal cells and pyramidal cells, although somata were triangular, ovoid, or round. Our criteria for classification of cells as principal cells and pyramidal cells consisted of the following: (1) a primary apical dendrite that was distinguishable from basal dendrites by position and diameter; (2) dendritic spines; (3) one or more axon collaterals that entered the alveus or an adjacent brain region; (4) apical dendritic branches that reached the fissure; and (5) electrophysiological properties that matched properties previously reported for subicular principal or pyramidal cells (e.g., Mason, 1993; Stewart and Wong, 1993; Taube, 1996; Behr et al., 1996; Greene and Totterdell, 1997), not interneurons. All cells, except several at the superficial edge of the cell layer had a single primary apical dendrite that was 2–5 times larger in diameter than any of the basal dendrites. The cells that did not possess a single pronounced apical dendrite, met all of the other criteria. Apical dendrites had at least one branch that reached the outer molecular layer and many branches reached the hippocampal fissure (Fig. 3A).
Fig. 3.
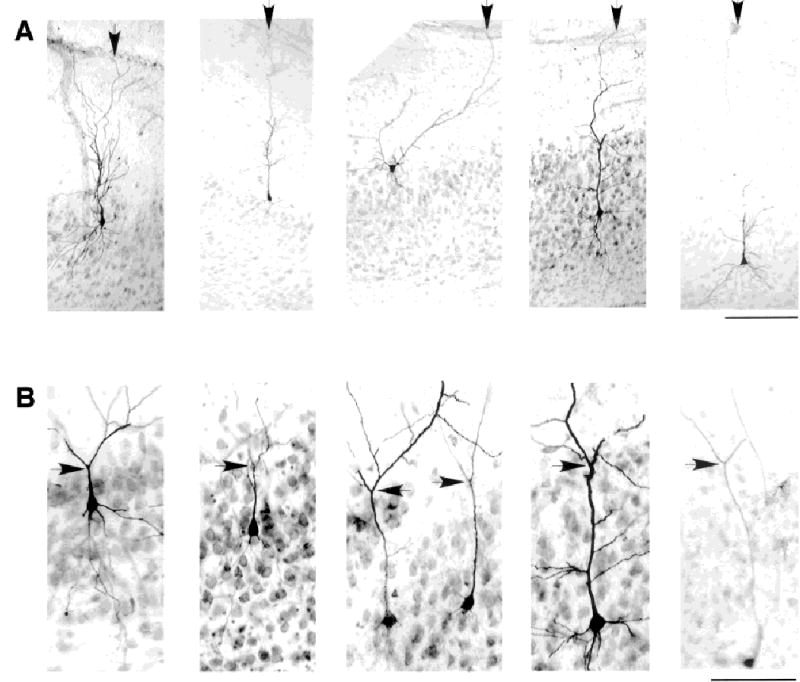
Apical dendrites of burst firing and regular spiking subicular neurons branch near the top of the cell layer and stretch to reach the hippocampal fissure. A: Assortment of regular spiking and intrinsic bursting neurons from different depths in the cell layer showing apical dendrites reaching the hippocampal fissure. Arrow in each panel points to the apical dendrite at the fissure. Electrophysiological identities of the cells in A, from left to right, are regular spiking (RS), RS, RS, intrinsic bursting (IB), and IB. B: Tendency for the first major branch point of the primary apical dendrite to be at the top of the cell layer. Arrow in each panel points to major division of the apical dendrite. Top of cell layer can be seen in thionin counterstain. Electrophysiological identities of the cells in B, from left to right, are: IB, IB, one of the pair was IB (likely the cell on the right), IB, and RS. Scale bars = 200 μm in A; 100 μm in B.
The subicular cell layer is thick, reaching almost 500 microns in some places. In Nissl sections, this can mean tens of cells along the apical to basal axis of the cell layer. We sampled cells all along this axis and along the CA1 to presubiculum axis. The locations of somata are shown in Figure 2. In agreement with the original description by Greene and Totterdell (1997), intrinsically bursting neurons tended to be located deep in the cell layer and regular spiking cells tended to be located superficially (Fig. 2, top). In a statistical comparison of cell position along the superficial–deep axis of the cell layer, the average depth of intrinsically bursting neurons (46 ± 22% from superficial edge of cell layer, n = 36; range, 6 to 97% of cell layer) was deeper than that for regular spiking neurons (30 ± 21%, n = 13; range, 6 to 61% of cell layer; P = 0.03, unpaired t test, two-tailed). This statistical difference in soma location along the superficial–deep axis of the cell layer should be used with caution to predict the electrophysiological identity of a subicular neuron. Clearly, a soma location near the deep edge of the cell layer will be more predictive of the electrophysiological cell class (IB) than a soma location near the superficial edge of the cell layer (see Fig. 2, top). Intrinsically bursting and regular spiking neurons did not differ statistically in their somata sizes (IB cells: height = 17 ± 3 μm, width = 14 ± 3 μm; RS cells: height = 18 ± 4 μm, width = 13 ± 2 μm; t test, paired, two-tailed, not significant; n = 13 IB, 13 RS).
There was a striking tendency for cells to have their first major branch point at the top of the cell layer (Fig. 3B). The first major branch point occurred within 50 μm (mean, 0 ± 20 μm) of the top of the cell layer in 51/60 cells where the primary branch point was visible in thionin-stained sections. This finding meant that the length of the primary dendrite varied as a function of cell depth in the cell layer. It also meant that cells at the top of the cell layer could have almost nonpyramidal cell morphologies (e.g., top center of Fig. 3A), although these cells were all found to have spiny apical dendrites with branches climbing to the fissure, and axons entering the deep white matter. However, not all cells followed the pattern of branching at the top of the cell layer. The first branch point in the other nine cells occurred between 55 and 150 μm of the top of the cell layer. In two of these cells, whose apical dendrites divided at 90 μm and 120 μm below the top of the cell layer, a second division occurred at the top of the cell layer. In two other cells, the primary apical dendrite never appeared to divide into equal-sized daughter branches. One example is shown in Figure 3A (second from the left).
Above the major division of the primary apical dendrite, higher order apical dendritic branches climbed through the molecular layer to reach the fissure. The overall spread of the apical dendritic arbor, perpendicular to the primary apical dendrite, had a wide range of 200 to 500 μm. This is almost certainly due in part to incomplete recovery of the full dendritic tree.
Although the first clear division of the apical dendrite into similarly sized branches occurred for most cells at the top of the cell layer, small branches were often found coming off the main apical dendrite. These smaller branches typically formed nearly right angles with the main apical dendrite and were distinguished from other dendritic division points by the differences in diameter. The diameter of the principal apical dendrite was 2–3.5 μm for regular spiking cells and 3 to 4 μm for intrinsically bursting neurons. The finer dendrites present along the primary dendrite ranged from 0.7 to 1.6 μm in diameter. These fine branches were longer than 100 μm in only two cells. Good examples of these small dendritic branches appear in Figures 3A,B, and 5D. Small branches were most common on cells with the longest primary apical dendrites.
Fig. 5.
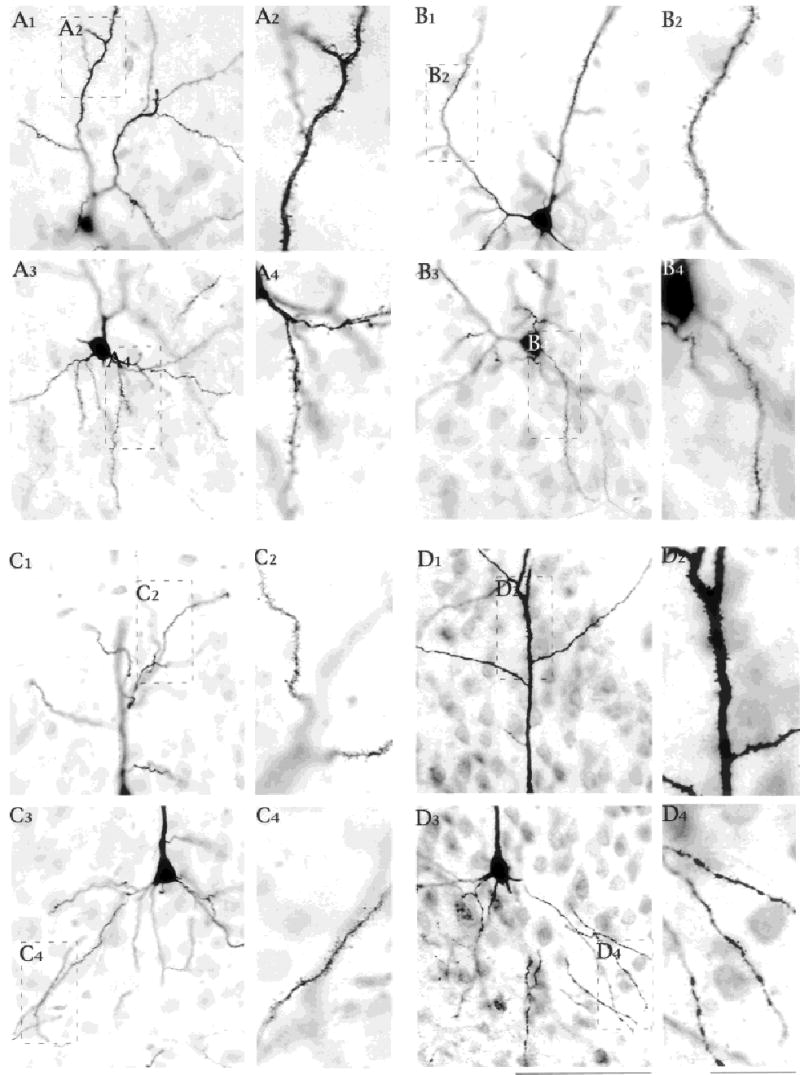
Spines on the apical and basal dendrites of an assortment of regular spiking (A,B) and intrinsic bursting (C,D) subicular neurons. Apical dendritic spines are shown in parts 1 and 2 of each set; basal dendrites are shown in parts 3 and 4 of each set. The most proximal portion of the apical dendrite was typically free of spines. This finding is best seen for the intrinsic bursting neurons in C3 and D1–D3. Note too that the most proximal parts of the basal dendrites are typically spine free. Scale bars = 100 μm in D3 (applies to parts 1 and 3 from all sets A–D); 25 μm in D4 (applies to parts 2 and 4 for all sets A–D).
Basal dendrites
The basal dendritic arbors were similar from cell to cell in both the number of branches and the overall size of the basal dendritic arbor (Fig. 4). The overall spread of the basal dendritic arbor ranged from 240 to 400 μm. All cells, regardless of location, apical dendritic morphology, or electrophysiological classification had multiple basal dendrites coming off the bottom half of the cell soma and branching within approximately 50 μm. The diameter of the principal basal dendritic branches ranged between 1.5 to 2.5 μm. Second- and third-order branching occurred less commonly. Basal dendritic branches over 100 μm long were common. In one cell (not shown), the basal dendritic branches were themselves seen to cross the alveus and enter the deep layers of entorhinal cortex.
Given the similarly sized basal dendritic arbors, superficially located cells had their basal dendritic arbors confined to the subicular cell layer. Only the deepest cells had basal dendrites that reached into the neuropil underlying the cell layer. On the other hand, all pyramidal cells had apical dendrites that reached the fissure.
Dendritic spines
All cells in this study were found to have spiny apical and basal dendrites (Fig. 5). The apical dendrite starts out aspiny (Fig. 5C3,D3). In many cells, spines were first visible after one or more small branches came off the major dendrite (Fig. 5B1,D1). The small perpendicular dendrites seen in the cell layer before the primary branch point were spiny, and the distal apical dendrites had many spines (Figs. 5A2,B2,C2,D2, 9B). Basal dendrites were spiny (e.g., Figures 5A4,B4,C4,D4, 9B, 10B), although the most proximal short pieces of basal dendrite were spine free (Fig.5A4,B3,C3).
Apical dendrites tilt
A surprising finding was that the apical dendrites of the most superficially located cells were not oriented perpendicular to the cell layer like the apical dendrites of the deeper cells were. There was a striking tendency for the superficial cells to be tilted toward either the subiculum–CA1 border or the subiculum–presubiculum border (e.g., Figs. 3, 7). The tendency for superficial cells to appear tilted was also seen in experiments using extracellular biocytin applications to label a population of neurons (Fig. 6). The deviation from “vertical” could be so severe as to suggest a nonpyramidal shape to the cell.
Fig. 7.
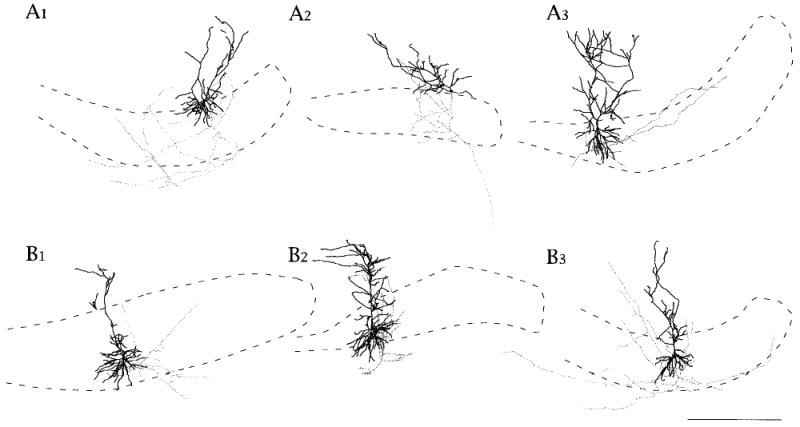
A,B: Neurolucida reconstructions of superficial and deep subicular cells. Somata and dendrites are shown in black. Dendritic spines are not drawn. Axons are shown in gray. Axonal varicosities and axonal extensions are not drawn. Local collaterals tended to be longer in the cell layer for superficially located cells. Deep cells tended to have axon collaterals that ascended close to the primary dendrite. All cells shown had axon collaterals that reached into the deep white matter, and in some cells, there were collaterals that crossed obliquely back across the cell layer toward CA1 or presubiculum. For this reason, all cells were considered to be projection cells. CA1 is to the left and presubiculum to the right in all figures. Cells in A1, A3, and B3 were reconstructed from horizontal slices. Cells in A2 and B2 were reconstructed from angled transverse slices like the one shown in Figure 1. Cell in B1 was reconstructed from a coronal slice. Scale bar = 500 μm in B3 (applies to all sets A,B).
Fig. 6.
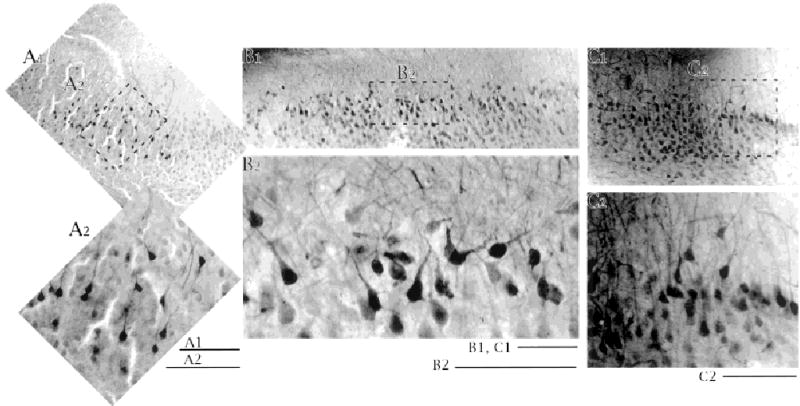
Patterns of apical dendrites of superficial pyramidal cells revealed by labeling many cells with extracellularly applied biocytin. A: Section taken near presubiculum. B: Section taken from near the center of the CA1–presubiculum axis. C: section taken from near CA1. Low-power images in the top row with higher power images below. Dotted outlines in the low-power images show the location of the high-power image. Note that the very superficial cells can have apical dendrites whose major axis appears tipped away from the perpendicular to the cell layer. The tendency was noted for cells to tip away from the midpoint of the CA1–presubiculum axis, seen best in the center panel. Scale bars = 200 μm in A1,B1,C1; 100 μm for A2,B2,C2.
The dendritic and axonal morphologies for a representative sample of superficially and deeply located subicular cells is shown in Figure 7. These Neurolucida reconstructions illustrate some of the properties of dendrites already discussed and axons (discussed below).
Axon collaterals in the cell layer
With relatively long survival times, we obtained good fills of axons for 53 of the 79 labeled cells. This fill included 23 intrinsic bursting, 6 regular spiking, 4 putative regular spiking, and 2 unclassified cells. All cells had multiple axon branches in the cell layer. Axons could be traced back to a single branch leaving the cell soma or the proximal part of a basal dendrite. Axons were readily distinguished from basal dendrites on the basis of their diameter (local axon collaterals were thinner than basal dendrites) and the presence or absence of spines (basal dendrites had spines, whereas the local axon collaterals had less frequent varicosities or axonal extensions suggestive of synaptic contacts). Branching within the cell layer could be extensive. Some cells had collaterals that stretched from nearly the border of subiculum with CA1 to the border of subiculum with presubiculum. These cells tended to be superficially located in the cell layer (e.g., Fig. 7, top left and top right). The three cells in the top row of Figure 7 are regular spiking cells. Burst firing cells are shown in the bottom row.
Cells located deep in the cell layer had thin axon collaterals with multiple varicosities and extensions climbed parallel to the primary apical dendrite, through the cell layer and into the apical dendritic region (described more below). The axon collaterals of deep cells were also less extensive in the plane of the cell layer when compared with superficial cells. Some of these cells were found to have collaterals running beneath the cell layer (e.g., Fig. 7, bottom left) or obliquely through the cell layer (Fig. 7, bottom right). These were axons that were likely to be part an efferent system. They were 2 to 5 times thicker than the local collaterals, suggesting more myelination, and had rare or absent varicosities (e.g., Fig. 10). The diameters of axon collaterals with varicosities in the cell layer or apical to the cell layer ranged between 0.25 and 0.67 μm, whereas the diameter of axons without varicosities, in the cell layer or basal to the cell layer, ranged between 1.02 and 1.68 μm. As indicated above, all but two labeled and electrophysiologically classified cells had axon collaterals that entered the alveus. Branches were seen going toward presubiculum (n = 15 IB, 1 RS cells); reaching presubiculum (n = 3 IB cells); toward CA1 (n = 6 IB cells); reaching CA1 (n = 2 IB cells, 1 RS cell); and toward entorhinal cortex (n = 2 IB, 2 RS cells).
Fig. 10.
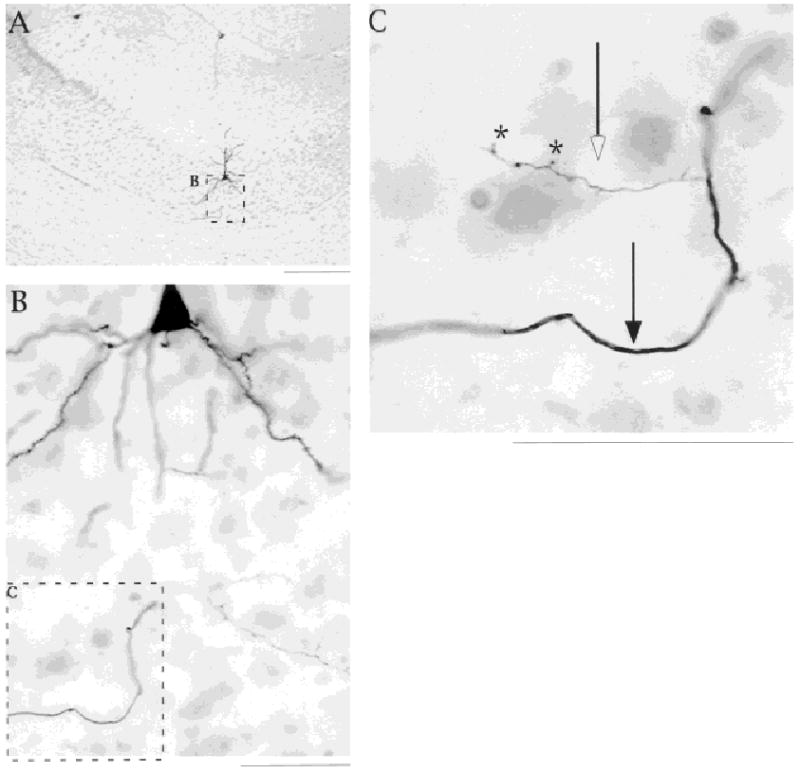
Local collaterals are thinner than collaterals that will leave the subiculum. A–C: Photomicrographs of a horizontal section show the local and efferent axons of an intrinsically bursting neuron. In C, open arrow points to a local collateral; note the axon extensions (marked by asterisks). The thicker collateral (marked by closed arrow) was in the alveus. Diameter of local collateral was 0.46 μm, and the diameter for the thick axon was 1.22 μm. Scale bars = 300 μm in A; 50 μm in B,C.
Examples of the patterns of axonal arbors in, above, and below the cell layer are shown in the Neurolucida drawings of Figure 7. Photomicrographs of axon collaterals in and above the cell layer are given in Figures 8, 9, and 10. Some varicosities on labeled axons were even seen in close proximity to triangular cell bodies (Fig. 8C).
Fig. 8.
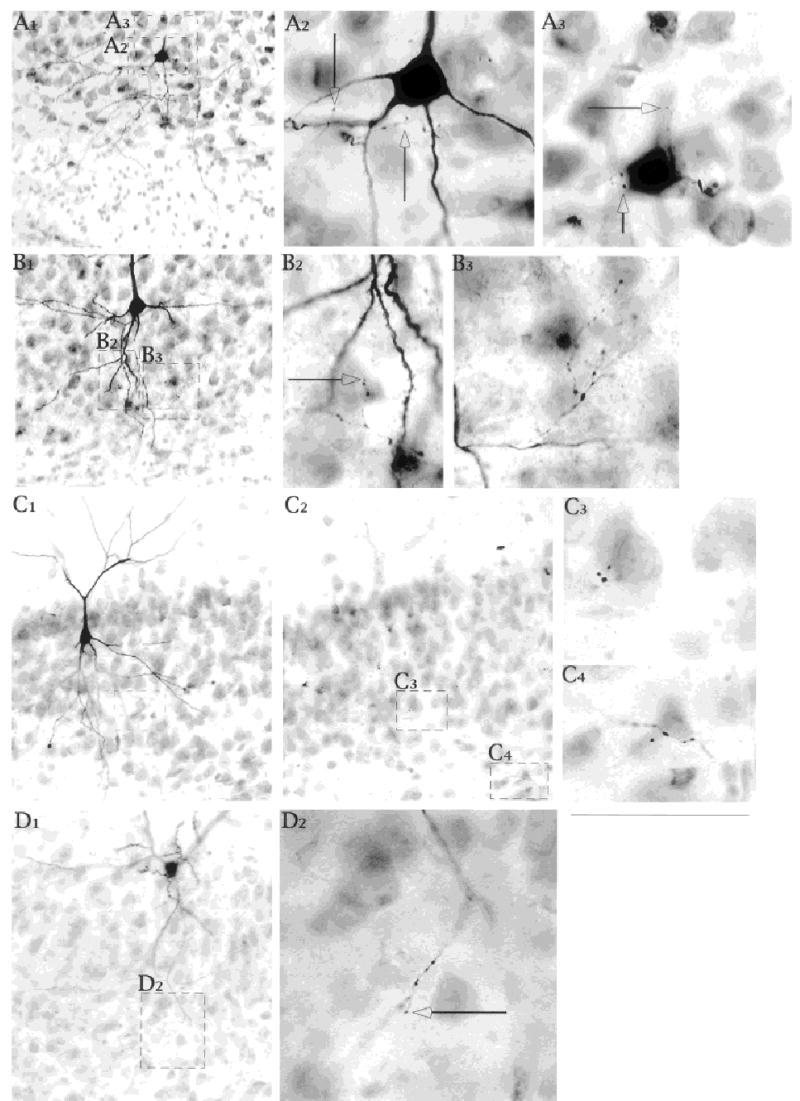
Axon collaterals and contacts in the cell layer. Photomicrographs of Neurobiotin labeled subicular pyramidal cells with thionin counterstain. A,B,C: Intrinsically bursting neurons. D A regular spiking cell. Open arrows in all panels identify axonal extensions that suggest possible contact points for the axon. They do not represent the cut ends of axon at one or the other face of the tissue specimen. Several different modes of termination are shown: (1) en passant endings near somata and proximal apical dendrite (A2,A3,C4), (2) cluster of terminals (B3), (3) scattered endings (B2,D2). Scale bars = 50 μm in D1 (applies also to A1,A2,B1,B2,C1,C2,D2); 50 μm in C4 (applies to B3,C3,C4).
Fig. 9.
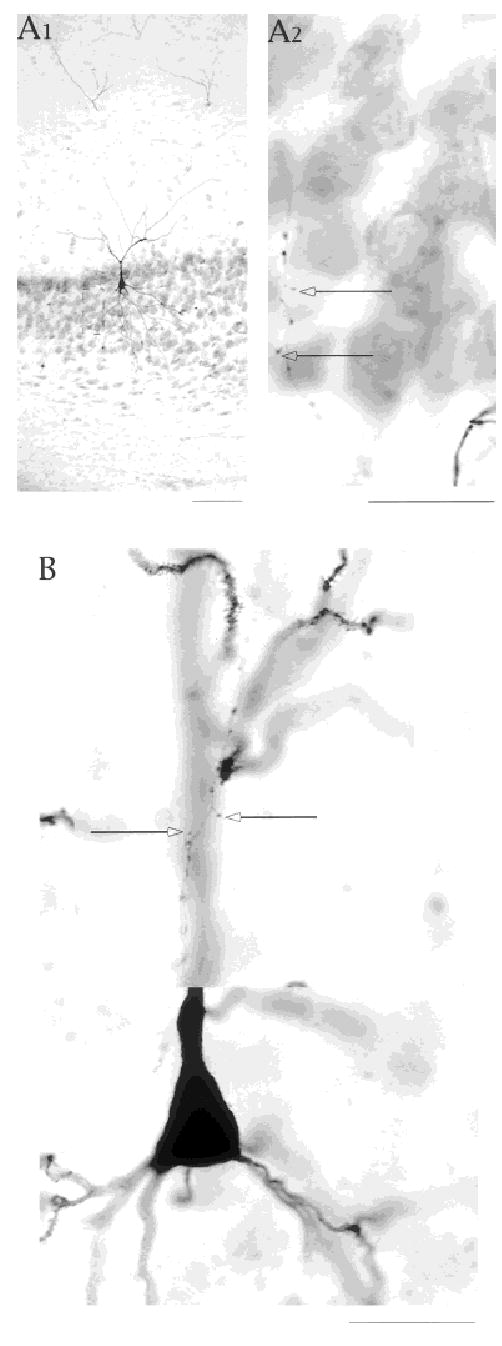
A,B: Ascending axon collaterals make contacts in the cell layer and in the apical dendritic zone. Open arrows identify axonal extensions suggesting synaptic contacts in the cell layer and in the vicinity of apical dendrites. The differences in diameter of local axon collateral and apical dendrites are highlighted in B. Scale bars = 25 μm in A1; 100 μm in A2; 25 μm in B.
Ascending collaterals
Thirty-five cells (including 23 IB and 6 RS cells) had at least one axon collateral that climbed into the apical dendritic region (Fig. 9). These thin ascending collaterals had multiple varicosities and extensions. Their paths in the cell layer, paralleling the primary apical dendrite, put deep cells in potential contact with superficial cells. In some sections, we could find a putative axonal contact point in the same plane of focus as a dendritic spine or dendritic shaft. In general for the deep cells, the extent of the axonal arbor paralleled the extent of the apical dendritic arbor. As indicated previously, superficial cells had much broader axonal arbors in the cell layer, but in the apical dendritic zone, their axonal arbors also paralleled their apical dendrites. Many cells had two to three ascending collaterals. In some, we located only a single ascending collateral. All ascending collaterals, like the collaterals reaching even long distances in the cell layer, were thinner than the axon collaterals that were headed out of the subiculum, suggesting that all local collaterals were un-myelinated or lightly myelinated. The difference between the local and efferent axon branches is shown in Figure 10.
Relative density of terminals
Our light microscopic evidence of synaptic contacts in the cell layer and in the apical dendritic region is supported by electrophysiological data in the companion paper. However, we do not have electron microscopic evidence to confirm these synaptic contacts with anatomic techniques. We emphasize that the relative density of varicosities and extensions on subicular axons is high. We support this point with two pieces of evidence. First, as we have mentioned, the local connections in subiculum are sufficient to support the generation and propagation of epileptiform activity in isolated subiculum. Second, we offer similar morphologic data from CA1 for contrast with the data presented for subiculum. Notice in the example shown in Figure 11, that the density of axonal varicosities and extensions is much less than what we have shown in all examples from subiculum.
Fig. 11.
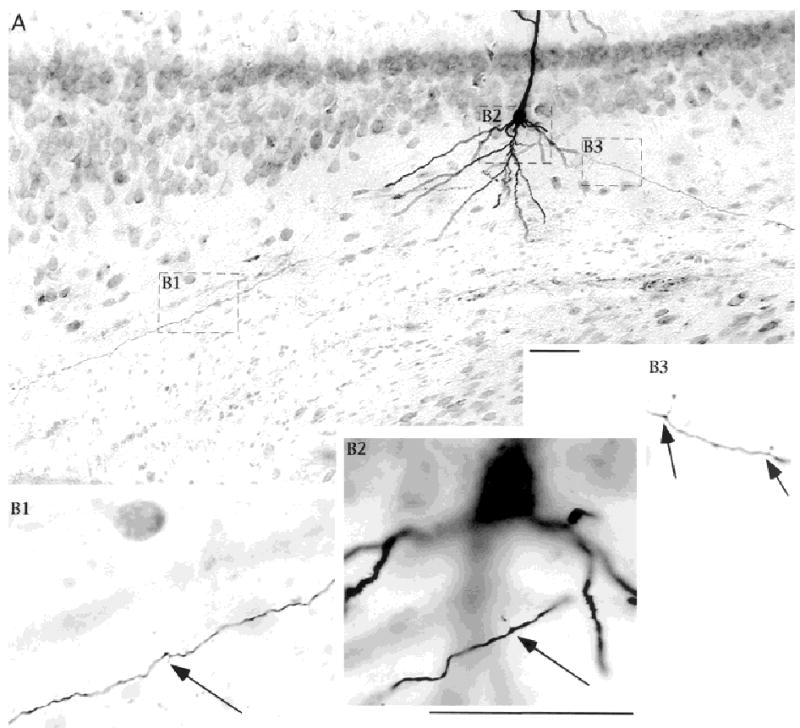
Relative density of axonal varicosities and extensions is less for CA1 pyramidal cell axons than for subicular cell axons. Neurobiotin-labeled CA1 pyramidal cell shown at low power in A, and segments at higher power are shown in B. Axonal extensions (arrows) were uncommon, in contrast to the examples shown for local axon collaterals of subicular cells. Note the absence of ascending axon collaterals. Scale bars = 50 μm in A; 50 μm in B2 (applies to B1–B3).
DISCUSSION
Neurobiotin, applied from intracellular recording electrodes, was used to label individual subicular neurons in rat brain slices. The dendritic and axonal morphologies of electrophysiologically classified cells were examined. This is the first combined morphology/electrophysiology study to demonstrate that intrinsic bursting and regular spiking subicular cells are projection cells and that both have extensive local collaterals. All labeled cells had long spiny apical dendrites that reached the outer molecular layer or the hippocampal fissure. The pattern of dendritic branching correlated better with the position of the cell soma within the thick subicular cell layer, than with the electrophysiological cell class. Similarly, patterns of axonal branching within subiculum correlated better with the position of the cell soma within the cell layer, than with electrophysiological cell class. Regular spiking and intrinsically bursting neurons did not differ in soma size, nor apical or basal dendritic diameters. Apical dendritic branching patterns of the most superficially located cells in the subicular cell layer support a division of the subiculum along the proximo–distal axis. Axonal arborization within the subiculum suggests that the intrinsic organization of the subiculum is different from that of CA1, CA3, or dentate. Deep cells tended to have ascending collaterals with limited spread over the proximo–distal axis, whereas superficial cells had much more extensive collateralization in the cell layer. These patterns suggested a crude columnar and laminar organization within the thick cell layer of subiculum.
Dendrites
In agreement with the studies by Mason (1993), Taube (1993), and Greene and Totterdell (1997), we found no morphologic feature of labeled neuron somata or dendrites that perfectly predicted a cell’s electrophysiological classification as regular spiking or intrinsically bursting. Cells with somata deeper in the cell layer had pronounced primary apical dendrites, whereas more superficially located cells branched closer to the soma. The locations of the first major branch point can be appreciated in the figures of Lorente de No (13, 30, 31; 1934), although it is easier to see the relation of the first branch point to the top of the cell layer in the individually labeled neurons together with a cell stain such as thionin.
Some very superficial cells branched immediately, giving them an atypical shape for pyramidal cells (see also Lorente de No, 1934). These cells were clearly principal neurons, because all were found to have axon collaterals that entered the alveus, indicating that they were at least projection cells. Additionally, all such cells were spiny. Finally, their electrophysiological properties were either regular spiking or intrinsic bursting. None exhibited the firing properties of interneurons described previously (Stewart and Wong, 1993; Greene and Totterdell, 1997).
It has been suggested that the dendritic structure may determine a cell’s firing pattern (Mainen and Sejnowski, 1996). Given the clear tendency for the first branch point to occur at the top of the cell layer, one would expect a relatively smooth progression of firing properties from the deepest to the most superficially located cells. Although there is a trend for burst firing cells to be deep and regular spiking cells to be superficial (Greene and Totterdell, 1997; this paper), there are numerous examples of regular spiking and burst firing cells throughout the thickness of the cell layer. Even within our class of burst firing neurons, where cells can exhibit tendencies to fire single spikes or spike doublets in response to injected current, we detected no trend in firing that correlated with the position of the soma in the cell layer or the branching pattern of the apical dendrites. It has been reported for CA3 pyramidal neurons that cells located deeper in the cell layer were more likely to burst in response to current injection (Bilkey and Schwartzkroin, 1990). One correlate that may relate to the dendrites is the finding that of 12 dye-coupled pairs of neurons, the recorded neuron of the pair was always a burst firing neuron. A similar result was found in CA1 (Baimbridge et al., 1991).
Axons
All labeled cells had axon collaterals with numerous axonal varicosities and extensions in the cell layer and in the apical dendritic region. In a study of similar structures on CA3 axons in CA1, 87% of varicosities were associated with at least one postsynaptic density, indicative of synapses (Shepherd and Harris, 1998). We conclude that both electrophysiological cell classes make synaptic contacts with other cells in subiculum. Furthermore, all but two of the classically shaped pyramidal cells had axon collaterals that left the subiculum, either by means of the deep white matter or after ascending obliquely across the subicular cell layer, confirming that both cell classes are projection cells.
Finch et al. (1983) were the first to show axon collaterals from recorded subicular neurons to demonstrate morphologically that the recorded cells were pyramidal projection cells. Although they did not distinguish different electrophysiological classes of recorded cells, they were able to show differences in the projection patterns of cells because they were able to recover very long pieces of axon. The two cells in which we could not trace axons into the deep white matter were both clearly pyramidal cells. Based largely on the work of Finch et al. (1983) where pyramidal cells were shown to be projection cells, we suggest that these two pyramidal cells are likely to have been projection cells as well. Whereas some of our cells with axons that projected out of subiculum support the notion of specific and separate projections by bursting and regular spiking cells (Stewart, 1997), we did not acquire enough data to statistically support the previous conclusions.
Our most important contribution, in terms of morphologic data about subicular cell axons, is the description of the local collaterals and the electrophysiological demonstration that these can mediate synchronization and propagation of activity in the subiculum itself (see companion paper for electrophysiology). It was possible to distinguish axon collaterals that may terminate in subiculum from those that would leave subiculum by means of the deep white matter or after ascending obliquely across the cell layer. Local collaterals were distinctly thinner than efferent fibers, suggesting little or no myelin for the local collaterals and at least some myelin on the efferent axons. Also consistent with modest degrees of myelination are the reported conduction velocities for subicular cell axons in the range of 0.25 to 0.8 m/sec (Stewart, 1997). There are no reported values for conduction velocities on the local collaterals. We can say from the generation and spread of epileptiform activity in the isolated subiculum that such local collaterals are functional (Behr and Heinemann, 1996; Harris and Stewart, companion paper).
We cannot know how much of an underestimate of the actual axonal arborization for each cell our data are. Many cells had axon branches that could be traced for over a millimeter. On the other hand, our data come from cells labeled in brain slices that were 400 μm thick. Dendritic and axonal arbors are truncated simply by the slicing techniques. This finding was the main reason why we filled cells in slices cut from in different planes. On the basis of the data obtained from reconstructions of cells from different slice types, we believe that our data on dendrites and local axon arbors are representative.
Architecture
One conclusion that we drew from the results was that there appears to be a columnar and laminar organization to the subiculum (Fig. 12). Cells located deep in the cell layer tended to have ascending axon collaterals that remained close to their apical dendrites, perhaps defining columns. By contrast, cells located superficially in the cell layer had extensive axon collaterals in the cell layer, providing the laminar organization. With the tendency for burst firing cells to be located deep in the cell layer and regular spiking cells to be located superficially in the cell layer, it is possible that burst firing neurons form a set of columns and the regular spiking cells serve to integrate their activity.
Fig. 12.
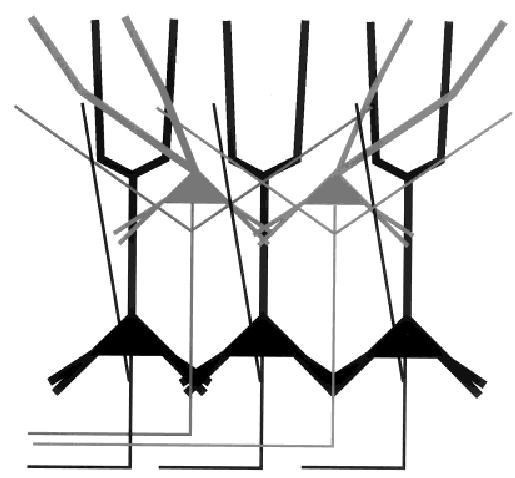
Model of the intrinsic organization of the subiculum. Deep cells have ascending axon collaterals that remain in close proximity to their apical dendrites, giving rise to a columnar pattern for the deep cells. Superficial cells have axon collaterals that can run long distances in the cell layer perhaps serving to organize the activity across columns. Very superficial cells can have apical dendritic arbors that tip off the vertical, which may permit them to integrate inputs from multiple deep layer cells. Both types of cells are projection cells. The properties of dendritic and axonal arbors correspond best with the cell location along the superficial–deep axis. Although there is a strong tendency for deep cells to be burst firing neurons, we emphasize that the correspondence of cell location with electrophysiological cell class is not without overlap. In particular, the superficial cells are a mixed population of regular spiking and burst firing neurons. The basal dendrites of superficial cells do not reach out of the cell layer, whereas deep cell basal dendrites reach into the basal neuropil. This is one location where laminar inputs might select for the bursting cell population. In the cell layer and in the apical dendritic zone all cells may receive laminar inputs, albeit to different parts of the cell. Superficial cells with tipped dendrites may receive more contacts from some laminar inputs.
Laminar inputs to the apical dendritic region of subiculum will be likely to contact superficial and deep cells alike, because all cells had apical dendrites that reached the fissure. On the other hand, laminar inputs to the cell layer or the basal dendritic region could differentially activate the basal dendrites of superficial or deep cells because the basal dendrites reached different distances down to the deep white matter.
Another interesting finding was that the most superficial cells had apical dendrites that were less and less “vertical” as one moved from the center of subiculum toward either the CA1 or the presubicular border. Because the apical dendrites tipped away from the center, toward presubiculum or toward CA1, this might be used to support a division of subiculum into functionally separate proximal (near CA1) and distal (near presubiculum) segments. A consequence of this organization is that the superficial cells that are tipped off the vertical would be the targets of a greater number of deep columnar inputs. These regions would be especially susceptible to seizure generation if the superficial cells getting convergent inputs from many deep cells were themselves burst firing neurons. This may explain why the foci for generating spontaneous epileptiform bursts were always found near the edges of isolated pieces of subicular tissue (Harris and Stewart, companion paper).
The evidence that the outputs of burst firing and regular spiking cells are separate (Stewart, 1997; see also Naber and Witter, 1998; and Donovan and Wyss, 1983) suggests that these different cell classes may serve different functions. With superficial cells at the center of the CA1–presubiculum axis having the most vertical apical dendrites, the center of the subiculum has the clearest columnar structure. One could argue that the center of subiculum has an intrinsic organization that differentiates it from the parts nearest the bordering cortices. What makes this finding especially interesting is that separate work on the inputs to subiculum from CA1 and the outputs of subiculum to the midline thalamic nuclei also support a division of subiculum into subsections along the proximo–distal axis. The outputs to the midline thalamus arise from the mid-transverse portion of the subiculum. The central projecting portion is bounded by two similarly sized “slabs” of subiculum that do not project to the midline thalamus. The inputs from CA1 to subiculum also have been suggested to support the conclusion that subiculum can be divided into three proximodistal portions (Witter and Groenewegen, 1992; Witter, 1993). Our morphologic data lend more support to such a division.
Much more needs to be examined with regard to the interaction of subicular cells with the other parts of the hippocampal formation to better understand the structural transition from allocortex to periallocortex and before the subiculum’s role as a relay, an integrator, and a distributor of activity in the hippocampal formation can be defined.
Acknowledgments
We thank Drs. M. Schwanzel-Fukuda, F. Scalia, and M. Halpern for technical advice and the use of equipment. We also thank Ms. E. Timmerman for her technical assistance with the Neurolucida system.
Footnotes
Grant sponsor: NIH; Grant number: MH11587; Grant number: NS38209; Grant sponsor: Research Foundation of SUNY; Grant sponsor: Netherlands Organization for Scientific Research; Grant number: NWO grant 903-47-008; Grant sponsor: Human Frontier Science Program.
References
- Baimbridg KG, Peet MJ, McLennan H, Church J. Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with Lucifer yellow dye coupling but not with the presence of calbindin-D28k. Synapse. 1991;7:269–277. doi: 10.1002/syn.890070404. [DOI] [PubMed] [Google Scholar]
- Behr J, Heinemann U. Low Mg2+ induced epileptiform activity in the subiculum before and after disconnection from rat hippocampal and entorhinal cortex slices. Neurosci Lett. 1996;205:25–28. doi: 10.1016/0304-3940(96)12360-0. [DOI] [PubMed] [Google Scholar]
- Behr J, Empson RM, Schmitz D, Gloveli T, Heinemann U. Electrophysiological properties of rat subicular neurons in vitro. Neurosci Lett. 1996;220:41–44. doi: 10.1016/s0304-3940(96)13242-0. [DOI] [PubMed] [Google Scholar]
- Bilkey DA, Schwartzkroin PA. Variation in electrophysiology and morphology of hippocampal CA3 pyramidal cells. Brain Res. 1990;514:77–83. doi: 10.1016/0006-8993(90)90437-g. [DOI] [PubMed] [Google Scholar]
- Donovan MK, Wyss JM. Evidence for some collateralization between cortical and diencephalic efferent axons of the rat subicular cortex. Brain Res. 1983;259:181–192. doi: 10.1016/0006-8993(83)91249-0. [DOI] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Res. 1983;271:201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Harris E, Stewart M. Re-entrant activity in a presubiculum-subiculum circuit generates epileptiform activity in vitro. Brain Res. 1999;849:139–146. doi: 10.1016/s0006-8993(99)02045-4. [DOI] [PubMed] [Google Scholar]
- Greene JRT, Mason A. Neuronal diversity in the subiculum: correlations with the effects of somatostatin on intrinsic properties and on GABA-mediated IPSPs in vitro. J Neurophysiol. 1996;76:1657–1666. doi: 10.1152/jn.1996.76.3.1657. [DOI] [PubMed] [Google Scholar]
- Greene JRT, Totterdell S. Morphology and distribution of electrophysiologically defined classes of pyramidal and nonpyramidal neurons in rat ventral subiculum in vitro. J Comp Neurol. 1997;380:395–408. doi: 10.1002/(sici)1096-9861(19970414)380:3<395::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Greene JRT, Lin H, Mason AJR, Johnson LR, Totterdell S. Differential expression of NADPH-diaphorase between electrophysiologically-defined classes of pyramidal neurons in rat ventral subiculum, in vitro. Neuroscience. 1997;80:95–104. doi: 10.1016/s0306-4522(97)00073-0. [DOI] [PubMed] [Google Scholar]
- Harris E, Stewart M. Propagation of synchronous epileptiform events from subiculum backward into area CA1 of rat brain slices. Brain Res. 2001;895:41–49. doi: 10.1016/s0006-8993(01)02023-6. [DOI] [PubMed] [Google Scholar]
- Jung H, Staff N, Spruston N. Ionic basis of intrinsic bursting in rat subicular neurons. Soc Neurosci Abstr. 1999;25:1740. [Google Scholar]
- Köhler C. Intrinsic projections of the retrohippocampal region in the rat brain: I. The subicular complex. J Comp Neurol. 1985;236:504–522. doi: 10.1002/cne.902360407. [DOI] [PubMed] [Google Scholar]
- Lin H, Totterdell S. Light and electron microscopic study of neuronal nitric oxide synthase-immunoreactive neurons in the rat subiculum. J Comp Neurol. 1998;395:195–208. doi: 10.1002/(sici)1096-9861(19980601)395:2<195::aid-cne4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. Studies on the structure of the cerebral cortex: II. Continuation of the study of the Ammonic system. J Psychol Neurol. 1934;46:113–177. [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Mason A. Electrophysiology and burst firing of rat subicular pyramidal neurons in vitro: a comparison with area CA1. Brain Res. 1993;600:174–178. doi: 10.1016/0006-8993(93)90418-m. [DOI] [PubMed] [Google Scholar]
- Martínez-Marcos A, Lanuza E, Halpern M. Organization of the ophidian amygdala: chemosensory pathways to the hypothalamus. J Comp Neurol. 1999;412:51–68. doi: 10.1002/(sici)1096-9861(19990913)412:1<51::aid-cne4>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Mattia D, Hwa GG, Avoli M. Membrane properties of rat subicular neurons in vitro. J Neurophysiol. 1993;70:1244–1248. doi: 10.1152/jn.1993.70.3.1244. [DOI] [PubMed] [Google Scholar]
- Naber PA, Witter MP. Subicular efferents are organized mostly as parallel projections: a double-labeling, retrograde-tracing study in the rat. J Comp Neurol. 1998;393:284–297. [PubMed] [Google Scholar]
- Naber PA, Witter MP, Lopes da Silva FH. Networks of the hippocampal memory system of the rat: the pivotal role of the subiculum. Ann N Y Acad Sci. 2000;911:392–403. doi: 10.1111/j.1749-6632.2000.tb06739.x. [DOI] [PubMed] [Google Scholar]
- Shepherd GMG, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart M. Antidromic and orthodromic responses by subicular neurons in rat brain slices. Brain Res. 1997;769:71–85. doi: 10.1016/s0006-8993(97)00690-2. [DOI] [PubMed] [Google Scholar]
- Stewart M, Wong RKS. Intrinsic properties and evoked responses of guinea pig subicular neurons in vitro. J Neurophysiol. 1993;70:232–245. doi: 10.1152/jn.1993.70.1.232. [DOI] [PubMed] [Google Scholar]
- Taube JS. Electrophysiological properties of neurons in the rat subiculum in vitro. Exp Brain Res. 1993;96:304–318. doi: 10.1007/BF00227110. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ, Kharazia VN, Nakane M, Schmidt HHHW. Neurons in rat hippocampus that synthesize nitric oxide. J Comp Neurol. 1993;331:111–121. doi: 10.1002/cne.903310107. [DOI] [PubMed] [Google Scholar]
- Witter MP. Organization of the entorhinal-hippocampal system: a review of current anatomical data. Hippocampus. 1993;3:33–44. [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ. Organizational principles of hippocampal connections. In: Trimble MR, Bolwig TG, editors. The temporal lobes and limbic system. London: Taylor & Francis Inc.; 1992. pp. 37–59. [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Wouterlood FG, Naber PA, van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann N Y Acad Sci. 2000;911:1–24. doi: 10.1111/j.1749-6632.2000.tb06716.x. [DOI] [PubMed] [Google Scholar]


