Abstract
Reelin (Reln) is a protein with some structural analogies with other extracellular matrix proteins that functions in the regulation of neuronal migration during the development of cortical laminated structures. In the cortex of adult animals, Reln is expressed primarily in γ-aminobutyric acid (GABA)ergic neurons and is secreted into perineuronal nets. However, only 50–60% of GABAergic interneurons express Reln. We have characterized this subpopulation of cortical GABAergic neurons that expresses Reln by using two strategies: (i) a double immunolabeling procedure to determine the colocalization of Reln with neuropeptides and Ca2+-binding proteins and (ii) a combination of Golgi staining and Reln immunolabeling to determine the morphology of the rat cortical cells that store Reln. Many interneurons that express Neuropeptide Y (NPY) or somatostatin (but none of those that express parvalbumin) are Reln-immunopositive. A small population of calbindin-positive interneurons and very few calretinin-positive cells express Reln immunopositivity. Golgi staining revealed that layer I horizontal cells, layer II–V bitufted neurons, and some deep cortical layer Martinotti cells express Reln. Basket and chandelier cells are often immunopositive to parvalbumin, but never to Reln. Although Reln is secreted by GABAergic neurons, its target are not the GABA receptors, but rather may be extrasynaptically located in perineuronal nets and concerned with the modulation of neuronal plasticity. Dab1, the target adapter protein that presumably mediates transcription regulation via the extrasynaptic actions of Reln, is expressed predominantly in pyramidal neurons, but it can also be detected in a small population of GABAergic neurons that are neither horizontal nor bitufted neurons.
Keywords: GABAergic interneurons, Ca2+-binding proteins, neuropeptides, chandelier cells, basket cells
The notation “reelin” (Reln) refers to a protein secreted into the extracellular matrix during embryonic development that is structurally related to other extracellular matrix proteins (1). In early development, Reln is selectively synthesized and secreted by specialized neurons (termed Cajal–Retzius) located in the marginal zone of the developing brain (1). A mutant mouse that fails to synthesize or secrete this protein shows a characteristic reeling gate and a disruption of the typical laminated pattern of telencephalic cortex (for review, see refs. 2 and 3). In these mice, the structure of the hippocampus and cerebellum is also disrupted, and the cerebellar size is grossly reduced. Another protein, which in embryos is normally expressed by the migrating cortical and hippocampal neurons and cerebellar Purkinje cells, is encoded by a gene termed Dab1, which is mutated in the scrambler mouse (4–6). This neurological phenotype, which is similar to the reeler mouse, shows a normal Reln expression in Cajal–Retzius cells and secretion in the extracellular matrix in embryonic development but neuroanatomical abnormalities reminiscent of those in the reeler mouse (4, 5). It is currently believed that the protein encoded by this gene acts downstream of Reln in a signaling pathway that controls laminar corticogenesis and the hippocampal and cerebellar development in mammalian brain (6).
In adult telencephalon, Reln is preferentially expressed in GABAergic neurons (7, 8), and Dab1 is expressed predominantly in pyramidal cells, as well as in a small population of non-pyramidal cells (9). Indeed, there is a complementary pattern in the distribution of Reln and Dab1 in the brain that suggests a functional link between these two proteins in the signaling pathway triggered extracellularly by Reln in adult mammalian brain.
Before addressing the functional significance of the pathway triggered by Reln extracellularly in adult brain, it is important to consider that perineuronal nets, which include extracellular matrix proteins (10, 11), may communicate with neurons via substrate adhesion molecules and integrins (for a review, see ref. 12). Hence, although the binding site of the released Reln may be extracellular in the perineuronal nets, it may influence neuronal trophic activity indirectly via an interaction with the Dab1 protein. This interaction requires phosphorylation, which allows this protein to act as an adapter protein operative in the translocation of Src kinases from the cytosol to the nucleus (13). Because a high percentage of Reln in the adult cerebral cortex is located in GABAergic interneurons, whose function in the various subtypes differs in a manner related to their morphology, it is important to characterize whether a morphologically specialized set of these interneurons expresses Reln. In an effort to further characterize which subtypes of GABAergic interneurons express Reln in the adult cortex, we have used a combination of immunocytochemical and Golgi-staining techniques. This study will allow us to suggest a possible interaction of Reln–Dab1 among specific populations of cortical GABAergic interneurons, or among these interneurons and pyramidal neurons. This work will also allow us to address the functional role of Reln released by GABAergic neurons into the extracellular matrix of perineuronal nets (10, 11).
MATERIALS AND METHODS
A total of 12 adult Fisher rats (180–240 g) were used in this study. They were anaesthetized with equithesin and perfused intracardially with saline followed by a cold solution of 4% paraformaldehyde in PBS (pH 7.4). Brains were removed, left in fresh fixative for 24 hours at 4°C, and washed in PBS. Brains to be processed for light microscopy immunolabeling were infiltrated in 30% sucrose, and 20-μm thick sections were obtained with a cryostat. For Golgi staining, blocks of cortex about 1–2 mm thick were cut with a razor blade.
Double Immunolabeling for Confocal Microscopy.
The colocalization of Reln and either somatostatin, neuropeptide Y (NPY), or calretinin was determined by using a double immunofluorescence approach. Because the antibodies against parvalbumin and calbindin were raised in the same species as the Reln antibody, and similarly the antibodies against Dab1 and GAD67 were raised in the same species, a combination immunogold/immunofluorescence was applied. This procedure allows the silver shell that amplifies the gold particles to completely engulf the antigenic site, preventing any cross-reactivity.
For double immunofluorescent labeling, sections were washed in PBS, blocked for 30 minutes in each of RPMI medium 1640 (GIBCO), 2% normal serum (Chemicon), and 1% BSA (Sigma) in PBS before incubation in a solution containing both the G-10 (14) primary antibody for Reln (1:1,000) and either rat anti-somatostatin (1:500; Chemicon), rabbit anti-calretinin (1:2,000; Chemicon) or rabbit anti-NPY (1:5,000; Sigma). After several washes, sections were incubated for 1 hour with an anti-mouse secondary antibody conjugated with fluorescein (1:100; Sigma), and with anti-rat or anti-rabbit secondary antibodies conjugated with rhodamine (1:100; Chemicon). Sections were then rinsed, mounted on slides, dried overnight, mounted with a glycerol/gelatin mounting medium (Sigma) containing 25 mg/ml diazabicyclo[2.2.2]octane (DABCO) antifading agent (Sigma), and coverslipped.
For the combined immunogold/immunofluorescent technique, Reln and Dab1 (4) were first labeled by immunogold following a protocol described (15). After silver intensification, sections were thoroughly rinsed with distilled H2O and blocked with 2% normal serum (30 min) and 1% BSA (30 min). Reln-labeled sections were then incubated overnight at 4°C with either mouse anti-calbindin D-28K (1:200; Sigma) or mouse anti-parvalbumin (1:2000; Sigma), and Dab1-labeled sections were incubated with either rabbit anti-GAD67 (1:2,000; Chemicon) or the G-10 antibody for Reln (1:1,000). After several rinses, sections were then incubated in anti-mouse secondary antibody conjugated with fluorescein (1:100; Sigma). Sections were then rinsed, mounted on slides, dried overnight, mounted with antifading agent (see above) and coverslipped.
For each of the above listed fluorochrome or gold/fluorochrome combinations, control sections were processed simultaneously in which the primary antibody was replaced with 1% BSA in PBS.
Golgi Staining and Reln Immunolabeling.
Blocks of tissue were Golgi-stained and deimpregnated as described (16), and then Reln was immunolabeled. Briefly, tissue blocks were immersed in a solution of 2% osmium tetroxide in buffer for 24 hours, followed by 2–4 days immersion in a 4% solution of potassium dichromate, and then washed and stored for 24 hours in a fresh solution of 0.75% silver nitrate. The tissue blocks were then transferred to a graded series of increasing glycerol concentration and stored in pure glycerol at 4°C. These blocks were embedded in 7% agar and cut at 80 μm using a vibratome. Sections were kept for 30 minutes under an illuminated device and studied under the microscope. Golgi-impregnated cells of interest were drawn and photographed at this time. Sections were then rehydrated, gold-toned, and deimpregnated by treating the sections with 1% sodium thiosulfate at 20°C for 80 minutes. This process removed the silver fill from the entire cell, replacing it with a thin shell of silver nitrate surrounding the cell soma and proximal dendrites. Each cell was examined at this step, and only cells whose cytoplasm was devoid of silver fill (pictures not included because of space limitations) were immunostained for Reln according to a previously described protocol (7). After diaminobenzidine immunostaining, 80-μm sections were embedded in LR-white resin. Semithin sections (0.5–1 μm in thickness) containing the neurons of interest were taken with an LKB-Nova Biochem ultramicrotome, mounted on slides, and examined under the light microscope for Reln-immunoreactivity. Photomicrographs of these same cells, now immunolabeled for Reln, were taken and deemed Reln-immunopositive if their cytoplasm was immunostained.
It is important to note here that Golgi staining does not readily infiltrate the axon myelin sheaths, therefore in adult brain it is sometimes difficult to trace the axonal arborizations. It is therefore necessary to impregnate many cells to make an accurate identification based on their axonal arborization.
Quantification of the Coexistence of Neuropeptides and Proteins in GABAergic Interneurons Epressing Reln.
To determine which percentage of frontoparietal cortex cells that express either a given neuropeptide or a Ca2+-binding protein also express Reln, three consecutive coronal sections from each of three animals were studied by using a Leica TCS-NT laser confocal microscope. For each neurochemical marker (e.g., calretinin, calbindin, parvalbumin, NPY, and somatostatin), every cell that contained that marker in the frontoparietal cortex was identified by using the appropriate filter, which was then switched to determine whether that same immunopositive cell also contained Reln immunoreactivity. The number of single-labeled (e.g., NPY) and double-labeled (e.g., NPY and Reln) cells were counted in layer I, layers II–III, and layers IV–VI of the frontoparietal cortex in both hemispheres of each section. The percentage of colocalization between each neuropeptide or Ca2+-binding protein and Reln was then tabulated over the three consecutive sections from each of three rat brains. The variation between animals was minimal, being no greater than the variation between the left and right hemispheres of each section.
Morphological Identification of Reln-Immunopositive Cortical Neurons.
Morphological identification of Golgi-impregnated cells was based on the following classical descriptions (for a review, see ref. 17): Horizontal cells of layer I generally have elongated cell soma that lie parallel to the pia and have dendrites that follow the same direction. Bitufted cells, which are only found in layers II–V, have an elongated soma that generally lies perpendicular to the pia, with most of the dendritic processes emerging from the top and bottom of the soma. Basket, chandelier, and dendritic-targeting neurons are generally multipolar cells that have a large soma, which are primarily located in layers III–V. They are distinguished by their axonal arborization: Basket-cell axons run across to pyramidal neuron somata, surrounding them and making synaptic contacts with them. Chandelier cells are so named for their axon terminals, which make morphologically specialized contacts with the initial segment of pyramidal cell axons, giving the appearance of candles (18). Dendritic-targeting cells, as their name implies, target the basal dendrites of pyramidal cells. Pyramidal cells, which are located in layers II–III, V, and VI, are easily distinguished by their pyramid-shaped somata, which have a large apical dendrite that runs perpendicular from the cell body in the direction of the pial surface. Stellate cells are relatively small star-shaped cells, predominantly found in cortical layer IV. In layer VI, the majority of nonspiny neurons are either fusiform (bitufted), or polygonal/rounded-shaped neurons. An important subpopulation of these layer V–VI bitufted and multipolar neurons, which send their axons to layer I where they contact the dendritic tufts of pyramidal cells, are referred to as Martinotti cells.
RESULTS
Coexpression in GABAergic Interneurons of Ca2+-Binding Proteins or Neuropeptides and Reln.
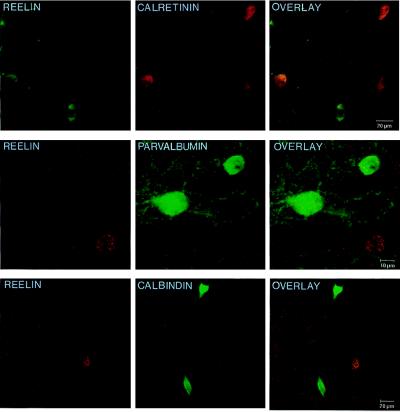
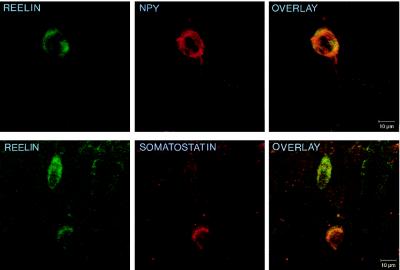
Table 1 lists the percentage of neuronal somata in the frontoparietal cortex that are immunopositive for a particular neuronal marker, as well as for Reln. These data show that a high percentage of GABAergic interneurons in the frontoparietal cortex that express either the neuropeptide NPY or somatostatin also express Reln. Conversely, a much smaller percentage of the GABAergic neurons that express the Ca2+-binding protein calretinin or calbindin also express Reln. However, GABAergic interneurons that express parvalbumin never express Reln. The results of these confocal studies in the frontoparietal cortex of adult rats are very similar to those recently reported by Alcantara and her colleagues in the adult mouse brain (22). In addition, this study also revealed that Reln does not colocalize well with either the neuropeptides cholecystokinin (CCK) or vasoactive intestinal peptide (VIP). The confocal images in Fig. 1 depict the scarcity of colocalization between Reln and the two Ca2+-binding proteins under study, while Fig. 2 gives an example of the higher frequency of colocalization between Reln and the neuropeptides NPY or somatostatin. The colocalization between Reln and the neuronal markers is limited by the variability of the concentration threshold to immunodetect these makers.
Table 1.
Percentage of Ca2+-binding protein or neuropeptide-immunopositive cells that colocalize with Reln in cortical layers of the frontoparietal cortex
| Neuronal marker | Layer I, % | Layer II–III, % | Layer IV–VI, % | Total, % |
|---|---|---|---|---|
| Calretinin | 57 | 26 | 0 | 22 |
| Calbindin | — | 22 | 27 | 25 |
| Parvalbumin | — | 0 | 0 | 0 |
| NPY | — | 82 | 89 | 85 |
| Somatostatin | 0 | 50 | 65 | 57 |
In each section of the frontoparietal cortex studied, every neuronal somata positive for a given neuropeptide or protein was verified for Reln-immunoreactivity. We studied each of three consecutive sections from each rat (see Materials and Methods), and the percentage of somata coexpressing one of the markers under study and Reln was tabulated.
Figure 1.
Confocal microscope images showing the double labeling for Reln and several Ca2+-binding proteins in layers II–III of 20-μm sections of the adult rat frontoparietal cortex. (Top) The double-immunofluorescent labeling for Reln (fluorescein; green) and calretinin (rhodamine; red). (Middle) Double immunolabeling of Reln (gold; red) and parvalbumin (fluorescein; green). (Bottom) The double immunolabeling of Reln (gold; red) and calbindin (fluorescein; green). Overlays (Right) show that Reln and these Ca2+-binding proteins are poorly colocalized in the adult rat frontoparietal cortex.
Figure 2.
Confocal microscope images showing the colocalization for Reln and neuropeptides in layers II–III of 20-μm sections of the adult rat frontoparietal cortex. (Upper) Colocalization of Reln (fluorescein; green) and NPY (rhodamine; red). (Lower) Colocalization between Reln (fluorescein; green) and somatostatin (rhodamine; red). Overlays at right are examples of the colocalization frequency between Reln and NPY, or somatostatin reported in Table 1.
Morphological Identification of the GABAergic Interneurons That Express Reln in the Frontoparietal Cortex.
The analysis of coronal sections of the frontoparietal cortex immunostained with the G-10 mAb against Reln reveals scattered immunoreactive somata in all cortical layers, as well as a diffuse immunostaining of the neuropil in layer I. The labeling in the somata and proximal dendrites appears to be granulated, very likely indicating a higher level of labeling in the rough endoplasmic reticulum and Golgi apparatus; such localization needs to be independently confirmed by electron microscopy.
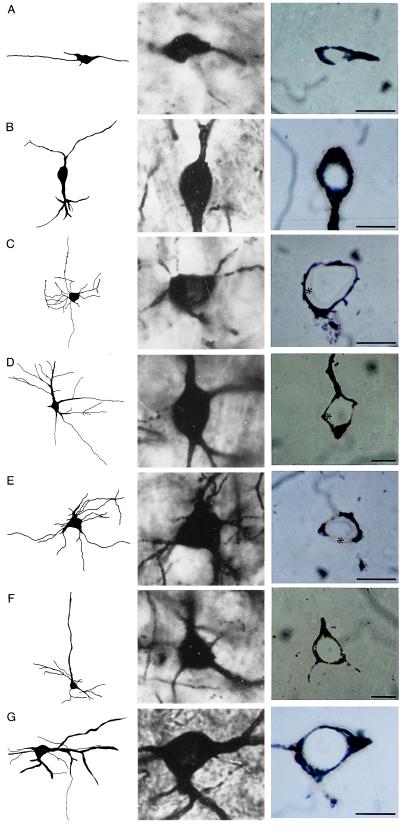
In layer I, Reln-immunopositive somata appear to be relatively abundant, especially considering the low cell-body density characteristic of this layer. The morphology of Reln immunoreactive somata of layer I is that of horizontal neurons (Fig. 3A), although there are also some immunopositive neurons with rounded or polygonal somata that are not parallel to the pial surface. Layers II and III also include a high number of Reln-immunoreactive neurons. Three different morphological subtypes can be appreciated: the most abundant are typical bitufted cells (Fig. 3B), however, there are also some immunoreactive rounded/polygonal multipolar neurons and some small pyramidal neurons. Layer IV includes some immunopositive multipolar neurons, possibly dendritic targeting neurons, and additional bitufted cells. In layer V, we observed a few scattered pyramidal cells that showed a slight Reln-immunoreactivity (Fig. 3F). In layer VI, the majority of Reln-immunopositive neurons have either fusiform somata or rounded or polygonal somata, many of which may be tentatively identified as Martinotti cells (Fig. 3G).
Figure 3.
(Left) Camera Lucida drawings of several morphologically identified neurons from the adult rat frontoparietal cortex. (Center) Photomicrograph of the somata of the cells drawn at Left. (Right) Photomicrographs of 1-μm sections taken through the same cells following removal of the silver impregnation, gold-toning, which outlines the cell soma with a gold shell, and immunolabeled for Reln. (A) Horizontal neuron of layer I. Note the cytoplasm immunostained for Reln (Right). (B) Bitufted neuron of layer III. Reln immunostaining is very evident in the cell cytoplasm (Right). (C) Chandelier neuron of layer IV. Note the unstained cytoplasm indicating a lack of immunoreactivity for Reln (∗). (D) Basket neuron of layer IV. Lack of immunoreactivity for Reln is evidenced by the unstained cytoplasm (∗). (E) Aspiny stellate cell of layer IV. Reln-immunonegative (∗). (F) Pyramidal neuron of layer V. A low level of immunoreactivity can be detected in the cytoplasm. (G) Martinotti cell of layer VI. Stained cytoplasm indicates immunoreactivity for Reln. (Bars = 10 μm.)
By using this combined Golgi-staining/Reln-immunolabeling technique, we could not detect Reln-immunoreactivity in any of the cells that can be morphologically identified as chandelier (Fig. 3C), basket (Fig. 3D), or stellate (Fig. 3E) cells.
Localization of Dab1 in the Adult Rat Frontoparietal Cortex.
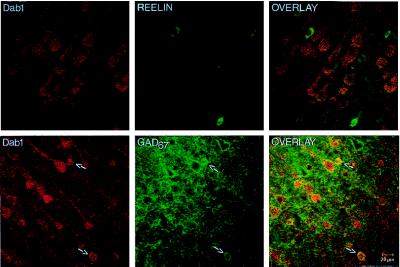
The confocal images in Fig. 4 show that Dab1 is not found in cells that contain Reln, but rather is predominantly found in pyramidal neurons. However, a colocalization of Dab1 with glutamic acid decarboxylase 67 (GAD67), the synthesizing enzyme for GABA, was also detected, suggesting that Dab1 may also be expressed in a subpopulation of GABAergic interneurons. These latter neurons do not have the typical elongated somata of the Reln-containing bitufted or horizontal cells and therefore may be the basket and/or chandelier cells that do not express Reln; this possibility is currently under investigation. However, not all pyramidal neurons contain Dab1, which is evidenced by the outline of GAD67-immunopositive terminals on typical pyramidal cell somata, which are not immunoreactive to the Dab1 antibody (see Fig. 4).
Figure 4.
Confocal microscope images taken from layer V of the adult rat frontoparietal cortex. (Upper) Double labeling of Dab1 (Gold; red) and Reln (fluorescein isothiocyanate; green). Note that Dab1 is predominantly located in pyramidal cells and is not found in Reln-expressing cells. (Lower) Double labeling of Dab1 (Gold; red) and GAD67 (fluorescein isothiocyanate; green). (Left) Whereas Dab1 is predominantly located in pyramidal cells, Dab1 is also found in a small population of nonpyramidal cells (arrows). (Center) Typical distribution pattern of GAD67 (fluorescein; green), both in GABAergic cells (arrows) and in the terminal endings surrounding the excitatory pyramidal cells. The overlay of the two images (Right) shows that these small Dab1-positive cells are GABAergic cells (arrows). Note also that there is a small population of pyramidal cells that do not contain Dab1, evidenced by the GAD67-labeled outline of pyramidal cells that are not Dab1-positive.
DISCUSSION
A previous report has shown that in adult rat cortex, at least 70% of the neurons that express Reln are GAD67-immunopositive (7). The Golgi-staining/Reln-immunolabeling method has allowed us to determine that Reln is expressed preferentially by horizontal cells in layer I, bitufted cells in layers II–V, and presumed Martinotti cells of layer VI. These findings correspond well with the histochemical colocalization studies showing that Reln co-exits with calbindin D-28K, a Ca2+-binding protein reportedly expressed by double bouquet cells (17, 20), as well as with somatostatin, a neuropeptide expressed by Martinotti cells (21). A population of multipolar neurons, as well as a very small population of pyramidal neurons, express Reln, but in pyramidal neurons, the Reln expression level is very low. The Golgi-impregnation studies fail to detect Reln expression in chandelier or basket cells, which is in agreement with the lack of Reln colocalization with parvalbumin, which is normally expressed in chandelier and basket cells (20, 22–25).
Some authors have postulated that only a few Cajal–Retzius cells (a neuronal phenotype that produces Reln from embryonic day 9 until 10–15 days postnatal) could survive in the adult brain and be located either in layer I or could have migrated to deeper layers (26–28). However, it is very likely that most of them die by the end of the second postnatal week in the rat (27, 29). Although it cannot be excluded that a few Cajal–Retzius cells might survive longer than two weeks postnatally and continue to express Reln into adulthood, the majority of Reln-positive GABAergic interneurons expressed in the adult brain belong to an entirely new generation of neurons that did not express Reln during development. This view in rodents also is supported by the observation that during the first postnatal week, when Cajal–Retzius cells are still abundant, additional cortical neurons expressing Reln are already evident (30). In the cortical marginal zone of humans at about 27 weeks of gestation, after Cajal–Retzius cells disappear, other Reln-expressing neuronal subtypes begin to emerge (31).
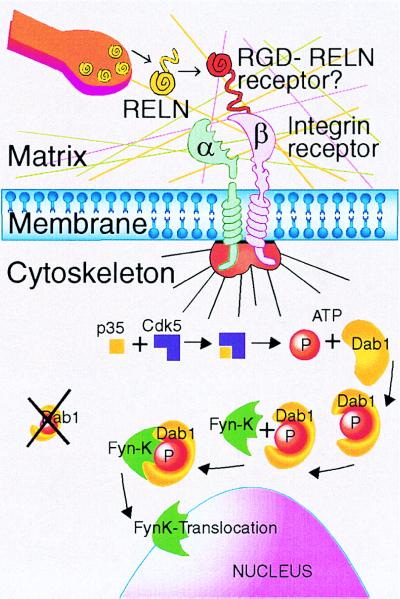
The Reln secreted by Cajal–Retzius cells and Reln secreted in adult life by other neurons share many structural characteristics and physicochemical properties with other extracellular matrix protein expressed in perineuronal nets (10, 11). Like these matrix proteins, Reln expresses eight epidermal growth factor-like motifs and is endowed with an N terminus signal peptide that is required for its secretion (1). In a previous study using electron microscopy (7), we have shown that Reln is expressed in cerebellar parallel fibers, suggesting it might be transported from the granule cell bodies to the molecular layer for its release extracellularly into the perineuronal nets in the proximity of the Dab1-expressing Purkinje cells and their dendrites (9). Based on this observation, one might infer that Reln released extracellularly from either axon terminals or dendrites of cortical interneurons may be anchored to the extracellular matrix structure expressed as a component of the perineuronal nets (10, 11) and connected to the dendrites of certain interneurons by substrate-adhesion molecules that link perineuronal nets to integrin receptors (see Fig. 5).
Figure 5.
Reln is released extracellularly from bitufted, horizontal, and Martinotti GABAergic cells of the frontoparietal cortex. Because a Reln-specific recognition site was not found in various GABA receptor subtypes, the integrin-dependent Reln stimulation of contiguous cells may occur via an RGD motif (Arginine, Glycine, Aspartate) expressed in matrix adhesion proteins (12). Thus, a Reln-dependent intraneuronal cascade of events is triggered, leading to the nuclear translocation of Src-family tyrosine kinases mediated by the adapter protein Dab1. This translocation can modulate the expression of specific patterns of DNA transcription. It is hypothesized that Reln, which structurally resembles other extracellular matrix proteins, may serve as an important regulatory link to harmonize regulation of specific genes to the frequency of columnary pyramidal cell firing. Those pyramidal or GABAergic neurons, which express Dab1, transduce the signal cascade triggered by extracellular Reln via the p35 activator protein of cdk5 (cyclin-dependent kinase 5) and cause binding of a nonreceptor tyrosine kinase (i.e., Fyn-K) to the adapter protein modified by phosphorylation (4, 6, 37). It is now being established beyond dispute that extracellular matrix proteins around the neurons are organized in a perineuronal net (10, 11). This net includes 3 layers: (i) integrin receptors form the layer contiguous with the intracellular milieu; (ii) the “matrix proper” where Reln is secreted and interacts with RGD; and (iii) an external layer rich in carbohydrates, such as hyaluronic and sialic acid (12).
It is important to note that Reln-containing GABAergic interneurons do not express the adapter protein Dab1. During embryonic brain development, Dab1 is expressed by neurons that migrate under Reln control (4). In the adult neocortex, Dab1 is primarily expressed in pyramidal neurons and in Purkinje cells and their dendrites (9), as well as in a small population of GABAergic interneurons in the adult rat cortex (Fig. 4). The dendrites of pyramidal cells are the main targets of GABAergic interneurons that secrete Reln extracellularly. The axons of layer I horizontal cells and Martinotti cells of layer VI, contact the dendritic tufts of pyramidal neurons in layer I (17). The transport of Reln in axons or dendrites, its possible extracellular release, and subsequent anchoring to specific sites in the extracellular matrix, could account for the diffuse Reln immunolabeling observed in layer I, not only in rodents (7) but also in humans (9). Bitufted cells project mostly to dendritic spines and shafts of pyramidal neurons, in both superficial and deeper cortical layers (17). In addition, bitufted cells contact other GABAergic interneurons such as a subpopulation of chandelier and basket cells, which may be those that express Dab1 (see Fig. 4). And although there is a small population of Reln-containing pyramidal cells, there is also a small population of pyramidal cells that do not contain Dab1 (Fig. 4). Further investigation is required to clarify whether the pyramidal neurons that are immunonegative for Dab1 are also those that are immunopositive for Reln.
Dab1 is mostly a cytoplasmic protein that may be transported in dendrites of pyramidal neurons, as it is transported in dendrites of Purkinje cells (7). After Dab1 phosphorylation, triggered by Reln anchored to the extracellular matrix (see Fig. 5), Dab1 may act as an adapter protein operative in the transport to the nucleus of Src family nonreceptor tyrosine kinases such as fyn (5, 13). When these proteins reach the nucleus, they participate in the regulation of protein synthesis related to neuronal plasticity. It is possible that Reln in bitufted cells may be secreted in the vicinity of Dab1-containing pyramidal or GABAergic cells, where its extrasynaptic actions may be involved in the regulation of protein synthesis. According to the model shown in Fig. 5, extracellular Reln could interact with RGD-containing substrate adhesion-promoting proteins that may participate in the neuronal internalization of the Reln transduction pathway via membrane integrin receptors. The activation of this receptor may prompt the interaction of the specific cyclin-dependent kinase 5 (cdk5) activator called p35. This in turn could cause the phosphorylation of Dab1, which is a rate-limiting event that enables the adapter function of this protein (13). In support of this speculation is the evidence that knockout mice of Dab1 protein (4), cdk5 (32), and p35 activator (33) can cause a corticogenic defect similar but not identical to that of the scrambler (34) and reeler (1) mice.
In summary, the activation of a nonreceptor tyrosine kinase by an unknown signaling cascade following integrin activation by extracellular matrix adhesion proteins could bring about a cascade of events affecting DNA transcription in pyramidal neurons, and presumably also in cortical GABAergic interneurons (i.e., chandelier and basket cells) that do not express Reln but may express Dab1. Among the actions that Reln may have in these cells is the regulation of the expression of GAD67, which consistently binds pyridoxal phosphates and can only be up-regulated by new synthesis (35). Thus, a deficit in Reln would bring about a deficiency of GAD67 expression. An example of a defect of such interaction might be present in schizophrenic brain, where a deficit in cortical Reln (9) and GAD (9, 36) expression has been detected.
In conclusion, we have demonstrated that Reln in the adult neocortex is preferentially expressed by a subset of GABAergic interneurons. These important results now allow us to carry out experiments directed to achieve a better understanding of the function of extracellularly secreted Reln in perineuronal nets of the adult brain, including its putative role in synaptic plasticity via integrin-dependent internalization of the Reln-Dab1 pathway (see Fig. 5).
Acknowledgments
We greatly appreciate the reviews of this manuscript made by Dr. Floyd Bloom (Department of Neuropharmacology, The Scripps Research Institute, La Jolla, CA) Dr. Edward Jones (Center for Neuroscience, University of California Davis), and Dr. Peter Somogyi (Medical Reserch Council Anatomical Neuropharmacology Unit, Oxford University, England). Their comments and critiques were extremely helpful. The authors also thank Dr. A. M. Goffinet (University of Namur, Belgium) for the generous gift of the G-10 antibody and Dr. B. W. Howell (Fred Hutchinson Cancer Research Center, Seattle, WA) for the generous gift of the Dab1 antibody. This work was supported by the Stanley Foundation and in part by National Institutes of Health Grants MH 49486-07 to A.G. and MH 56500-01 to E.C.
ABBREVIATIONS
- GABA
γ-aminobutyric acid
- NPY
neuropeptide Y
- GAD
glutamic acid decarboxylase
References
- 1.D’Arcangelo G, Miao C G, Chen S C, Soares H D, Morgan J J, Curran T. Nature (London) 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 2.Rakic P, Caviness V S., Jr Neuron. 1995;14:1101–1104. doi: 10.1016/0896-6273(95)90258-9. [DOI] [PubMed] [Google Scholar]
- 3.Curran T, D’Arcangelo G. Brain Res Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 4.Howell B W, Hawkes R, Soriano P, Cooper J A. Nature (London) 1997;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 5.Sheldon M, Rice D S, D’Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell B W, Cooper J A, Goldowitz D, Curran T. Nature (London) 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- 6.Rice D S, Sheldon M, D’Arcangelo G, Nakajima K, Goldgowitz D, Curran T. Development. 1998;125:3719–3729. doi: 10.1242/dev.125.18.3719. [DOI] [PubMed] [Google Scholar]
- 7.Pesold C, Impagnatiello F, Pisu M G, Uzunov D P, Costa E, Guidotti A, Caruncho H J. Proc Natl Acad Sci USA. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesold C, Pisu M G, Impagnatiello F, Uzunov D P, Caruncho H J. Brain Res Brain Res Prot. 1998;3:155–160. doi: 10.1016/s1385-299x(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 9.Impagnatiello F, Guidotti A, Pesold C, Dwivedi Y, Caruncho H J, Pisu M G, Uzunov D, Smalheiser N, Davis J, Pandey G, et al. Proc Natl Acad Sci USA. 1998;95:15718–15723. doi: 10.1073/pnas.95.26.15718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celio M R, Blumcke I. Brain Res Rev. 1994;19:128–145. doi: 10.1016/0165-0173(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Celio M R, Spreafico R, De Biasi S, Vitellaro-Zuccarello L. Trends Neurosci. 1998;21:510–515. doi: 10.1016/s0166-2236(98)01298-3. [DOI] [PubMed] [Google Scholar]
- 12.Ruoslahti E, Pierschbacher M D. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 13.Howell B W, Gertler F B, Cooper J A. EMBO J. 1997;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DeBergeyck V, Nakajima K, Lambert de Rouvroit C, Naerhuyzen B, Goffinet A M, Miyata T, Ogawa M, Mikoshiba K. Mol Brain Res. 1997;50:85–90. doi: 10.1016/s0169-328x(97)00166-6. [DOI] [PubMed] [Google Scholar]
- 15.Pesold C, Caruncho H J, Impagnatiello F, Berg M J, Fritschy J-M, Guidotti A, Costa E. Neuroscience. 1997;79:477–487. doi: 10.1016/s0306-4522(96)00609-4. [DOI] [PubMed] [Google Scholar]
- 16.Kisvarday Z F, Gulyas A, Beroukas D, North J B, Chubb I W, Somogyi P. Brain. 1990;113:793–812. doi: 10.1093/brain/113.3.793. [DOI] [PubMed] [Google Scholar]
- 17.Nieuwenhuys R. Anat Embryol. 1994;190:307–337. doi: 10.1007/BF00187291. [DOI] [PubMed] [Google Scholar]
- 18.Somogyi P. Brain Res. 1977;136:345–350. doi: 10.1016/0006-8993(77)90808-3. [DOI] [PubMed] [Google Scholar]
- 19.Alcantara S, Ruiz M, D’Arcangelo G, Ezan F, de Lecea L, Curran T, Sotelo C, Soriano E. J Neurosci. 1998;18:7779–7799. doi: 10.1523/JNEUROSCI.18-19-07779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendry S H, Jones E G, Emson P C, Lawson D E, Heizmann C W, Streit P. Exp Brain Res. 1989;76:467–472. doi: 10.1007/BF00247904. [DOI] [PubMed] [Google Scholar]
- 21.Kawaguchi Y, Kubota Y. Neuroscience. 1998;85:677–701. doi: 10.1016/s0306-4522(97)00685-4. [DOI] [PubMed] [Google Scholar]
- 22.Defelipe J, Hendry S H, Jones E G. Proc Natl Acad Sci USA. 1989;86:2093–2097. doi: 10.1073/pnas.86.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freund T F, Magloczky Z, Soltestz I, Somogyi P. Neuroscience. 1986;19:1133–1159. doi: 10.1016/0306-4522(86)90129-6. [DOI] [PubMed] [Google Scholar]
- 24.Kawaguchi Y, Kubota Y. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 25.Lund J S, Lewis D A. J Comp Neurol. 1993;328:282–312. doi: 10.1002/cne.903280209. [DOI] [PubMed] [Google Scholar]
- 26.Marin-Padilla M. Trends Neuroscience. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 27.Meyer G, Soria J M, Martinez-Galan J R, Martin-Clemente B, Fairen A. J Comp Neurol. 1998;397:493–518. [PubMed] [Google Scholar]
- 28.Super H, Soriano E, Uylings H B M. Brain Res Rev. 1998;27:40–64. doi: 10.1016/s0165-0173(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 29.Derer P, Derer M. Neuroscience. 1992;36:83–856. [Google Scholar]
- 30.Schiffmann S N, Bernier B, Goffinet A M. Eur J Neurosci. 1997;9:1055–1071. doi: 10.1111/j.1460-9568.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 31.Meyer G, Goffinet A M. J Comp Neurol. 1998;397:29–40. [PubMed] [Google Scholar]
- 32.Ohshima T, Ward J M, Huh C G, Longnecker G, Veeranna P H C, Pant H C, Brady R O, Martin L J, Kulkarni A B. Proc Natl Acad Sci USA. 1996;93:11173–11178. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chae T, Kwon Y T, Bronson R, Dikkes P, Li E, Tsai L-H. Neuron. 1997;18:29–42. doi: 10.1016/s0896-6273(01)80044-1. [DOI] [PubMed] [Google Scholar]
- 34.Sweet H O, Bronson R T, Johnson K R, Cook S A, Davisson M T. Mamm Genome. 1996;7:798–802. doi: 10.1007/s003359900240. [DOI] [PubMed] [Google Scholar]
- 35.Erlander M G, Tillakaratne N J K, Feldblum S, Patel N, Tobin A J. Neuron. 1991;7:91–100. doi: 10.1016/0896-6273(91)90077-d. [DOI] [PubMed] [Google Scholar]
- 36.Akbarian S, Kim J J, Potkins S G, Hagman J O, Tafazzoli A, Bunney W E, Jr, Jones E G. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 37.Goffinet A M. Nature (London) 1997;389:668–669. doi: 10.1038/39453. [DOI] [PubMed] [Google Scholar]