Abstract
New neurons continue to be generated in the adult dentate gyrus of the hippocampus. Corticosterone (CORT), a steroid secreted by the adrenal glands, had been shown to regulate progenitor proliferation. High levels of CORT suppress proliferation while low levels of the steroid stimulate it. Here we present an investigation into the regulation of survival by corticoids, with emphasis on the differential effects of the pre-mitotic and post-mitotic corticoid environments. Post-mitotic adrenalectomy increased subsequent survival of progenitors at 28 days, while additional CORT administered during the post-mitotic period decreased survival. In contrast, a corticoid-free environment prior to progenitor division resulted in a reduced survival rate of new cells and, similarly, high levels of CORT before proliferation reduced subsequent survival. In addition, phased treatment with CORT during a 27-day post-mitotic interval showed that newly formed cells lose their sensitivity to administered CORT after about 18 days. These results are the first to show that the corticoid environment both before and after cell division regulates survival.
Abbreviations : ADX, adrenalectomized; DHEA, dehydroepiandrosterone; PBS, phosphate-buffered saline
Introduction
Increasing evidence fosters the recent acceptance of adult hippocampal neurogenesis in a variety of species including humans (Eriksson et al, 1998). The process of forming new neurons in the dentate gyrus of the adult hippocampus can be divided into several phases, though they overlap. The rate of proliferation of progenitor cells determines the formation of new but undifferentiated daughter cells. Current estimates put this at about 9000 per day in the rat (Cameron & McKay, 2001). Proliferation is controlled by a variety of factors, including age (Kuhn et al., 1996), physical activity (van Praag et al., 1999) and exposure to novel environment (Kempermann et al., 1998), but most prominently by stress (Tanapat et al., 2001; Pham et al., 2003), a response associated with increased levels of glucocorticoids (Reul et al., 1987a). Various factors regulate proliferation in the dentate gyrus, but the survival of these newly formed cells is also a critical event. Recent estimates suggest that only about a half of newly formed cells survive for at least 28 days in the rat (Dayer et al., 2003). Relatively little is known about determinants of survival, though the adrenal steroid dehydroepiandrosterone (DHEA) is known to enhance it as well as survival of other types of stem cell (Karishma & Herbert, 2002; Suzuki et al., 2004). During the survival period, newly formed cells differentiate into either neurons (estimated at about 70%) or glia (Cameron et al., 1993b). Whether endocrine factors control differentiation in the dentate gyrus has hardly been studied, though if factors exist that direct differentiation into one or other pathway these would be important. Finally, it has been shown that newly formed neurons grow axons that make functional synaptic contact with the CA3 pyramidal cells of the hippocampal cortex (Hastings & Gould, 1999), a process which itself may be subject to specific controls.
In this paper, we focus on the role of glucocorticoids in the survival of newly formed cells in the dentate gyrus. The levels of glucocorticoids secreted by the adrenal glands are highly susceptible to environmental factors, including the daily light/dark cycle (Reul et al., 1987a) as well as intermittent events such as physical or psychological stress (Reul et al., 1987b). Previous work has established beyond doubt that excess glucocorticoid, whether resulting from administered hormones or experimental stressors, reduces the rate of cell division (Gould et al., 1992; Cameron & Gould, 1994; Tanapat et al., 2001; Pham et al., 2003; Czeh et al., 2002 Malberg & Duman, 2003), whereas adrenalectomy enhances this process (Gould et al., 1992; Cameron & Gould, 1994). However, the role of glucocorticoids in the survival of the new cells (and whether this role, if demonstrable, is distinct from that on proliferation) remains unknown and is thus the objective of this study. It is clear that survival is a critical phase in the process whereby progenitor cells are translated into adult functional neurons in the adult hippocampus. The question of whether glucocorticoids also alter the differentiation of these newly formed cells will be taken up in a subsequent paper.
Materials and methods
Animals
All procedures were carried out under Home Office (UK) licence; Lister hooded male rats (Harlan, UK), weighing about 250–300 g at the beginning of the experiment, were used. Rats were housed three–four per cage on reversed 12 h light : dark cycles (lights off at 11.00 h). Ambient temperature was maintained at 21 ± 2 °C. Food and water were available ad libitum. All surgeries were carried out under general anaesthesia using isoflurane. Adrenalectomized animals were given a choice of 0.9% saline and water to replenish salt loss.
BrdU injection and corticosterone level manipulations
BrdU (Sigma), a thymidine analogue incorporated into dividing cells during DNA synthetic phase (S-phase) of the cell cycle, was dissolved in 0.9% saline and injected i.p. (200 mg/kg) at midday. A single BrdU injection paradigm was chosen to ensure that the population of cells labelled would come from the same time frame, and thus all new-born cells are in the same developmental phase at the start of the drug treatment.
To remove circulating corticoids, bilateral adrenalectomies were performed under isofluorane anaesthesia. Basal level of corticosterone was replaced with subcutaneous implant of 1 : 1 corticosterone/cholesterol (Sigma) melted and moulded into pellets weighing 200–220 mg. To increase plasma corticosterone above ‘basal’ levels, animals were given additional s.c. injection of corticosterone suspended in peanut oil at 40 mg/kg/day, a dose shown to reduce neurogenesis.
Experimental procedures
Experiment 1
Four groups of animals were used in this experiment (n = 80). They all received a single injection of BrdU (200 mg/kg, i.p.) to label the new-born cells. In Group 1 (n = 8), animals were killed 24 h after BrdU labelling, whereas in Group 2 (n = 24) they were sham-operated 24 h after BrdU and implanted s.c. with a cholesterol pellet. Eight rats were perfused at 7, 18 and 28 days after surgery. Group 3 (n = 24) was bilaterally adrenalectomized (ADX) 24 h after BrdU and implanted with a cholesterol pellet. Rats (n = 8) were sampled at the same intervals. Group 4 (n = 24) were also ADX, but received a 50% corticosterone pellet (200–250 mg). They were sampled at the same intervals (Fig. 1A).
Fig. 1.
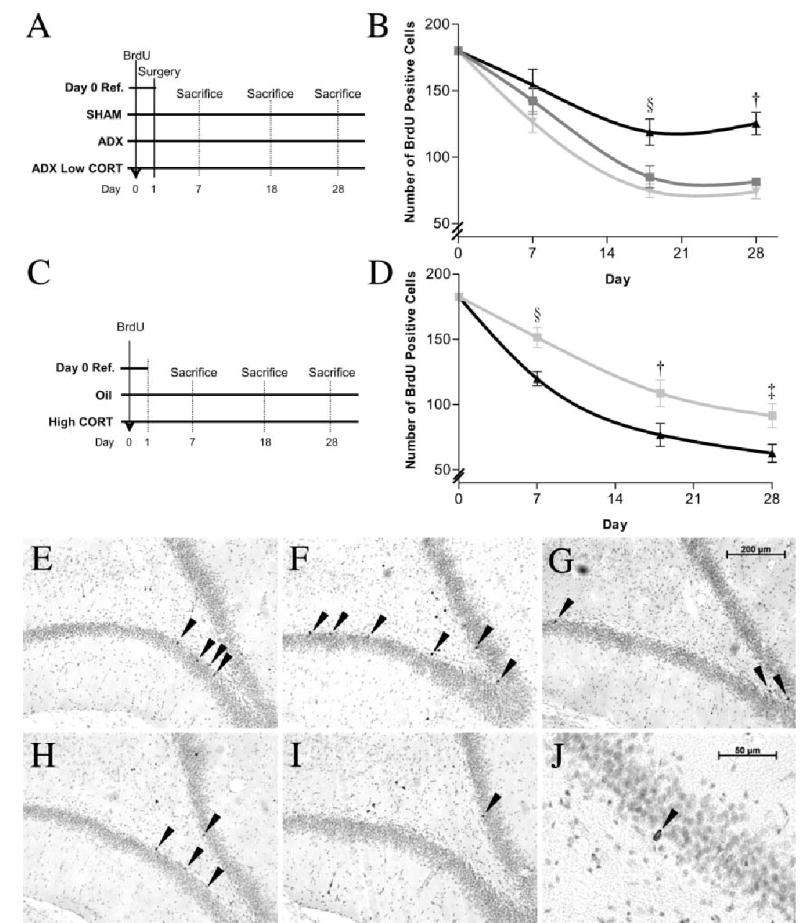
Endogenous corticosterone decreases post-mitotic survival. (A) Design of Experiment 1. (B) Mean ± SE of surviving BrdU-labelled cells over time for the three treatment groups: sham-operated (▪), adrenalectomized (ADX) (▴) and ADX with basal corticosterone replacement (ADX Low corticosterone) (▾) (§P < 0.023; †P< 0.0001). Additional corticosterone reduces post-mitotic survival. (C) Design of Experiment 2. (D) Mean ± SE of surviving BrdU-labelled cells over time for animals receiving daily injection of oil (▪) or high corticosterone (40 mg/kg) (▴) (§P < 0.007; †P < 0.04; ‡P < 0.029). (E–G) Twenty-eight-day-old BrdU-labelled cells in dentate gyrus in sham, ADX and ADX Low corticosterone animals, respectively. (H, I) Twenty-eight-day-old BrdU-labelled cells in oil or high corticosterone-treated animals, respectively. (J) Morphology of BrdU-labelled cells at high magnification.
Experiment 2
Forty-two animals, given a single injection of BrdU as before, were divided into three groups. Group 1 (n = 6) was killed 24 h after the BrdU injection. Group 2 (n = 18) received a daily injection of peanut oil, beginning 24 h after BrdU. Group 3 (n = 18) received 40 mg/kg/day corticosterone suspended in peanut oil. Animals (n = 6) from each treatment group were sampled at the same time intervals as in the last experiment (Fig. 1C).
Experiment 3
Fifty-six intact rats were given BrdU as above, and then received various regimes of daily s.c. injections of either corticosterone (40 mg/kg/day) or oil for 27 days. They were divided into seven groups of eight rats. Group 1 received oil throughout. The next three groups received corticosterone for 9 days each, and oil otherwise. The start of corticosterone was staggered by 9 days in each group (group 2 days 1–9, group 3 days 10–18, group 4 days 19–27). Group 5 received corticosterone on days 1–18, group 6 days 10–27 and group 7 days 1–27. All rats were killed on day 28 (Fig. 2A).
Fig. 2.
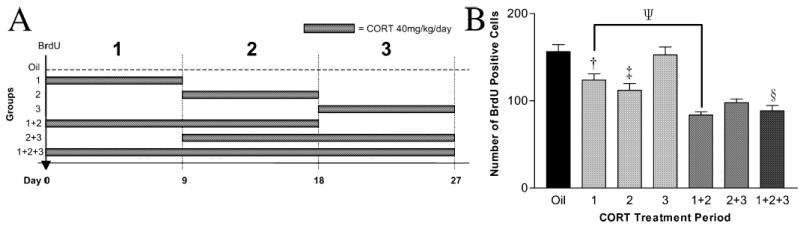
Effect of phased treatment with corticosterone on survival of newly formed cells. (A) Animals received phased excess corticosterone treatment (oil otherwise) following the regimes depicted. (B) Mean ± SE of BrdU cells at time of death; group 1 + 2 + 3 confirms long-term treatment with high corticosterone post-BrdU reduces survival (§P < 0.0001). Phased 9-day treatment during period 1 and 2, but not 3, significantly reduced survival of the newly formed cells (†P < 0.029, ‡P < 0.001), suggesting progenitors are only sensitive to high corticosterone during the first 18 days after mitosis. Further phased high corticosterone treatment to the first 9 days (group 1 vs. 1 + 2) resulted in additional reduction (ΨP < 0.003). However, no significant difference was observed comparing groups 2 and groups 2 + 3.
Experiment 4
Rats were divided into two major groups (n = 24 each). The first group was given daily oil injections for 10 days (pre-treatment), and then two injections of BrdU, 3 h apart, on day 10. This double injection protocol served to label a larger pool of proliferating cells to compensate the suppressive effect of corticosterone on cell division. The second group (n = 24) was given daily injections of corticosterone (40 mg/kg/day) for 10 days, and BrdU as above. Each group was then divided into three subgroups. Groups 1a and 2a were killed 24 h after the BrdU injection. Groups 1b and 2b were given another 28 days (post-treatment) of oil injections. Groups 1c and 2c were given 28 days of corticosterone (40 mg/kg/day). The latter four subgroups were perfused 24 h after the last injection (day 29) (Fig. 3A).
Fig. 3.
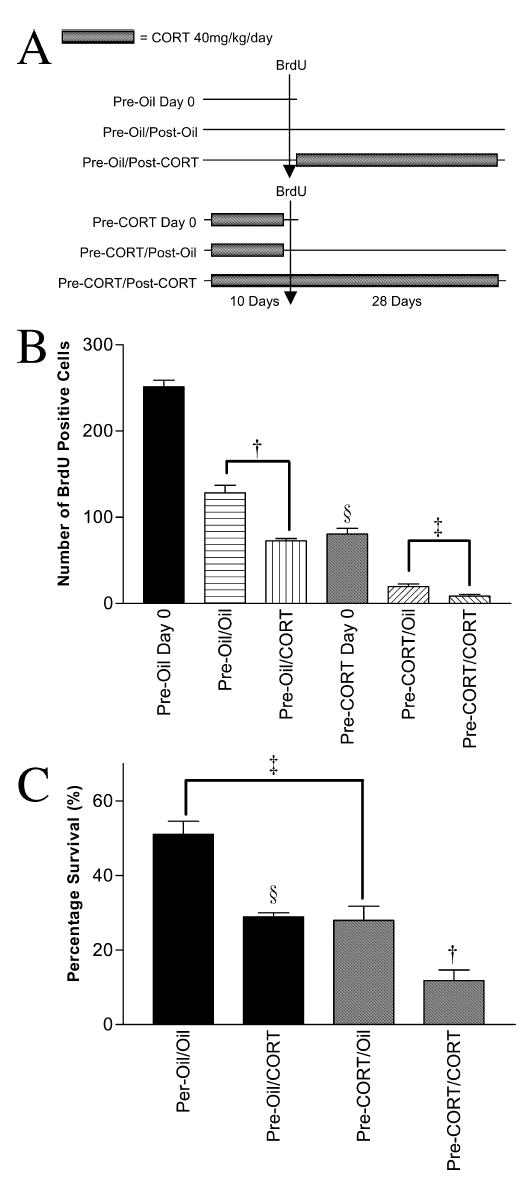
(A) Schematic diagram showing oil/corticosterone/BrdU treatments given to each group. (B) Mean ± SE of BrdU cells at time of death. Comparison of oil and high corticosterone pre-treatment confirms the suppressive effect of excessive corticosterone on proliferation (§P< 0.0001). Post-BrdU treatment with high corticosterone for 28 days significantly reduced survival regardless of type of pre-treatment given (†P < 0.0001, ‡P < 0.009). (C) Mean survival percentages (to corresponding day 0) demonstrate pre-BrdU high corticosterone treatment reduced subsequent survival of the progenitor cells. Both corticosterone pre-treatment (‡P < 0.0001) and post-mitotic corticosterone (§P < 0.0001, †’P < 0.004) reduced survival.
Experiment 5
All rats were ADX (n = 48), half were given basal corticosterone replacement (s.c. implant of 50% corticosterone pellet) and the others received a cholesterol pellet. Ten days after surgery, all animals received a single injection of BrdU. Each of the two original groups was divided into three subgroups. The first two subgroups (la, 2a: n = 8 per group) were killed 24 h after the BrdU injection. The second subgroups (1b, 2b: n = 8) had their corticosterone or cholesterol pellets removed and replaced with new, corticosterone pellet 24 h after BrdU. The third subgroups (1c, 2c; n = 8) had their corticosterone or cholesterol pellets removed and replaced with cholesterol pellets. Subgroups b and c were perfused 29 days after BrdU (Fig. 4A).
Fig. 4.
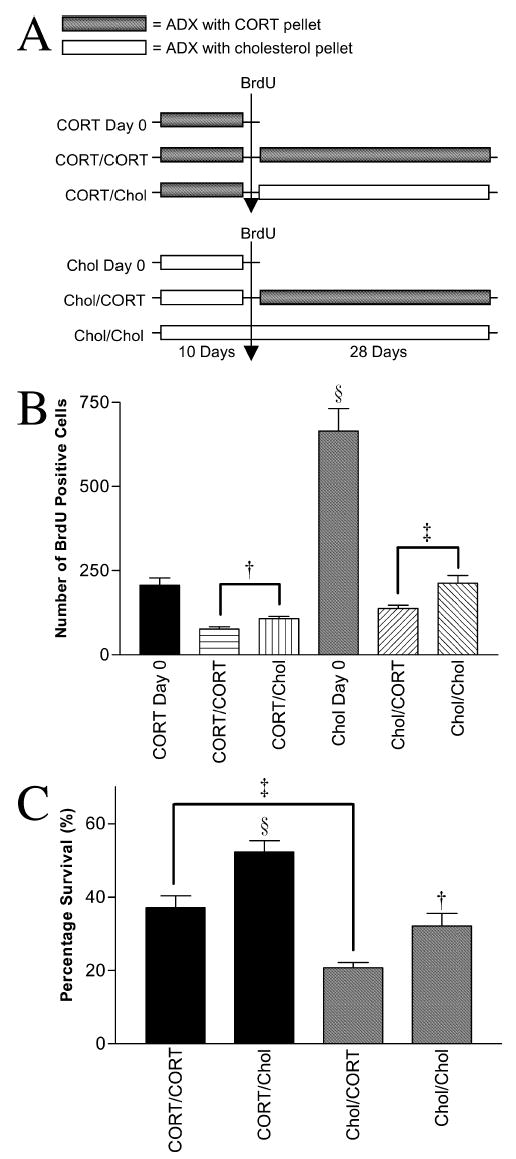
(A) Schematic diagram showing corticosterone manipulations given to each group. (B) Mean ± SE of BrdU cells at time of death in adrenalectomized (ADX) rats. Corticosterone replacement (basal levels) before BrdU confirms the stimulating effect of ADX on proliferation (§P < 0.0001). Removal of corticosterone post-BrdU enhanced survival of progenitors at day 28 irrespective of pre-BrdU treatment (†P < 0.024, ‡P < 0.005). (C) Mean of survival counts expressed as percentage of corresponding day 0 group. A glucocorticoids-free environment before BrdU labelling resulted in a decrease in subsequent survival compared with corticosterone pre-treatment (‡P < 0.001), while the same environment during the post-mitotic period enhanced survival (§P < 0.039, ‡P < 0.001). This suggests that although corticosterone removal stimulates proliferation, a much smaller proportion of these new cells survive.
BrdU immunohistochemistry
Animals were given an overdose of pentobarbitone sodium and perfused transcardially with 0.1 M phosphate buffer followed by 4% paraformaldehyde in 0.01 M KPBS (phosphate-buffered saline, pH 7.4). Brains were removed, post-fixed for 4 h, and then immersed in 20% sucrose in KPBS overnight for cryoprotection against formation of water ice crystals during freezing. Coronal sections were cut using a freezing microtome through the entire dentate gyrus at 40 μm and sections were stored in antifreeze (1:1:2 glycerol : ethyleneglycol : 0.1 M PBS). All rinses were done in KPBS. Sections were first mounted onto poly-lysine-coated slides and dried overnight. They were then antigen-retrieved in 0.01 M citric acid for 15 min at 97 °C, cooled for 30 min, incubated in 3% H2O2 for 10 min, digested with trypsin 250 (0.025% in KPBS) for 10 min, denatured in 2 N HCl for 30 min at 37 °C, and incubated overnight at room temperature in mouse monoclonal antibody raised against BrdU (Novocastra; 1 : 200 in 0.3% Triton, 1% normal horse serum). The slides were then taken through a mouse IgG ABC kit procedure (Vector), and reacted for 10 min with a diaminobenzidine (Sigma). The slides were counter-stained with Cresyl violet, dehydrated and cover slipped under DPX.
Quantification
All slides were randomized and coded prior to quantitative analysis. Slides were examined using a 40 × objective and BrdU-labelled cells were counted on every 12th section through the entire dentate gyrus bilaterally (about nine sections per animal). Only cells on the border of the subgranular zone and hilus were counted. BrdU cells in all focal planes through the 40-μm section were included. The data shown are that obtained from this one-in-12 section sample. We intentionally avoided the stereology method as it generates data with high variance (Alonso, 2000).
Statistical analysis
Data were analysed by one-, two- or three-way ANOVA; the data were transformed before analysis if the variances were not homogenous. Instances in which non-parametric tests were used are specified in the Results section.
Results
Effect of post-mitotic glucocorticoid environment on survival
In Experiment 1 we examined the effect of post-mitotic glucocorticoids manipulations on survival of the newly formed cells. Figure 1B shows that the numbers of surviving BrdU-labelled cells decreased progressively from 24 h after BrdU administration to day 7 and then until day 18 in all three groups. Two-way ANOVA showed a highly significant effect of the three post-BrdU treatments (sham-operation, ADX, ADX with corticosterone replacement) on the number of labelled cells from day 7 to day 28 (F2,77 = 15.3, P < 0.0001). There was also a significant effect of time (days 0, 7, 18, 28; F2,77 = 26.4, P < 0.0001). Post-hoc comparisons showed that survival of cells in ADX rats without corticosterone replacement was significantly higher (Bonferroni, P < 0.002) than the other two groups, which did not differ from each other (P = 0.864). One-way ANOVA of the sham and ADX groups at different time points revealed that adrenalectomy significantly increased survival at day 18 (F1,15 = 6.8, §P < 0.023) and day 28 (F1,15 = 25.3, †P < 0.0001), but not day 7 (F1,15 = 0.57, P = 0.47). These data demonstrate that only half of the new cells born in the adult dentate gyrus survive over a period of 28 days under normal physiological conditions (sham). Removal of endogenous corticoids by adrenalectomy favours survival of the new cells. Replacing corticosterone in ADX animals restores the survival profile to that similar to sham, which further confirms that corticosterone is the major regulator controlling post-mitotic cell survival.
To further characterize the effect of corticosterone on survival of newly formed cells, Experiment 2 was carried out to examine the effect of excess corticosterone on post-mitotic survival. Figure 1D shows the progressive reduction in labelled cells at increasing sampling periods in both groups. There was a significant effect of both post-BrdU treatment (oil/corticosterone; F1,41 = 11.9, P < 0.001) and time (days 7, 18, 28; F2,41 = 15.4, P < 0.0001) on the number of BrdU-labelled cells. One-way ANOVA showed that there were significantly fewer surviving cells in the corticosterone-treated group on all three days sampled post-BrdU (day 7: F1,11 = 11.4, §P < 0.007; day 18: F1,11 = 5.56, †P < 0.04; day 28: F1,11 = 6.47, ‡P < 0.029). These results show that increased plasma corticosterone reduces survival of newly formed cells during the post-mitotic development period. Taken together, these two experiments make the case for a major controlling action of glucocorticoids on post-mitotic survival of neural progenitors in the adult dentate gyrus.
Time-dependent effect of corticosterone on post-mitotic survival
The first experiment (Fig. 1B) seemed to suggest that the progressive decline of labelled cells tailed off after 18 days in the controls, as did the disinhibiting effect on survival of removing endogenous glucocorticoids. However, the second experiment (Fig. 1D) showed that numbers of BrdU-labelled cells were still declining in corticosterone-treated rats. Therefore we did a further experiment (Experiment 3) in which rats received periods of corticosterone treatment at varying intervals following an initial injection of BrdU (Fig. 2A). This experiment showed that survival was reduced by corticosterone given during the first 18 days after BrdU labelling but not during the final 9 days before death (Fig. 2B). Three-way ANOVA, with each period as the independent variable, showed an overall treatment effect (F6,55 = 20.7, P< 0.0001), and that both the first 9 (F1,55 = 16.5, P< 0.0001) and the second 9 days (F1,55 = 67.2, P < 0.0001) of corticosterone treatment reduced survival, but that the third period (days 19–27) had no effect (F1,55 = 0.195, P = 0.661). Post-hoc analyses showed that corticosterone treatment from days 1–27 significantly reduced survival (Bonferroni, §P < 0.0001). Corticosterone treatment for either days 1–9 or 10–18 reduced survival (compared with oil treatment) (Bonferroni, †P < 0.029, ‡P < 0.001, respectively), but these two periods did not differ from each other (Bonferroni, P = 1.00). Corticosterone treatment for days 19–27 had no significant effect (Bonferroni, P = 1.00). Both 1–18 and 10–27 days of corticosterone treatment suppressed survival (Bonferroni, P< 0.0001, P< 0.0001, respectively). Extending the treatment from 1–9 to 1–18 days further reduced survival (Bonferroni, ΨP < 0.003, 1 vs. 1 + 2), but an additional 9-day treatment from day 18 gave no further reduction (Bonferroni, P = 1.00, 2 vs. 2 + 3). This experiment shows that newly formed cells are sensitive to corticosterone for about 18 days after labelling with BrdU, but become insensitive during the final 9 days presumably as one result of the maturation process.
Effect of glucocorticoid environment at time of progenitor proliferation on subsequent survival
Having established the effect of corticosterone on post-mitotic survival, the next two experiments (4 and 5) aimed to examine if changing the glucocorticoid environment prior to progenitor proliferation would affect subsequent survival. Figure 3B shows that there were marked differences in the number of BrdU-labelled cells in the different groups. As expected, pre-BrdU-treatment with 40 mg/kg/day corticosterone reduced BrdU labelling in samples taken 24 h later in comparison with oil pre-treatment, demonstrating the suppressive effect of corticosterone on proliferation (F1,15 = 279.4, §P < 0.0001). Analysis of the four groups surviving for 28 days showed significant effects of both pre- and post-BrdU treatment with corticosterone (F1,31 = 420.4.0, P < 0.0001; F1,31 = 49.9, P< 0.0001, respectively). Corticosterone treatment given post-BrdU labelling significantly reduced survival in both pre-treatment groups (one-way ANOVA, oil: F1,15 = 41.4, †P < 0.0001; corticosterone: F1,15 = 9.53, ‡P < 0.009). This was reflected in the overall survival, calculated as the mean percentage labelled cells at 29 days of the corresponding groups killed 24 h post-BrdU (Fig. 3C). The mean survival percentages for each group were pre-oil/oil = 51.1%; pre-oil/corticosterone = 28.9%; pre-corticosterone/oil = 27.9%; pre-corticosterone/corticosterone = 11.8%. Two-way ANOVA showed significant effect on reduced survival for both corticosterone pre-treatment (F1,31 = 47.5, P < 0.0001) and post-treatment (F1,31 = 43.1, P< 0.0001). One-way ANOVA with post-hoc tests also showed that post-treatment with corticosterone suppressed survival (Bonferroni, §P < 0.0001, pre-oil/oil vs. pre-oil/corticosterone; Bonferroni, †P < 0.004, pre-corticosterone/oil vs. pre-corticosterone/corticosterone). It is important to note that pre-treatment with corticosterone (and oil treatment after BrdU) also resulted in reduced survival (Bonferroni, ‡P < 0.0001); this was evident when comparing pre-oil/oil with pre-corticosterone/oil groups (Fig. 3C).
As shown in Experiment 1, endogenous corticosterone suppressed post-mitotic survival; removal of circulating corticoids increased the number of surviving BrdU cells 28 days later. Therefore, the next question we addressed was whether removing corticosterone prior to progenitor division would enhance subsequent survival of those cells proliferating in a corticosterone-free environment. Figure 4B shows that ADX animals (ADX day 0) perfused 24 h after BrdU injection had more labelled cells compared with ADX animals who received basal corticosterone replacement (Repld day 0) (F1,15 = 42.2, §P < 0.0001). This confirms the stimulating effect of adrenalectomy on progenitor proliferation. Two-way ANOVA revealed significant effect on survival as caused by a corticoids-free environment, both prior (F1,31 = 41.5, P < 0.0001) and after (F1,31 = 19.1, P < 0.0001) BrdU labelling. One-way ANOVA and post-hoc tests stated survival enhancement in the post-mitotic period (Bonferroni, †P < 0.024, corticosterone/corticosterone vs. corticosterone/Chol; Bonferroni, ‡P < 0.005, Chol/corticosterone vs. Chol/Chol). Because pre-treatment altered the number of labelled cells, survival can also be expressed as a percentage of this number (based on the two groups killed at day 0, Fig. 4C). The mean survival percentages of the four groups were corticosterone/corticosterone = 37.1%, corticosterone/Chol = 52.3%, Chol/corticosterone = 20.7% and Chol/Chol = 32.1%. Two-way ANOVA revealed significant effect on survival as caused by a corticoids-free environment, both prior (F1,31 = 41.5, P< 0.0001) and after (F1,31 = 19.1, P< 0.0001) BrdU labelling. One-way ANOVA and post-hoc tests stated survival enhancement in the post-mitotic period (Bonferroni, §P < 0.039, corticosterone/corticosterone vs. corticosterone/Chol; Bonferroni, †P < 0.001, Chol/corticosterone vs. Chol/Chol). Although post-mitotic glucocorticoids depletion enhanced survival, a glucocorticoids-free environment before and at the time of division destined lowered survival (Bonferroni, ‡P < 0.001, corticosterone/corticosterone vs. Chol/corticosterone). It appears that, in addition to the regulating role of corticosterone during the post-mitotic period, the glucocorticoid environment in which progenitor divisions take place also governs the consequent survival of the newly formed cells.
Discussion
Despite the fact that glucocorticoids are well known to regulate the rate of proliferation in adult dentate gyrus, there has been no evidence yet to suggest they also have separable, but major, effects on the survival of newly formed cells. Our first experiment demonstrated that removing endogenous corticoids by bilateral adrenalectomy immediately following a single injection of BrdU substantially increased survival of previously labelled cells. Because replacing endogenous corticoids with exogenous corticosterone reversed this result, it seems highly likely that this controlling action lies mostly with glucocorticoids, though further experiments on the role of mineralocorticoids, and their possible interaction with glucocorticoids, remain to be done. Moreover, because high levels of corticosterone have been shown to reduce proliferation (Cameron & Gould, 1994), we were interested to see if this would have a similar effect on post-mitotic survival. The second experiment showed clearly that labelling proliferating cells with BrdU and then treating the rats with excess corticosterone reduced survival at the three time points sampled. These two experiments illustrate that just as decreasing post-BrdU corticoids increases survival, the opposite is true when corticosterone levels are increased.
In the next experiment, animals received phased treatment of corticosterone at varying intervals following an initial injection of BrdU. This showed clearly that there was a marked difference in sensitivity to corticosterone between the first 18 and the final 9 days of the survival period. Corticosterone given during the first or second 9 days reduced subsequent survival at 28 days. Treatment with corticosterone during the final 9 days had no effect. A recent study shows that BrdU-labelled cells begin to express NeuN, a marker of mature neurons, at about day 18 (Brown et al., 2003). This coincides with our data that newly formed cells lose sensitivity to glucocorticoids 18 days after labelling. Therefore, it seems plausible that newly formed cells become less dependent on the surrounding corticoid environment over time as one result of the maturation process. Further experiments are required to define the sensitive period more exactly, and to reveal the mechanism for it.
A recent study on the properties of newly-born cells in the adult dentate gyrus showed that the BrdU-labelled cells continue to divide for up to 4 days after BrdU injection (Dayer et al., 2003). Because glucocorticoids exert powerful effects on proliferation, it is arguable that the effects observed on survival were simply the consequence of glucocorticoids regulating post-labelling divisions. However, the fact that there was no significance between groups at day 7 in the first experiment (Fig. 1B) argues against this possibility, and there is further evidence from the time-phase experiment, which showed that animals receiving corticosterone treatment for days 9–18 after labelling (a period with presumably no dividing pre-labelled BrdU cells) had significantly fewer surviving BrdU cells at day 27. Together, these experiments point to corticosterone as a major regulator of post-mitotic survival of new-born cells in the adult dentate gyrus independent of its effects on proliferation.
Besides the effect of corticosterone on post-mitotic survival, we also investigated the effect of exposing proliferating cells to excess corticosterone before BrdU labelling and/or during the subsequent survival period. Administration of excess corticosterone prior to progenitor labelling reduced subsequent survival, irrespective of post-mitotic exposure, to levels similar to animals receiving only post-BrdU excess corticosterone, suggesting that the glucocorticoid environment of the dentate gyrus both before and at the time of proliferation determines consequent survival of the newly formed cells. The converse experiment shows that adrenalectomy during the post-mitotic interval increased survival of BrdU pre-labelled progenitors. However, a corticosterone-free environment before and at the time of proliferation, whilst increasing proliferation, destined new-born cells to a reduced survival rate. This is the first study to compare directly the effect of pre- and post-treatment with glucocorticoids on survival of newly formed cells in the dentate gyrus, and further demonstrates that the endocrine environment in which progenitors divide is crucial to their future survival. It seems highly likely that this environmental regulation of survival acts through neighbouring cells as new-born cells in the dentate gyrus do not express corticoid receptors (Cameron et al., 1993a). Reports on the importance of the local environment in regulating proliferation and survival support this idea (Seki, 2002; Song et al., 2002; Hastings & Gould, 2003), but further work on this topic is required before a solid conclusion can be drawn.
It is well documented that there is an inverted U-shaped relationship between corticosteroid levels and hippocampal functions such as spatial learning (Diamond et al., 1992; Yau et al., 1995; Conrad et al., 1999). Moderate elevation of plasma corticosterone levels has been shown to favour hippocampal-dependent memory consolidation (Hui et al., 2004) and memory retrieval (Okuda et al., 2004), while excess corticosterone impairs these processes (Newcomer et al., 1994; Endo et al., 1996; de Quervain et al., 1998; McLay et al., 1998; Coburn-Litvak et al., 2003). Our results on the effects of the corticoid environment at the time of proliferation on subsequent survival support a similar relation: either excess or reduced corticoids impair survival at 28/29 days. However, the post-mitotic corticoid environment does not show this relationship. Recent evidence suggests that corticosterone regulates adult hippocampal neurogenesis through activation of both the mineralocorticoid and glucocorticoid receptors, in a pattern similar to that in hippocampal-dependent learning (Conrad et al., 1999; Czeh et al., 2002; Montaron et al., 2003). Whether these mechanisms also apply to post-mitotic survival remains to be studied.
Our results demonstrate that the endocrine control of neurogenesis extends into the post-mitotic interval. They also show that the corticoid environments prevailing at both progenitor division and during the post-mitotic process are important modulating signals determining the survival of the new-born cells, but that this is limited to earlier stages of the maturation trajectory. This raises the possibility that other modulators of neurogenesis, such as growth factors (Wagner et al., 1999; Aberg et al., 2000), exercise (van Praag et al., 1999) or learning tasks (Gould et al., 1999; Shors et al., 2001), may affect survival in a manner similar to glucocorticoids. As well as survival of progenitors, there are clear indications that differentiation of the newly formed cells can be varied by the local environment of these cells. Analyses to address the question of whether glucocorticoids affect neuronal differentiation are now under way (Palmer et al., 2000 Belachew et al., 2003). We have already reported that a second adrenal steroid, DHEA, present at high levels in primates, promotes survival of newly formed cells in the dentate gyrus (Karishma & Herbert, 2002), so it is becoming clear that the steroid environment in which post-mitotic cells find themselves regulates the number that are available for differentiation and subsequent connection to CA3. How far this can be attributed to defined cellular events such as apoptosis remains to be determined. Future work will determine the identity of the cellular and molecular elements in the microenvironment of the progenitors (Seki, 2003) that are responsible for the effects we describe in this study.
Acknowledgments
We thank Helen Shiers and Sarah Cleary for technical assistance. This work was supported by a grant from the Wellcome Trust. Edmund Y.H. Wong received a scholarship from the Cambridge Commonwealth and Cambridge Overseas Trust.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Belachew S, Chittajallu R, Aguirre AA, Yuan X, Kirby M, Anderson S, Gallo V. Postnatal NG2 proteoglycan-expressing progenitor cells are intrinsically multipotent and generate functional neurons. J Cell Biol. 2003;161:169–186. doi: 10.1083/jcb.200210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993a;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993b;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Coburn-Litvak PS, Pothakos K, Tata DA, McCloskey DP, Anderson BJ. Chronic administration of corticosterone impairs spatial reference memory before spatial working memory in rats. Neurobiol Learn Mem. 2003;80:11–23. doi: 10.1016/s1074-7427(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for a bimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Muller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Bennett MC, Fleshner M, Rose GM. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Endo Y, Nishimura JI, Kimura F. Impairment of maze learning in rats following long-term glucocorticoid treatments. Neurosci Lett. 1996;203:199–202. doi: 10.1016/0304-3940(95)12296-6. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Neurons inhibit neurogenesis. Nat Med. 2003;9:264–266. doi: 10.1038/nm0303-264. [DOI] [PubMed] [Google Scholar]
- Hui GK, Figueroa IR, Poytress BS, Roozendaal B, McGaugh JL, Weinberger NM. Memory enhancement of classical fear conditioning by post-training injections of corticosterone in rats. Neurobiol Learn Mem. 2004;81:67–74. doi: 10.1016/j.nlm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Karishma KK, Herbert J. Dehyrodepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly-formed neurons and prevents corticosterone-induced suppression. Eur J Neurosci. 2002;16:445–453. doi: 10.1046/j.1460-9568.2002.02099.x. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Zadina JE. Chronic corticosterone impairs memory performance in the Barnes maze. Physiol Behav. 1998;63:933–937. doi: 10.1016/s0031-9384(97)00529-5. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Craft S, Hershey T, Askins K, Bardgett ME. Glucocorticoid-induced impairment in declarative memory performance in adult humans. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neural. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- Reul JM, van den Bosch FR, de Kloet ER. Differential response of type I and type II corticosteroid receptors to changes in plasma steroid level and circadian rhythmicity. Neuroendocrinology. 1987a;45:407–412. doi: 10.1159/000124766. [DOI] [PubMed] [Google Scholar]
- Reul JM, van den Bosch FR, de Kloet ER. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J Endocrinol. 1987b;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- Seki T. Hippocampal adult neurogenesis occurs in a microenvironment provided by PSA-NCAM-expressing immature neurons. J Neurosci Res. 2002;69:772–783. doi: 10.1002/jnr.10366. [DOI] [PubMed] [Google Scholar]
- Seki T. Microenvironmental elements supporting adult hippocampal neurogenesis. Anat Sci Int. 2003;78:69–78. doi: 10.1046/j.0022-7722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Wright LS, Marwah PL, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci USA. 2004;101:3202–3207. doi: 10.1073/pnas.0307325101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neural. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Wagner JP, Black IB, DiCicco-Bloom E. Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci. 1999;19:6006–6016. doi: 10.1523/JNEUROSCI.19-14-06006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JL, Olsson T, Morris RG, Meaney MJ, Seckl JR. Glucocorticoids, hippocampal corticosteroid receptor gene expression and antidepressant treatment: relationship with spatial learning in young and aged rats. Neuroscience. 1995;66:571–581. doi: 10.1016/0306-4522(94)00612-9. [DOI] [PubMed] [Google Scholar]


