Abstract
New neurons are produced continually in the dentate gyrus of the hippocampus. Numerous factors modulate the rate of neuron production. One of the most important is the adrenal-derived corticoids. Raised levels of corticoids suppress proliferation of progenitor cells, while removal of corticoids by adrenalectomy reverses this. The exact mechanisms by which corticoids mediate such regulation are unknown, but corticoids are believed to act through the receptors for mineralocorticoids (MR) and glucocorticoids (GR). Previous reports regarding the roles of these receptors in regulating cell proliferation came to contrasting conclusions. Here we use both agonists and antagonists to these receptors in adult male rats to investigate and clarify their roles. Blockade of MR with spironolactone in adrenalectomised male rats implanted with a corticosterone pellet to reproduce basal levels enhanced proliferation, whereas treatment with the GR antagonist mifepristone had no effect. However, mifepristone reversed the suppressive effect of additional corticosterone in intact rats. Both aldosterone and RU362, agonists of MR and GR, respectively, reduced proliferation in adrenalectomised rats, and combined treatment with both agonists had an additional suppressive action. These results clearly show that occupancies of both receptors act in the same direction on progenitor proliferation. The existence of two receptors with different affinities for corticoids may ensure that proliferation of progenitor cells in the adult dentate gyrus is regulated across the range of adrenal corticoid activity, including both basal and stressful contexts. Although a small proportion of newly formed cells may express GR and MR, corticosterone probably regulates proliferation indirectly through other local cells.
Keywords: adrenal-derived corticoids, adrenalectomy, dentate gyrus, mifepristone, neurogenesis, spironolactone
Abbreviations: ADX, adrenalectomy or adrenalectomised; ALDO, aldosterone; CORT, corticosterone; GR, glucocorticoid receptor; KPBS, potassium phosphate buffered saline; MIFE, mifepristone; MR, mineralocorticoid receptor; SPIR, spironolactone
Introduction
Adult neurogenesis occurs in the adult brain in diverse mammalian species including rodents (Kuhn et al., 1996), monkeys (Gould et al., 1999) and humans (Eriksson et al., 1998). Two of the regions in the adult brain demonstrating constitutive adult neurogenesis are the subventricular zone (Corotto et al., 1993) and the dentate gyrus in the hippocampus (Kuhn et al., 1996), a region which mediates memory formation (Izquierdo & Medina, 1997) and consolidation (Nadel & Moscovitch, 1997). Although the roles of these newly generated neurons in the hippocampus are unknown, recent findings have suggested that new neurons may play important roles in learning (Shors et al., 2001). The process of neurogenesis can be divided into three distinct phases, namely proliferation, survival and differentiation. The experiments described in this report focus on the first phase of this developmental trajectory.
The rate of proliferation of new neurons can be modulated by various factors including changes in the external environment (Kempermann et al., 1997), the endocrine system (Brezun & Daszuta, 1999; Kulkarni et al., 2002) and neurological disorder or injury (Liu et al., 1998; Haughey et al., 2002). Corticoids represent one of the most potent regulatory factors of progenitor proliferation so far known. Corticoids, principally mineralocorticoids such as aldosterone (ALDO) and glucocorticoids such as corticosterone (CORT), are steroids produced by the adrenal glands in rodents. Under stressful conditions, corticoids are released from the adrenal glands into the bloodstream and subsequently enter the brain. It has been previously demonstrated that stress, whether psychological (Tanapat et al., 2001) or physical (Pham et al., 2003), reduces the rate of progenitor proliferation in the dentate gyrus, and that this down-regulation in proliferation is mediated by raised levels of corticoids (Tanapat et al., 2001).
Experimental manipulation of circulating corticoid levels have shown that raised level of glucocorticoids suppress proliferation of progenitor cells in the dentate gyrus (Gould et al., 1992; Gould et al., 1997; Ambrogini et al., 2002; Hellsten et al., 2002), whereas removal of corticoids by adrenalectomy stimulates this process, suggesting that even endogenous corticoids regulate progenitor division (Cameron & Gould, 1994; Gould et al., 1992). Similar findings have been reported in studies of hippocampal-dependent learning (Conrad et al., 1999).
High levels of the two types of corticoid receptor, mineralocorticoid (MR) and glucocorticoid (GR), are present in the dentate gyrus (Van Eekelen et al., 1988). A major difference between the two corticoid receptors is that MRs have a 10-fold higher affinity for corticosterone than GRs (Reul & de Kloet, 1985). Although it is known that corticoids regulate proliferation of progenitors in the adult dentate gyrus, the roles of the two receptors in mediating this process are unclear. It has previously been reported that activation of MR stimulates proliferation (Fischer et al., 2002). These authors observed that adrenalectomy stimulated progenitor proliferation and that treatment with an MR agonist further increased cell proliferation. However, there is opposing data from a different group who found that treating adrenalectomised animals with an MR agonist resulted in proliferation rates similar to that under physiological conditions (Montaron et al., 2003). To re-examine the role of the two corticoid receptors in the regulation of progenitor division we employed two different approaches, using both agonists and antagonists in adrenalectomised rats with replacement doses of corticosterone, to test the effect of activating or inactivating the two receptors individually, or in combination, on the proliferation rate of progenitor cells in the dentate gyrus.
Materials and methods
Animals
All procedures were carried out under Home Office (UK) licence; adult Lister hooded male rats (Harlan, UK), weighing ≈ 250–300 g at the beginning of the experiment, were used. Rats were housed three per cage on reversed 12-h light–12-h dark cycles (lights off at 11.00 h). Ambient temperature was maintained at 21° ± 2 °C. Food and water were available ad libitum. All surgery was carried out under general anaesthesia. Adrenalectomised animals were given a choice of 0.9% saline and water to replenish salt loss. Blood samples were obtained by heart puncture.
Corticoid manipulation and drug treatments
Corticoids
To remove endogenous corticoids, dorsal bilateral adrenalectomies (ADX) were performed under isofluorane anaesthesia. ‘Basal’ levels of corticosterone were replicated with subcutaneous implants of 3 : 7 corticosterone : cholesterol (Sigma) mixture, melted and moulded into pellets weighing 200–220 mg. To increase plasma corticosterone above basal levels, animals were given additional daily subcutaneous injections of corticosterone suspended in peanut oil at 10 or 40 mg/kg/day.
Labelling dividing progenitors
BrdU (Sigma, UK), a thymidine analogue incorporated into dividing cells during the DNA synthetic phase (S-phase) of the cell cycle, was dissolved in 0.9% saline and four daily doses injected intraperitoneally (50 mg/kg/day) at mid-day.
Drug treatments
All drugs were suspended in propylene glycol (Sigma), and the doses were chosen with reference to reported effects on hippocampal functions (McCullers et al., 2002a,b). Spironolactone (SPIR; Sigma, UK) and mifepristone (MIFE; Sigma, UK) are specific antagonists for MR and GR, respectively. SPIR was given at a concentration of 50 mg/kg/day and a dose of 25 mg/kg/day was used for MIFE. Aldosterone (Merck, Germany) is a specific agonist of MR, and RU362 (a gift of Adventis, Germany) activates GR. Aldosterone was given at a dose of 250 μg/kg/day and RU362 at 1 mg/kg/day.
Experimental procedures
Experiment 1
Three groups of animals (n = 4 each group) were used for this experiment. Groups 1 (Sham) and 3 (High CORT) were sham-operated, whereas group 2 (ADX) was bilaterally adrenalectomised. Animals were allowed six days to recover, after which the Sham and ADX groups received daily subcutaneous injections of peanut oil while the High CORT group had corticosterone (40 mg/kg/day) for 5 days. On the last four days of oil or corticosterone treatment, animals were given intraperitoneal injections of BrdU (50 mg/kg/day). They were killed 24 h after the last BrdU injection with an overdose of pentobarbitone sodium intraperitoneally (Fig. 1a). Blood samples were collected within 2 min after injection and before perfusion for radioimmunoassay of plasma corticosterone. Thymus glands were also removed and weighed.
Fig. 1.
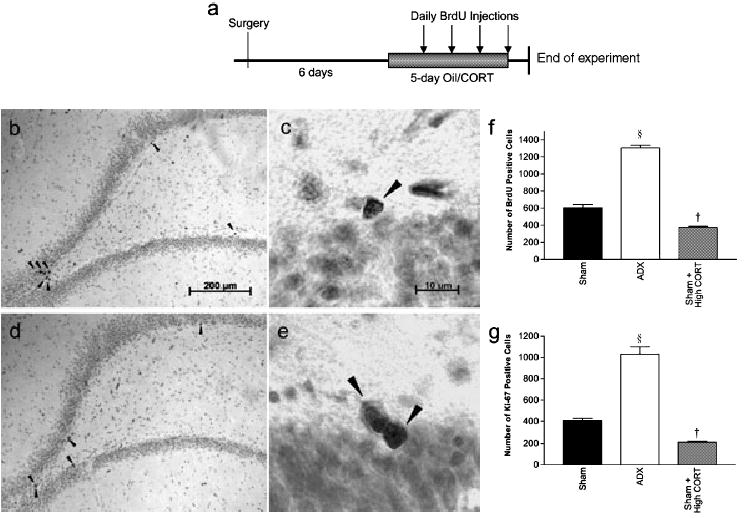
(a) Diagram showing timescale of treatments, (b–d) Cells immunoreactive for (b and c) BrdU and (d and e) Ki-67 in the dentate gyrus of the hippocampus. Adrenalectomy significantly increased proliferation of progenitors (§P < 0.0001) whilst raised corticosterone suppressed this process (†P < 0.0001), as shown by (f) BrdU and (g) Ki-67 cell counts.
Experiment 2
Thirty-six animals, divided into six groups (n = 6), were used in this experiment. Group 1 (Sham) was sham-operated and Group 2 (ADX group) was bilaterally adrenalectomised. Both Sham and ADX animals were implanted subcutaneously under the armpit with a cholesterol pellet (200–220 mg). The four other groups, namely Groups 3 (Vehicle), 4 (SPIR), 5 (MIFE), and 6 (SPIR + MIFE), were all ADX and given a 30% corticosterone pellet implant at the time of ADX. All animals were given daily subcutaneous injections for 10 days beginning 24 h post-ADX. Sham, ADX and Vehicle groups were injected with vehicle (propylene glycol) while SPIR, MIFE and SPIR + MIFE had SPIR (50 mg/kg/day), MIFE (25 mg/kg/day) and both, respectively. To label the dividing progenitors, animals were given BrdU intraperitoneally (50 mg/kg/day) on the last 4 days of the drug treatment. They were killed 24 h after the last BrdU injection (Fig. 2a). Blood samples and thymus glands were collected before perfusion as above.
Fig. 2.
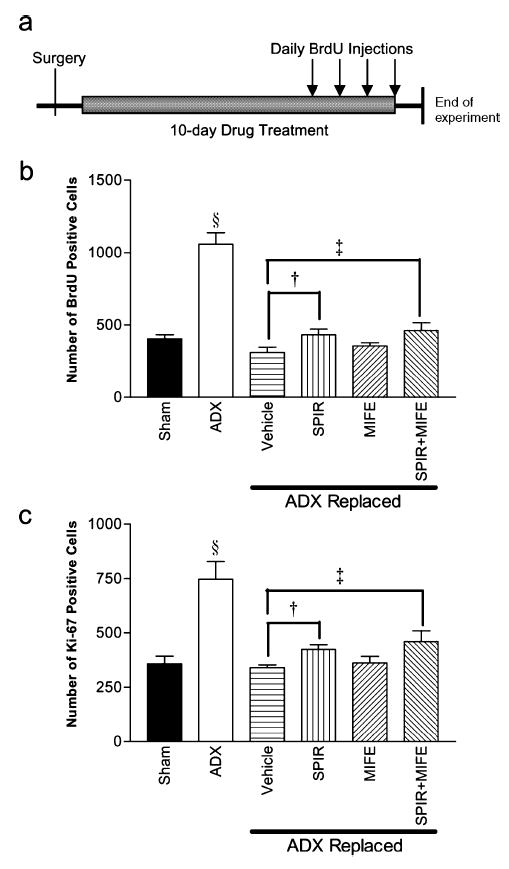
(a) Diagram showing timescale of treatments, (b and c) Effects of the MR antagonist SPIR and GR antagonist MIFE on proliferation as demonstrated by (b) BrdU and (c) Ki-67 cell counts. Adrenalectomy significantly stimulated proliferation (§P < 0.001), and corticosterone replacement with a 30% corticosterone pellet restored proliferation to basal levels. SPIR significantly enhanced proliferation (†P < 0.007), but there was no observed effect of MIFE. ‡P < 0.043.
Experiment 3
Three groups of rats (n = 4) were adrenalectomised and implanted with a 30% corticosterone pellet (200–220 mg). The first group (Vehicle) received daily subcutaneous injection of propylene glycol for 10 days. The second group (CORT) were given daily subcutaneous injections of corticosterone (10 mg/kg/day) in the same vehicle. The third group (CORT + MIFE) were given corticosterone (10 mg/kg/day) and MIFE (25 mg/kg/day). The drug treatment began 24 h after surgery and lasted for 10 days. On the last 4 days of the drug treatment, animals were given a daily injection of BrdU (50 mg/kg/day, i.p.). Rats were killed 24 h after the last BrdU injection (Fig. 3a). Trunk blood and thymus glands were collected before perfusion.
Fig. 3.
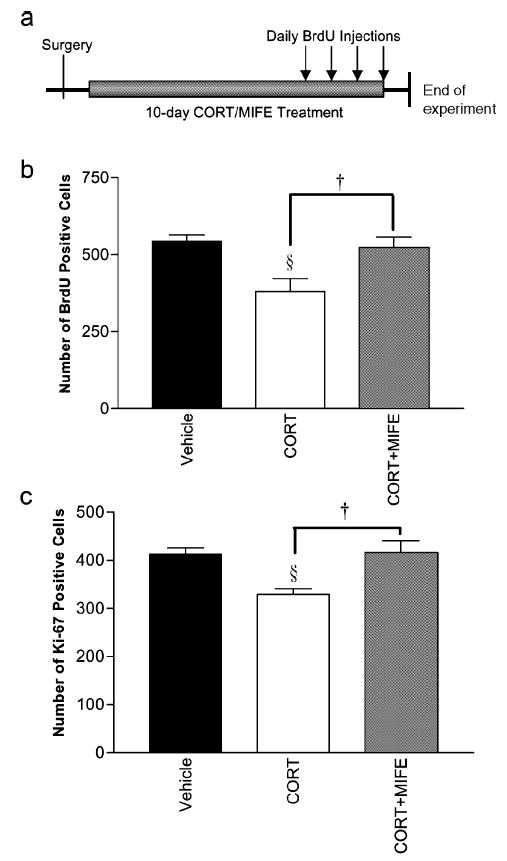
(a) Diagram showing timescale of treatments, (b and c) Efficacy of MIFE in antagonizing suppressive effect on proliferation by raised corticosterone. All animals were adrenalectomised and given corticosterone replacement pellets, (b) BrdU and (c) Ki-67. Corticosterone suppressed cell division (§P < 0.014); additional treatment with MIFE significantly reversed the effect of corticosterone (†P = 0 vs. vehicle).
Experiment 4
Five groups of rats (n = 6) were used. Group 1 (Sham) was sham-operated, while all the other animals were ADX. Drug treatments began 24 h after surgery; Groups 1 and 2 (ADX) were given vehicle (propylene glycol) for 10 days while the others received either aldosterone (ALDO, 250 μg/kg), RU362 (1 mg/kg) or both, subcutaneously. Intraperitoneal BrdU injections (50 mg/kg) were given on the last 4 days of drug treatments. Animals were killed 24 h post-BrdU (Fig. 4a). Thymus glands and trunk blood samples were taken as above.
Fig. 4.
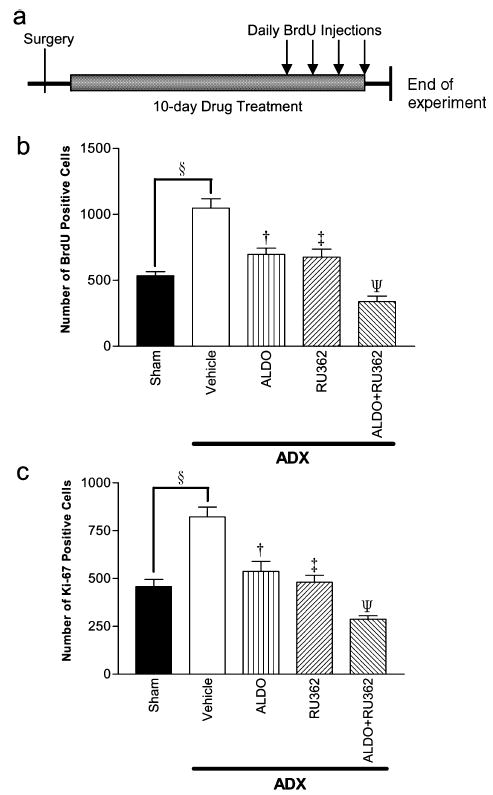
(a) Diagram showing timescale of treatments, (b and c) Effect of the MR agonist aldosterone and GR agonist RU362 on progenitor division as shown by (b) BrdU and (c) Ki-67. Adrenalectomy increased proliferation rate as before (§P < 0.001). Both aldosterone and RU362 restored the cell division rate to basal levels (sham-operated animals), in contrast to the Vehicle group (†P < 0.001 and ‡P < 0.001). Treatment with both agonists further suppressed proliferation to below basal levels (ΨP < 0.001).
Tissue preparation
Animals were given an overdose of pentobarbitone sodium and perfused transcardially with 0.1 M PBS followed by 4% paraformaldehyde in 0.01 m KPBS (pH 7.4). Thymus glands were extracted and weighed. Brains were removed, postfixed for 4 h and immersed in 20% sucrose in KPBS overnight. Coronal sections were cut using a freezing tissue sheer through the entire dentate gyrus at 40 μm and sections were stored in antifreeze (1:1:2 glycerol : ethyleneglycol : 0.1 M PBS) at−20 °C.
Immunohistochemistry
One in every 12 brain sections collected from each animal was used for immunohistochemistry.
BrdU
Sections were first mounted onto polylysine-coated slides and dried overnight. They were then antigen-retrieved in 0.01 M citric acid for 15 min at 97 °C, cooled for 30 min, incubated in 3% H2O2 for 10 min, digested with trypsin 250 (0.025% in KPBS) for 10 min, denatured in 2 N HCl for 30 min at 37 °C and incubated overnight at room temperature in mouse monoclonal antibody raised against BrdU (Novocastra, UK; 1 : 200 in 0.3% Triton with 1% normal horse serum). The slides were then taken through a mouse IgG ABC kit procedure (Vector) and reacted for 10 min with diaminobenzidine (DAB; Sigma). The slides were counterstained with Cresyl Violet, dehydrated and cover-slipped under DPX. All rinses were done in 0.01 M KPBS.
Ki-67
Ki-67 is a marker of cell proliferation and is expressed throughout the cell cycle except G0. Sections were mounted on to polylysine-coated slides as before and boiled in 0.01 M citrate buffer at 97 °C for 40 min. The sections were then quenched in hydrogen peroxide and incubated overnight in monoclonal mouse anti-Ki-67 primary anti-body (Novocastra, UK; 1 : 100) with 0.2% Triton X-100 and 1% normal horse serum. The remaining steps were the same as those described above.
Corticosterone radioimmunoassays
Twenty microlitres of serum was first extracted with 0.025 M phosphate gelatin buffered saline (PGB) and ethanol. 1.5 mL of ethanol was added to the mixture was vortexed briefly. Samples were then centrifuged at 4 °C at 500 g for 10 min, and the supernatant was then decanted into a fresh tube and dried in a vortex evaporator. The extracted corticosterone was then reconstituted in 500 μL of PGB. Nine corticosterone standards ranging from 15.625 to 4000 pg/100 μL were prepared. Corticosterone antiserum (100 μL) used for the assay was raised against a corticosterone-3- (O-carboxymethyl)oxime-bovine serum albumin conjugate (Bioclinical Services Ltd, UK) and 100 μL of the label 3[H] corticosterone (specific activity range 70–100 Ci/mmol) were added to controls, standards and serum samples. Charcal-dextran separation was performed the next day and scintillant was added. The samples were then counted for 1 min in the scintillation counter. The percentage bound values were compared to the standard curve to obtain the value which was then converted to ng/mL. Intra-assay variances were 7.37, 5.48 and 6.77.
Quantification
All slides were randomised and coded prior to quantitative analysis by the experimenter and the codes were not broken until all the slides have been counted. Sections were examined using a 40× objective and BrdU-labelled and Ki-67-stained cells were counted every twelfth section on separate series through the entire dentate gyrus bilaterally (approximately nine sections per animal). Only cells on the border of the subgranular zone and hilus were counted (BrdU-positive cells approximately two nuclei from the border were not included). BrdU cells in all focal planes through the 40-μm section were included. The data shown is the total count obtained from this one-in-12 section sample. We intentionally avoided the stereology method as it generates data with high variance (Alonso, 2000).
Statistical analysis
Data was analysed by one- or two-way ANOVA; the variances were tested for homogeneity.
Results
Experiment 1: effect of corticoids on proliferation of progenitors
Experiment 1 was intended to confirm the reported effect of corticoids on proliferation of progenitors in the dentate gyrus. Proliferating cells immunoreactive for BrdU or Ki-67 in the dentate gyrus are shown in Fig. 1b–e. Figure 1f (BrdU) and Fig. 1g (Ki-67) demonstrate that removal of endogenous corticoids by adrenalectomy stimulated progenitor proliferation by ≈ 100% (one-way ANOVA: BrdU, F2,9 = 204.5, P < 0.0001; Ki-67, F2,9 = 194.5, P < 0.0001). Pairwsie comparisons showed that administration of 40 mg/kg corticosterone (High CORT) significantly reduced the number of new cells formed to ≈ 50% of Sham (BrdU, Bonferroni P < 0.0001; Ki-67, Bonferroni P < 0.001). Thymus weights increased in ADX animals, and decreased in the high CORT group compared to controls (oneway ANOVA, F2,9 = 41.2, P < 0.001; ADX, Bonferroni P < 0.001; High CORT, Bonferroni P < 0.0001). There was an inverse relationship between plasma corticosterone levels and thymus weights (Table 1).
Table 1.
Plasma corticosterone levels and thymus gland weights at time of killing
| Ttreatments | Plasma CORT (ng/mL) | Thymus (% of body weight) |
|---|---|---|
| Experiment 1 | ||
| Sham | 117.0 ± 7.8 | – |
| ADX | 1.2 ± 0.2 | – |
| High CORT | 281.5 ± 21.9 | – |
| Experiment 2 | ||
| Sham | 158.0 ± 21.0 | 0.15 ± 0.01 |
| ADX | 3.2 ± 1.2 | 0.21 ± 0.02 |
| Vehicle | 68.8 ± 7.2 | 0.16 ± 0.01 |
| SPIR | 70.2 ± 11.9 | 0.15 ± 0.02 |
| MIFE | 85.2 ± 8.9 | 0.10 ± 0.01 |
| SPIR+MIFE | 63.2 ± 13.1 | 0.10 ± 0.01 |
| Experiment 3 | ||
| Vehicle | – | 0.11 ± 0.01 |
| CORT | – | 0.06 ± 0.01 |
| CORT+MIFE | – | 0.08 ± 0.01 |
| Experiment 4 | ||
| Sham | – | 0.16 ±0.01 |
| Vehicle | – | 0.25 ± 0.01 |
| ALDO | – | 0.20 ± 0.02 |
| RU362 | – | 0.02 ± 0.003 |
| ALDO+RU362 | – | 0.02 ± 0.01 |
Values are expressed as mean ± SEM. Adrenalectomy significantly removed circulating corticoids and stimulated growth of thymus glands, whilst additional corticosterone significantly raised circulating corticoid levels and decreased weights. ADX, adrenalectomy or adrenalectomised; ALDO, aldosterone; CORT, corticosterone; MIFE, mifepristone; SPIR, spironolactone.
Experiment 2: effect of SPIR or MIFE on proliferation in ADX-corticosterone-replaced rats
Experiment 2 aimed to determine the roles of MR and GR in mediating the effects of corticosterone on the regulation of proliferation, by blocking the receptors with their specific antagonists either individually or in combination. Fig. 2b and c shows the BrdU and Ki-67 cell counts from this experiment. The data were analysed as follows: the six groups were compared using a one-way ANOVA and the ADX groups given a 30% corticosterone implant (groups 3–6) were analysed using a two-way ANOVA, taking SPIR and MIFE as the two factors. One-way ANOVA showed a highly significant difference between the groups: Brdu, F5,30 = 22.6, P < 0.001; Ki-67, F5,30 = 10.0, P < 0.001. Pairwise comparisons showed that, as expected, ADX without replacement corticosterone stimulated proliferation (Brdu and Ki-67, Bonferroni P < 0.001). Implanting a 30% corticosterone pellet reduced proliferation compared to ADX with a cholesterol implant (Brdu and Ki-67, Bonferroni P < 0.001). SPIR had no effect (Bonferroni P = 0.54), neither did MIFE (P = 0.86 for both BrdU and Ki-67). Giving both antagonists also had no significant effect. However, two-way ANOVA limited to the 30% corticosterone pellet-implanted groups (3–6) showed that, while MIFE had no effect (F1,20 = 1.2 and 0.46 for BrdU and Ki-67, respectively), that of SPIR was significant (F1,20 = 9.1 and 8.4, respectively; P < 0.007 and < 0.009). There were no significant interactions between the two antagonists. Thymus weights were increased by ADX without CORT treatment (F5,30 = 12.5, Bonferroni P = 0.05), but restored by ‘basal’ CORT replacement (P = 1.0; Table 1). SPIR had no effect in the thymus weights of ADX rats implanted with 30% corticosterone, but thymus weights were decreased by MIFE and by the combined treatment: MIFE, Bonferroni P = 0.03; SPIR + MIFE, P = 0.006. Plasma corticosterone levels were virtually undetectable in the ADX group (F = 54.2, P < 0.0001) but were restored to levels within the normal range in the CORT-implanted groups. The addition of either or both antagonists had no effect on measured corticosterone levels.
Experiment 3: effect of MIFE in intact corticosterone-supplemented rats
Figure 3b and c shows the respective cell counts of BrdU- and Ki-67-labelled cells. Corticosterone (10 mg/kg/day) significantly decreased both BrdU and Ki-67 cell counts (one-way ANOVA, F2,9 = 8.3 and 7.1, P = 0.009 and 0.014, respectively; Bonferroni P = 0.02 in both cases). Giving MIFE reversed this effect, so that there was no longer a significant difference between vehicle-treated rats and those given corticosterone + MIFE (Bonferroni P = 1.0). Corticosterone treatment significantly increased circulating corticosterone levels in both CORT and MIFE + CORT groups (CORT, F1,7 = 9.72, P < 0.021; MIFE + CORT, F1,7 = 7.89, P < 0.031; Table 1). Corticosterone treatment also reduced thymus weights as before (F1,7 = 39.2, P < 0.001) but additional treatment with MIFE counteracted this effect (F1,7 = 25.2, P < 0.002).
Experiment 4: effect of aldosterone or RU362 on proliferation in ADX rats
In this experiment, we used specific agonists to activate the two receptors separately in a corticoid-free environment. Animals were first adrenalectomised and then given either the MR agonist aldosterone or the GR agonist RU362. Figure 4b and c shows the respective cell counts of BrdU- and Ki-67-immunoreactive cells after treatments. There was an overall significant effect of the treatments (one-way ANOVA, F4,25 = 24.7 and 22.2 for BrdU and Ki-67; P < 0.001 in both). Pairwise comparisons showed that ADX significantly increased proliferation of progenitors compared to intact animals, as expected (Bonferroni P < 0.001 for both BrdU and Ki-67). ALDO significantly reduced proliferation compared to ADX-vehicle-treated animals (P = 0.001 for both BrdU and Ki-67); the counts were not significantly different from those of the intact group (Bonferroni P > 0.05 for both markers). RU362 had similar effects: counts were reduced compared to ADX-vehicle-treated rats (P < 0.001 for both markers) but were no different from intact controls (P = 1.0 for both markers). Giving both ALDO and RU362 had additional effects, reducing proliferation to even lower levels compared with either agonist alone (Bonferroni P = 0.001 for both markers). Two-way ANOVA restricted to the ADX groups gave similar results. There were significant main effects for both ALDO (F1,20 = 37.4 and 32.0, P < 0.001 for both BrdU and Ki-667) and RU362 (F1,20 = 41.8 and 49. 1, P < 0.001 for the two markers, respectively) but no significant interaction between them. Corticosterone was virtually undetectable in ADX animals (Table 1). Adrenalectomy increased thymus weight (Vehicle, F1,11 = 74.5, P < 0.0001); however, treating animals with specific agonists reduced this increase (ALDO, F1,11 = 7.4, P < 0.021; RU362, F1,11 = 1273.7, P < 0.0001; ALDO + MIFE, F1,11 =795.7, P < 0.0001).
Correlation between BrdU and Ki-67 cell counts
Because we used two methods of assessing rates of proliferation in the dentate gyrus we were able to compare the counts resulting from the four daily BrdU injections paradigm (a cumulative measure of proliferation) with those using the Ki-67 antibody (which represent proliferation at the time of killing). Figure 5 shows that the two markers of proliferation are highly correlated and this was confirmed by a highly significant Pearson correlation coefficient (r = 0.91, P < 0.0001).
Fig. 5.
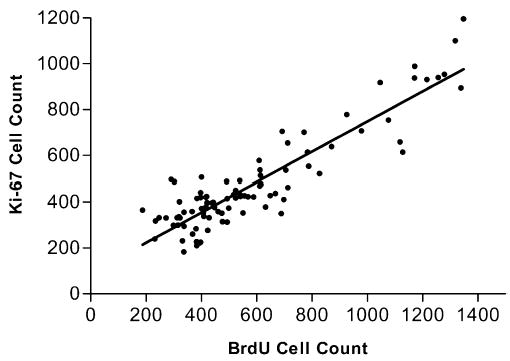
Ki-67 cell counts are plotted against BrdU cell counts. Pearson’s correlation test showed that the two markers for proliferation correlated positively (r = 0.91, P < 0.0001).
Discussion
The steroidal environment of the hippocampus is one of the most potent factors regulating adult neurogenesis. Adult neurogenesis can be subdivided into three separate events: proliferation, survival and differentiation. The reduction in proliferation of progenitor cells by corticoids is well documented, but we have shown that the corticoid environment subsequent to proliferation also determines survival of these newly formed cells (Wong & Herbert, 2004). This paper employs specific agonists and antagonists to demonstrate the inhibitory role of both MR and GR on proliferation of progenitor cells in the dentate gyrus. Whether these receptors have separable actions on survival or differentiation of these proliferating cells awaits further study, though we have found that corticosterone also reduces the proportion of newly formed cells that form phenotypic neurons within 28 days (E. Y. H. Wong & J. Herbert, unpublished data).
It has been proposed that activation of MR stimulates cell division in the dentate gyrus (Fischer et al., 2002), but contradictory findings suggesting that MR activation suppresses proliferation have also been reported (Montaron et al., 2003). The role of GR in this respect is less controversial (Montaron et al., 2003). In this study, Experiment 1 confirmed the well-known and converse effects of ADX and additional CORT on increasing or decreasing proliferation in the dentate gyrus, under the conditions used here. In Experiment 2, the replacement corticosterone pellet resulted in levels of circulating corticosterone similar to the circadian nadir in intact rats, so it is likely that the replaced corticosterone occupied the two receptors in a way similar to that under basal physiological conditions. Even though its penetration into the brain is limited, the MR antagonist SPIR significantly increased proliferation rate (two-way ANOVA), implying that saturation of the MRs leads to constitutive suppression of progenitor cell division. On the other hand, the GR antagonist MIFE did not affect proliferation. This might be because at these lower levels of CORT there was little activation of GR. The addition of MIFE to SPIR did not increase the latter’s effect. This reinforces the conclusion that GRs were playing little part in regulating proliferation at this (‘basal’) level of CORT.
In Experiment 3, MIFE effectively antagonized the suppressive power on cell division of additional corticosterone given to intact rats. This shows that the dose of MIFE used in Experiment 2 was sufficient to antagonize the GRs. It also supports the conclusion that GRs control proliferation at higher (stress-related) levels of CORT. The respective roles of MR and GR were directly tested in Experiment 4. The results agreed with those of Experiment 2: MR activation down-regulates proliferation of progenitor cells. In addition, this experiment clearly shows that activation of the GRs alone is powerful enough to negatively regulate proliferation (because RU362 has a highly specific action on this receptor type). Activating both receptors with the two agonists aimed to simulate high levels of circulating corticosterone, a time when a large proportion of both the MR and the GR are occupied. Treatment with aldosterone and RU362 significantly reduced proliferation rate to below basal levels, an observation similar to that in Experiment 1 when high levels of corticosterone were given. This experiment thus showed clearly that, as predicted from the earlier experiments, agonists of either MR or GR can reduce proliferation rates in ADX rats. Assuming no cross-reactivity between agonists, it also shows that activating both sets of receptors together results in profound suppression of proliferation.
Our findings show indisputably that, in the antagonist experiments, inactivation of MR or GR can modulate progenitor proliferation positively. The converse of this is also true: activation of MR or GR in the replacement paradigm negatively regulated the rate of progenitor division. Importantly, activation of both MR and GR reduced proliferation to a level half that of the MR or GR agonist alone. Under physiological conditions ≈ 80% of MRs are occupied and GRs are progressively occupied when corticosterone levels are high (> 300 ng/mL in the rat), as occurs during physical or psychological stress (Reul & de Kloet, 1985). This two-receptor system may provide a sensitive way to detect changes in circulating corticoids across an extended range, covering both basal and stressful situations.
In the experiments reported here, we used two methods of assessing proliferation rates. This was to provide confirmatory data but, perhaps more importantly, to obviate the possibility that alteration of BrdU uptake resulting from manipulating the corticoid environment, rather than changes in the function of the corticoid receptors themselves, might have been held responsible for some or all of our results. Because in all cases the results from the two methods were the same, and they correlated closely with each other, this can be confidently ruled out. We measured both plasma corticosterone and thymus weights, so we were able to conclude that treatment with either MIFE or SPIR did not alter corticosterone levels but did have the expected effect on the thymus, a well-known target tissue for adrenal steroids. This reinforces the conclusion that the results we obtained were due to interactions at the level of the receptors in the brain and not secondary to changes in peripheral steroid levels.
Newly formed cells in the dentate gyrus have been found not to express corticoid receptors (Cameron et al, 1993). This suggests that the effects of corticoids on proliferation are indirect and are likely to be mediated by mature receptor-expressing dentate granule cells or glia surrounding the progenitors (Song et al., 2002). The local environment of the progenitors determines proliferation and survival of these cells (Hastings & Gould, 2003; Seki, 2003). Possible candidates which regulate this ‘mature to immature’ transition include growth factors and neurotrophins such as insulin-like growth factor-1, brain-derived neurotrophin factor, neurogenesin-1 and epidermal growth factor, all of which have been implicated in modulating adult hippocampal neurogenesis (Kuhn et al., 1997; Aberg et al., 2000; Lee et al., 2002; Ueki et al., 2003). Variations in circulating corticoids have been shown to influence expression of these proteins and their receptors (Gubba et al., 2004; Islam et al., 1998; Schaaf et al., 2000) but the precise mechanisms by which the cellular environment in general, and corticoids in particular, regulate the development of the progenitors remain to be studied.
On the other hand, in contrast to the results of Cameron et al. (1993), a recent study (Garcia et al., 2004) found that a small proportion of newly formed cells (≈15%) in fact express GR 24 h after division. By day 4, ≈25% of the newly formed cells possess both MR and GR. This poses the possibility that, in our experimental paradigm (4-day BrdU injection), a small portion of the BrdU-labelled cells could be capable of responding directly to the treatments given (agonists or antagonists). However, whether the observed results were direct consequences of activation or inactivation of GR and MR on the newly formed cells or an indirect effect on the local environment surrounding the newly formed cells remain to be investigated.
This report shows that activity of MR and GR control the first stage in the neurogenetic process, proliferation of progenitors in the dentate gyrus. Future work will determine how the two receptors modulate survival as well as differentiation of newly formed cells in the dentate gyrus. In addition, it is clearly important to determine whether the two receptors share the same downstream molecular pathway in either process. Because raised corticoids are known to be a risk factor for the subsequent development of major depression (Goodyer et al., 2000; Harris et al., 2000), a condition which has been linked to the phenomenon of neurogenesis in the adult hippocampus (Malberg, 2004; Sapolsky, 2004), unravelling the actions of corticoids on proliferation and survival of newly formed neurons in the dentate gyrus has clinical as well as experimental interest.
Acknowledgments
We thank Helen Shiers and Sarah Cleary for technical assistance. This work was supported by a grant from the Wellcome Trust. E.Y.H.W. received a scholarship from the Cambridge Commonwealth and Cambridge Overseas Trust.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-I selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Ambrogini P, Orsini L, Mancini C, Ferri P, Barbanti I, Cuppini R. Persistently high corticosterone levels but not normal circadian fluctuations of the hormone affect cell proliferation in the adult rat dentate gyrus. Neuroendocrinology. 2002;76:366–372. doi: 10.1159/000067581. [DOI] [PubMed] [Google Scholar]
- Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999;89:999–1002. doi: 10.1016/s0306-4522(98)00693-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Conrad CD, Lupien SJ, McEwen BS. Support for abimodal role for type II adrenal steroid receptors in spatial memory. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- Corotto FS, Henegar JA, Maruniak JA. Neurogenesis persists in the subependymal layer of the adult mouse brain. Neurosci Lett. 1993;149:111–114. doi: 10.1016/0304-3940(93)90748-a. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Fischer AK, von Rosenstiel P, Fuchs E, Goula D, Almeida OF, Czeh B. The prototypic mineralocorticoid receptor agonist aldosterone influences neurogenesis in the dentate gyrus of the adrenalectomized rat. Brain Res. 2002;947:290–93. doi: 10.1016/s0006-8993(02)03042-1. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. First-episode major depression in adolescents. Affective, cognitive and endocrine characteristics of risk status and predictors of onset. Br J Psychiatry. 2000;176:142–149. doi: 10.1192/bjp.176.2.142. [DOI] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Nat! Acad Sci USA. 1999;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Cameron HA. Adrenal steroids suppress granule cell death in the developing dentate gyrus through an NMDA receptor-dependent mechanism. Brain Res Dev Brain Res. 1997;103:91–93. doi: 10.1016/s0165-3806(97)00079-5. [DOI] [PubMed] [Google Scholar]
- Gubba EM, Fawcett JW, Herbert J. The effects of corticosterone and dehydroepiandrosterone on neurotrophic factor mRNA expression in primary hippocampal and astrocyte cultures. Brain Res Mol Brain Res. 2004;127:48–59. doi: 10.1016/j.molbrainres.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Harris TO, Borsanyi S, Messari S, Stanford K, Cleary SE, Shiers HM, Brown GW, Herbert J. Morning cortisol as a risk factor for subsequent major depressive disorder in adult women. Br J Psychiatry. 2000;177:505–510. doi: 10.1192/bjp.177.6.505. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Neurons inhibit neurogenesis. Nat Med. 2003;9:264–266. doi: 10.1038/nm0303-264. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A. Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci. 2002;16:283–290. doi: 10.1046/j.1460-9568.2002.02093.x. [DOI] [PubMed] [Google Scholar]
- Islam A, Ayer-LeLievre C, Heigenskold C, Bogdanovic N, Winblad B, Adem A. Changes in IGF-1 receptors in the hippocampus of adult rats after long-term adrenalectomy: receptor autoradiography and in situ hybridization histochemistry. Brain Res. 1998;797:342–346. doi: 10.1016/s0006-8993(98)00389-8. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Leam Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation, of granule cell progenitors in the adult rat hippocampus. Eur J Neurosci. 2002;16:2008–2012. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. J Neurosci. 1998;18:7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE. Implications of adult hippocampal neurogenesis in antidepressant action. J Psychiatry Neurosci. 2004;29:196–205. [PMC free article] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Mifepristone protects CA1 hippocampal neurons following traumatic brain injury in rat. Neuroscience. 2002a;109:219–230. doi: 10.1016/s0306-4522(01)00477-8. [DOI] [PubMed] [Google Scholar]
- McCullers DL, Sullivan PG, Scheff SW, Herman JP. Traumatic brain injury regulates adrenocorticosteroid receptor mRNA levels in rat hippocampus. Brain Res. 2002b;947:41–49. doi: 10.1016/s0006-8993(02)02904-9. [DOI] [PubMed] [Google Scholar]
- Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, Abrous DN. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Is impaired neurogenesis relevant to the affective symptoms of depression? Biol Psychiatry. 2004;56:137–139. doi: 10.1016/j.biopsych.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Schaaf MJ, De Kloet ER, Vreugdenhil E. Corticosterone effects on BDNF expression in the hippocampus. Implications for memory formation. Stress. 2000;3:201–208. doi: 10.3109/10253890009001124. [DOI] [PubMed] [Google Scholar]
- Seki T. Microenvironmental elements supporting adult hippocampal neurogenesis. Anat Sci Int. 2003;78:69–78. doi: 10.1046/j.0022-7722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Song H, Stevens CF, Gage FH. Astroglia induce neurogenesis from adult neural stem cells. Nature. 2002;417:39–44. doi: 10.1038/417039a. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neural. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z, Maragakis NJ, Hevner RF, Miura N, Sugimura H, Sato K. A novel secretory factor, Neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci. 2003;23:11732–11740. doi: 10.1523/JNEUROSCI.23-37-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eekelen JA, Jiang W, De Kloet ER, Bohn MC. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J Neurosc Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- Wong EYH, Herbert J. The corticoids environment: a determining factor for neural progenitors survival in the adult hippocampus. Eur J Neurosci. 2004;20:2491–2498. doi: 10.1111/j.1460-9568.2004.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


