Abstract
The neurobiological mechanisms underlying overeating in obesity are not understood. Here, we assessed the neurobiological responses to an Implantable Gastric Stimulator (IGS), which induces stomach expansion via electrical stimulation of the vagus nerve to identify the brain circuits responsible for its effects in decreasing food intake. Brain metabolism was measured with positron emission tomography and 2-deoxy-2[18F]fluoro-d-glucose in seven obese subjects who had the IGS implanted for 1–2 years. Brain metabolism was evaluated twice during activation (on) and during deactivation (off) of the IGS. The Three-Factor Eating Questionnaire was obtained to measure the behavioral components of eating (cognitive restraint, uncontrolled eating, and emotional eating). The largest difference was in the right hippocampus, where metabolism was 18% higher (P < 0.01) during the “on” than “off” condition, and these changes were associated with scores on “emotional eating,” which was lower during the on than off condition and with “uncontrolled eating,” which did not differ between conditions. Metabolism also was significantly higher in right anterior cerebellum, orbitofrontal cortex, and striatum during the on condition. These findings corroborate the role of the vagus nerve in regulating hippocampal activity and the importance of the hippocampus in modulating eating behaviors linked to emotional eating and lack of control. IGS-induced activation of regions previously shown to be involved in drug craving in addicted subjects (orbitofrontal cortex, hippocampus, cerebellum, and striatum) suggests that similar brain circuits underlie the enhanced motivational drive for food and drugs seen in obese and drug-addicted subjects, respectively.
Keywords: brain activation, obesity
The cerebral mechanisms underlying the behaviors that result in pathological overeating and obesity are poorly understood. The regulation of food intake is a complex balance between excitatory and inhibitory processes. The excitatory processes arise from the body’s needs for nutrients and calories. The inhibitory processes arise from satiety signals (i.e., electrical and chemical) after food consumption (1). The vagus nerve is one of the ways by which satiety signals are conveyed to the brainstem (2). Gastric and duodenal vagal afferents increase their firing in response to the mechanical pressure from the ingested nutrients and in response to food-induced release of a variety of brain gut peptides (i.e., cholecystokinin and ghrelin). In addition, several neurotransmitters (e.g., serotonin, dopamine, norepinephrine, and opiates) and peptides (i.e., cholecystokinin and corticotrophin releasing factor) are also involved in feeding behaviors (1). It is also recognized that in addition to food’s role in fulfilling nutrient requirements, eating also may serve to mitigate stress (comfort food) (3). Disruption in the sensitivity of the brain to these signals could lead to obesity.
Recently, the Transcend Implantable Gastric Stimulator (IGS) system, which generates electric signals to induce the expansion of the fundus, has been shown to produce increased satiety, which leads to decreased food intake and reduced body weight in obese subjects (4). It is hypothesized that electrical stimulation by the IGS triggers the gastrointestinal system to release satiety signals equivalent to those conveyed after a meal by the vagus nerve. The purpose of this study was to measure brain glucose metabolic responses to the IGS by using positron emission tomography (PET) and 2-deoxy-2[18F]fluoro-d-glucose (FDG) to assess brain circuits mediating its effects on food intake. Inasmuch as vagal stimulation affects the activity of regions involved with emotional regulation such as hippocampus (5), orbitofrontal cortex (6), and striatum (7), we hypothesized that the therapeutic benefits from IGS in obesity would involve activation of these brain regions.
Results
The body mass index of the subjects was 46.0 ± 6.2 kg/m2 (range 36.7–52) before the implantation of the IGS. The subjects had lost an average of 11.6 ± 7.7% (range 0–25%) in body mass index because of the implantation 1–2 years prior. Six subjects had maintained >5% of the weight loss at the time of the study. Evaluation of eating behaviors by using the Three-Factor Eating Questionnaire-Eating Inventory revealed small but significant (P < 0.04) decreases on the scores on emotional eating during the on relative to the off condition (21% lower) and no differences on the scores for cognitive restraint or uncontrolled eating (Table 1).
Table 1.
The results of Three-Factor Eating Questionnaire when the IGS was activated (on) and deactivated (off)
| Questionnaire | IGS on | IGS off | P |
|---|---|---|---|
| Cognitive restraint | 13.4 ± 4.4 | 14.6 ± 4.6 | 0.24 |
| Uncontrolled eating | 9.7 ± 3.0 | 10.0 ± 3.0 | 0.72 |
| Emotional eating | 6.1 ± 3.0 | 7.7 ± 3.2 | 0.04 |
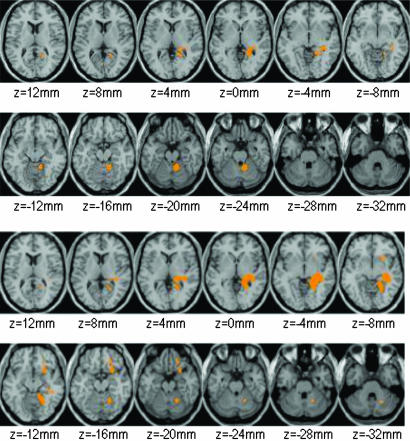
Statistical parametric mapping (SPM) analysis of the brain metabolic images (absolute measures) showed significantly higher activity in the on than off conditions in the right hippocampal region and in right anterior cerebellum (P < 0.01) (Fig. 1). Similar findings were obtained for the SPM analysis done on the normalized brain metabolic images (relative measures), which also showed significantly higher metabolism in the right hippocampal region and in right cerebellum but, in addition, revealed higher metabolism in right orbitofrontal cortex (OFC) and right striatum (P < 0.01) (Fig. 1 and Table 2). The SPM comparison for the off > on was not significant.
Fig. 1.
Results of SPM analysis obtained with the absolute metabolic (Upper) and with the normalized (Lower) images (ratio of pixel to whole brain). Threshold corresponds to P < 0.01 (uncorrected), T = 3.14, and k = 20 voxels.
Table 2.
Coordinates for regions that differed significantly between the on and off IGS conditions obtained in the SPM comparisons of the normalized metabolic images
| Activated regions (on > off) | Peak coordinates |
Z | Activated voxels | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Rt. hippocampus/parahippocampus | 24 | −50 | 4 | 3.32 | 399 |
| Rt. anterior cerebellum | 21 | −53 | −20 | 2.58 | 373 |
| Rt. orbitofrontal (BA 47) | 28 | 14 | −16 | 3.02 | 52 |
| Rt. orbitofrontal (BA 11) | 22 | 42 | −20 | 2.92 | 30 |
| Rt. striatum | 21 | 11 | −7 | 2.32 | 27 |
Significance set at P < 0.01 (uncorrected), T = 3.14, and k = 20 voxels. Z value represents peak voxel statistical significance. Rt., right.
The regions of interest (ROI) analysis corroborated that during the on condition, metabolism was significantly higher in right hippocampus, right striatum (putamen and ventral striatum), and right orbitofrontal cortex (Brodmann area 11), but the difference was not significant in right cerebellum (Table 3). However, failure to corroborate the cerebellar effect is likely to reflect the fact that the ROI quantified metabolic activity in a much greater volume (whole right cerebellum) that the relatively small cerebellar volume detected by SPM. Note also that whereas the ROI analysis revealed significant differences in right ventral striatum, putamen, and orbitofrontal cortex, the SPM on the absolute metabolic image did not (Fig. 1). This apparent discrepancy has to do with the threshold used to report significance for SPM, which was P < 0.01, versus that used for the individual ROI comparisons, which was P < 0.05. Indeed, if we bring significance for the SPM analysis to P < 0.03, this result reveals the differences in right ventral striatum, putamen, and orbitofrontal cortex.
Table 3.
Regional brain metabolic measures (micromoles per 100 g per min) obtained by using ROI when the IGS was activated (on) and deactivated (off)
| ROI | IGS on | IGS off | P |
|---|---|---|---|
| Right putamen | 56.9 ± 5.4 | 51.0 ± 3.0 | 0.03 |
| Left putamen | 56.7 ± 8.4 | 53.7 ± 4.3 | 0.46 |
| Right ventral striatum | 50.3 ± 7.0 | 43.7 ± 4.2 | 0.04 |
| Left ventral striatum | 49.8 ± 8.5 | 47.8 ± 6.3 | 0.73 |
| Right orbitofrontal cortex | 41.7 ± 1.8 | 37.8 ± 3.1 | 0.02 |
| Left orbitofrontal cortex | 44.6 ± 4.2 | 38.8 ± 3.3 | 0.07 |
| Right hippocampus | 39.2 ± 5.2 | 33.1 ± 5.3 | 0.01 |
| Left hippocampus | 37.1 ± 5.8 | 33.1 ± 5.1 | 0.14 |
| Right cerebellum | 40.8 ± 6.0 | 45.7 ± 4.7 | 0.12 |
| Left cerebellum | 41.6 ± 5.3 | 45.2 ± 3.2 | 0.16 |
Boldface numbers represent statistical significance (P < 0.005).
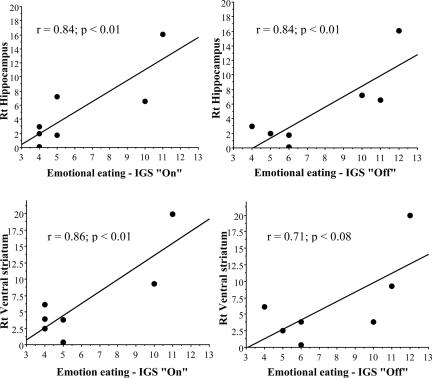
Changes in regional metabolism in right hippocampus were correlated significantly (P < 0.01) with the scores on emotional eating for the scores obtained both during the on and off conditions (Fig. 2) and with the scores on uncontrollable eating obtained both during the on (r = 0.81, P < 0.02) and off conditions (r = 0.77; P < 0.05). Changes in right ventral striatum also were correlated with scores on emotional eating during the on condition (P < 0.01) and showed a trend for the off condition (P < 0.08) (Fig. 2). Cognitive control was not associated with any of the regional metabolic changes. Neither the correlations between the metabolic changes and the changes in the scores of emotional eating (difference between the on and off) nor the correlations between metabolic changes and body mass index reductions were significant.
Fig. 2.
Correlation between changes in metabolism (from off to on) and the scores on the emotional eating obtained during the on and off conditions.
Discussion
Here, we show that chronic IGS induced significant changes in regional brain metabolism. Specifically, the SPM done on the absolute and normalized metabolic images revealed significantly higher metabolism in the right hippocampus and the right cerebellum during IGS stimulation (on condition). In addition, the SPM on the normalized images and the ROI analyses on the absolute measures also revealed increases in right orbitofrontal cortex and right striatum.
The right hippocampal region was the brain area that was the most sensitive to the effects of IGS (18% increases in metabolism). This result is consistent with prior preclinical and clinical studies showing that vagal stimulation changed hippocampal activity (reviewed in ref. 5). Moreover, therapeutic response to chronic vagal nerve stimulation in epileptic patients was associated with hippocampal changes in GABAA receptor density (8). In addition, with chronic IGS, we also show significant activation of other limbic regions, namely right OFC and right striatum as well as activation of the right anterior cerebellum. IGS stimulation is believed to mimic the response of the vagus nerve to meal-related signals, which it transmits to the nucleus tractus solitarius in the brainstem from where signals are conveyed to various cortical and subcortical regions, including hippocampus and OFC (2, 9, 10). The pattern of activation obtained in our study with chronic IGS differed from that previously reported with gastric distention (balloon inflation method), which also simulates vagal nerve and activated brainstem, inferior frontal gyrus, insula, and subgenual anterior cingulate cortex (11). The differences are likely to reflect the fact that the IGS stimulation we used was chronic, whereas the balloon inflation procedure was an acute intervention. Thus, the response to the IGS is likely to reflect the pattern of activation linked with continuous, as opposed to transient, vagal stimulation. Vagal nerve stimulation has been used for other indications (e.g., epilepsy, depression, and Alzheimer’s disease) (12). Imaging studies to evaluate the pattern of activation observed when vagal nerve stimulation is used for these therapeutic purposes have shown effects in some of the same brain regions reported in this study (i.e., hippocampus and OFC) but also in other brain regions (reviewed in ref. 5). The sensitivity of the hippocampus and the OFC to vagal stimulation, regardless of the disease condition for which it was used, suggests that these two regions may be some of the main targets conveyed by the vagus nerve through the nucleus tractus solitarius. On the other hand, the differences in regional effects between studies are likely to reflect differences in the location of nerve stimulation, parameters used (i.e., frequencies and intensity), and the unique neuropathology of the disease.
The hippocampal region, which was the region with the largest change induced by IGS activation, traditionally has been associated with learning and memory (13). However, this brain region also is involved in sensorimotor processing (14) and emotional behaviors (15). There also is evidence from preclinical studies that the hippocampus is involved with eating behaviors because hippocampal damage can result in hyperphagia (16). Moreover, it has been postulated that the hippocampus participates in a specific type of memory inhibition function that could contribute to the suppression of food intake (17). The hippocampus also is likely to contribute to eating behaviors by processing satiety signals such as those produced by cholecystokinin (18), ghrelin (19), and motilin (20) and by its involvement in incentive motivation (21) and on behavioral control (22). The hippocampus modulates dopamine (DA) release in the ventral striatum (23), which is a means by which the saliency of stimuli is modulated (24). It also regulates activity in prefrontal regions involved with inhibitory control (25, 26). Imaging studies have shown that the desire to eat a specific food was associated with activation of the hippocampus, which was interpreted to reflect the involvement of this region in the memories of the desired food (27). A recent study showed that tasting a liquid meal resulted in decreased activity in posterior hippocampus in obese, and previously obese but not in lean, subjects, implicating the hippocampus in the neurobiology of obesity (28). The effects of gastric stimulation on the hippocampus therefore could contribute to the decrease in food intake by interfering with the retrieval of food reward memories, decreasing saliency, and/or strengthening inhibitory control. The association between IGS-induced changes in hippocampal metabolism and the scores on emotional eating and uncontrolled eating support this hypothesis.
The OFC is a brain region involved in the integration of food-related sensory, visceral, and reinforcing information (29). Visceral and other satiety-related signals reach the OFC (from the nucleus tractus solitarius via thalamic relays) and there modulate the representation of food resulting in an output that reflects the reward or appetitive value of each food (19). Studies using functional MRI (30) and PET-FDG (31) showed that OFC activation was associated with the perception of hunger and desire for food. Moreover, a decrease in OFC activation when the food was eaten to satiety (32) suggests that the saliency value of the food is represented in the OFC. The DA system has a recognized role in reinforcing and motivating feeding behavior. Specifically, mesolimbic DA projections into striatum are hypothesized to regulate food intake by modulating appetitive motivational processes (33). We previously had shown that changes in extracellular DA in striatum in response to food stimulation were significantly correlated with subjective reports of hunger and with the desire for food (34). Mesencephalic DA cells also regulate the function of the OFC both by direct and indirect striato-cortical projections (35). The enhanced OFC activation previously reported with food stimulation may reflect downstream effects from DA stimulation, which would imply that DA’s involvement in the motivation for appetitive behaviors in part is mediated through the OFC that, in turn, also is modulated by vagal nerve stimulation. Thus, it is possible that IGS-induced weight loss reflects, in part, the decreased motivational value of food that is mediated through the OFC and the striatum.
The right cerebellum, which was the only nonlimbic brain region activated by chronic IGS also receives projections from the vagus nerve (36). In addition to its involvement in motor control, it also participates in visceroception (37) and learning (38). The cerebellum also has been implicated in modulating food responses, specifically those when food is consumed to satiety (39). Moreover, as for the results with the hippocampus, these responses were more accentuated in obese than in lean subjects, implicating an involvement of the cerebellum in the neurobiology of obesity.
The brain regions activated by IGS have all been associated with the responses to drugs and to drug-associated cues in addicted subjects. Specifically, activation of the OFC and the striatum has been linked with the craving and desire for the drug (drug priming and conditioned cues) (40), and activation of the cerebellum and hippocampus have been linked with mnemonic processes associated with prior drug experiences (38). Thus, results from this study corroborate the similarities in the neurobiological substrates underlying the intense wanting triggered by drugs and drug cues in addiction and by food in obesity. The overlapping substrates between these disorders could explain why drugs that are effective in animal models of obesity also have been shown to decrease drug intake in animal models of addiction (i.e., cannabinoid 1 antagonist, corticotrophin-releasing factor antagonist, and GABA-enhancing agents) (41).
Metabolic changes induced by IGS were not associated with the difference in behavioral scores; instead, it was the scores themselves obtained either on the on or off conditions for emotional eating and for uncontrolled eating that were associated with metabolic changes in hippocampus and ventral striatum. This association would suggest that the sensitivity of the right hippocampus and ventral striatum to vagal stimulation might underlie the vulnerability to emotional eating and sensitivity in the hippocampus to uncontrollable eating. In this study, the IGS resulted in increased activation in limbic brain regions and cerebellum in the absence of food stimulation. Future studies comparing responses to food stimulation between the on versus the off IGS conditions could allow researchers to determine whether chronic vagal stimulation blunts the limbic responses to food or to food-conditioned stimuli. The fact that similar regions were activated by vagal nerve stimulation in obese subjects and by drug cues in addicted subjects suggests an overlap in the neurocircuitry that underlies the responses mediated by vagal stimulation in obese subjects and those mediated by conditioned drug cues in addicted subjects. Because IGS stimulation is effective in decreasing food intake, this phenomenon raises the possibility that it also may interfere with drug administration. Preclinical studies to assess this hypothesis merit investigation.
A limitation for this study was the small number of subjects. These were very difficult subjects to recruit; we required that they be medically healthy and we excluded subjects with hypertension, diabetes, or cardiovascular disease because we wanted to minimize confounds from these conditions. In this study, we did not have a balanced randomization (six of the seven subjects had the on scan first), and we did not have a control group that underwent FDG scans on a test-retest condition with no intervention. However, previous studies from our group measuring test-retest variability showed no significant test-retest differences in metabolic measures for hippocampus, OFC, striatum, or cerebellum (42).
These findings provide insight into the brain circuits involved in processing the satiety signals that originate from the stomach of obese subjects. The brain regions activated by gastric stimulation overlap with those reported during craving responses in addicted subjects, supporting the commonalities in the neurocircuitry that underlie compulsive food intake and compulsive drug intake.
Methods
Subject Eligibility.
We studied six female obese subjects and one male obese subject (47.8 ± 6.3 years of age; range, 37–55 years old) who had the IGS implanted for 1–2 years. Subjects with body mass index >30 kg/m2 at the time of IGS implantation were included. The exclusion criteria were as follows: urine positive for psychoactive drugs, dependence on alcohol or other drugs of abuse (except for caffeine <5 cups per day or nicotine <1 pack per day), neurological disorder of central origin or major psychiatric disorder, use of anorexic medications for weight loss in the past 6 months, use of medications in the past 1 month that can affect brain function, uncontrolled cardiovascular disease (e.g., hypertension), uncontrolled endocrinological disease (including diabetes), and acute or chronic medical illness that may affect brain function.
The Institutional Review Boards at Brookhaven National Laboratory and State University of New York–Stony Brook approved the protocol, and written informed consent was obtained from all subjects.
Study Design.
Each subject was tested two times with PET-FDG scan on 2 separate days within 2 weeks. Subjects were asked to fast for ≈14–16 h before the PET scans. The referring physicians screened subjects for eligibility at Transneuronix clinical study sites (such as in New York, Atlanta, Kansas City, and Boston) and activated (remained on) or deactivated (turned off) the IGS. The subjects were blinded to the status of the IGS (remained activated or deactivated) after the screening visit. For Study Arm 1, the IGS remained activated (on), and for study Arm 2, the IGS remained deactivated (off) for the 2 weeks before the first PET scans. For six of the subjects, the on scan was done first, and for one of the subjects, the on scan was done second.
Scanning.
Subjects were scanned with FDG by using an HR+ Siemens (Iselin, NJ) tomograph by using the 3D acquisition mode. Details on procedures for positioning of the subjects, arterialized venous and venous catheterization, quantification of radiotracer and transmission and emission scans have been published in ref. 43. Briefly, one emission scan (20-min duration) was taken starting at 35 min after an i.v. injection of ≤185 MBq of FDG. During the study, subjects were positioned supine in the PET camera with their eyes open; the room was dimly lit, and noise was kept to a minimum.
Behavioral Assessment.
The Three-Factor Eating Questionnaire-Eating Inventory was obtained on the day that the scans were performed. This questionnaire measures three aspects of eating behavior: cognitive restraint, uncontrolled eating, and emotional eating (44).
Image and Data Analysis.
Differences between the IGS on and off conditions were tested by using the software package for Statistical Parametric Mapping (SPM99) (45). Before the analysis, each subject’s PET image was mapped onto the Montreal Neurological Institute template and smoothed via a Gaussian kernel with full width half maximum at 16 mm. Pixels significantly different between the IGS on and the IGS off were identified with respect to the Talairach and Tournoux stereotactic coordinates and displayed on the axial MR images. To ensure that the regional differences were not driven predominantly by subjects with either very high or very low whole-brain metabolism, SPM analysis was obtained on both the absolute and the normalized metabolic images. The normalized (relative) image was obtained by dividing the signal level of each voxel with the global mean, which was the average signal level of all voxels in the PET image. The level of significance was set at P < 0.01, uncorrected, cluster threshold >20 voxels.
In parallel, we also obtained ROI for the right and left orbitofrontal cortex, anterior cingulate, anterior insula, caudate, putamen, ventral striatum, thalamus, hippocampus, and cerebellum by using a template, which we had published in ref. 33.
Differences in the behavioral components of eating behavior and in the ROIs between the on and off conditions were tested by using the paired t test, two-tailed. To assess the associations between changes in metabolism induced by IGS and the behavioral scales, we measured the correlations between the regions for which absolute metabolic measures were affected significantly by IGS and the behavioral scores obtained during the on and the off conditions as well as the differences between on and off. P values <0.05 were considered significant if they were corroborated by the association with both the on and the off conditions or P < 0.01 if they were observed in only one condition or in the difference between conditions.
Acknowledgments
We thank all of the subjects who participated in this study; M. Bessler, J. Champion, S. Champion, A. Daud, A. Melanson, I. Sarosiek, and S. Shikora for subject referrals; K. Torres for Institutional Review Board correspondence and study compliance; D. Schlyer and M. Schueller for Cyclotron operations; D. Alexoff, P. Vaska, and D. Warner for PET operations; R. Ferrieri, C. Shea, Y. Xu, and P. King for radiotracer preparation and analysis; P. Carter, B. Hubbard, and M. Jayne for patient care; K. Pradhan and M. Michaelides for data analysis; and A. Ruggiero for manuscript submission. This work was supported by U.S. Department of Energy Office of Biological and Environmental Research Grant DE-ACO2-98CH10886, National Institute on Drug Abuse Grants DA6891 and DA6278, National Institute on Alcohol Abuse and Alcoholism Grants AA9481 and Y1AA3009, and the General Clinical Research Center at University Hospital Stony Brook (National Institutes of Health Grant M01RR 10710).
Abbreviations
- DA
dopamine
- FDG
2-deoxy-2[18F]fluoro-d-glucose
- IGS
Implantable Gastric Stimulator
- OFC
orbitofrontal cortex
- PET
positron emission tomography
- ROI
region of interest
- SPM
statistical parametric mapping.
Footnotes
Author contributions: G.-J.W., J.Y., N.D.V., P.K.T., and J.S.F. designed research; F.T. performed research; G.-J.W., N.D.V., Y.M., W.Z., C.T.W., D.T., and P.K.T. analyzed data; G.-J.W. wrote the paper; and N.D.V. and J.S.F. revised the manuscript.
Conflict of interest statement: J.Y. was an employee of Transneuronix, Inc. (Mt. Arlington, NJ) at the time of the experiment; however, the company did not provide funding for the study.
This article is a PNAS direct submission.
References
- 1.Woods SC. Physiol Behav. 2005;86:709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz GJ. Nutrition. 2000;16:866–873. doi: 10.1016/s0899-9007(00)00464-0. [DOI] [PubMed] [Google Scholar]
- 3.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Proc Natl Acad Sci USA. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cigaina V. Obes Surg. 2002;12(Suppl 1):12S–16S. doi: 10.1007/BF03342141. [DOI] [PubMed] [Google Scholar]
- 5.Groves DA, Brown VJ. Neurosci Biobehav Rev. 2005;29:493–500. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 6.MacLean PD. The Triune Brain in Evolution: Role in Paleocerebral Functions. New York: Plenum; 1990. [DOI] [PubMed] [Google Scholar]
- 7.Dedeurwaerdere S, Cornelissen B, Van Laere K, Vonck K, Achten E, Slegers G, Boon P. Epilepsy Res. 2005;67:133–141. doi: 10.1016/j.eplepsyres.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Epilepsy Res. 2003;55:59–70. doi: 10.1016/s0920-1211(03)00107-4. [DOI] [PubMed] [Google Scholar]
- 9.Miyashita T, Williams CL. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Altschuler SM, Bao XM, Bieger D, Hopkins DA, Miselis RR. J Comp Neurol. 1989;283:248–268. doi: 10.1002/cne.902830207. [DOI] [PubMed] [Google Scholar]
- 11.Stephan E, Pardo JV, Faris PL, Hartman BK, Kim SW, Ivanov EH, Daughters RS, Costello PA, Goodale RL. J Gastrointest Surg. 2003;7:740–749. doi: 10.1016/s1091-255x(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 12.Chae JH, Nahas Z, Lomarev M, Denslow S, Lorberbaum JP, Bohning DE, George MS. J Psychiatr Res. 2003;37:443–455. doi: 10.1016/s0022-3956(03)00074-8. [DOI] [PubMed] [Google Scholar]
- 13.McGaugh JL. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 14.Bast T, Feldon J. Prog Neurobiol. 2003;70:319–345. doi: 10.1016/s0301-0082(03)00112-6. [DOI] [PubMed] [Google Scholar]
- 15.Dolcos F, LaBar KS, Cabeza R. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 16.Forloni G, Fisone G, Guaitani A, Ladinsky H, Consolo S. Physiol Behav. 1986;38:321–326. doi: 10.1016/0031-9384(86)90101-0. [DOI] [PubMed] [Google Scholar]
- 17.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Davidson TL, Jarrard LE. Neurosci Biobehav Rev. 2004;28:261–271. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Diano S, Farr SA, Benoit SC, McNay E, da Silva I, Horvath B, Gaskin SK, Nonaka N, Jaeger LB, Bank WA, et al. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 20.Guan Y, Tang M, Jiang Z, Peeters TL. Brain Res. 2003;984:33–41. doi: 10.1016/s0006-8993(03)03016-6. [DOI] [PubMed] [Google Scholar]
- 21.Tracy AL, Jarrard LE, Davidson TL. Behav Brain Res. 2001;127:13–23. doi: 10.1016/s0166-4328(01)00364-3. [DOI] [PubMed] [Google Scholar]
- 22.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Physiol Behav. 2005;86:731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Louilot A, Le Moal M. Neuroscience. 1994;59:495–500. doi: 10.1016/0306-4522(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 24.Berridge KC, Robinson TE. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 25.Peleg-Raibstein D, Pezze MA, Ferger B, Zhang WN, Murphy CA, Feldon J, Bast T. Neuroscience. 2005;132:219–232. doi: 10.1016/j.neuroscience.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Gurden H, Takita M, Jay TM. J Neurosci. 2000;20:RC106. doi: 10.1523/JNEUROSCI.20-22-j0003.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. NeuroImage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 28.DelParigi A, Chen K, Salbe AD, Hill JO, Wing RR, Reiman EM, Tataranni PA. Int J Obes Relat Metab Disord. 2004;28:370–377. doi: 10.1038/sj.ijo.0802558. [DOI] [PubMed] [Google Scholar]
- 29.Rolls ET. Physiol Behav. 2005;85:45–56. doi: 10.1016/j.physbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Morris JS, Dolan RJ. J Neurosci. 2001;21:5304–5310. doi: 10.1523/JNEUROSCI.21-14-05304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang G-J, Volkow ND, Telang F, Jayne M, Ma J, Rao M, Zhu W, Wong CT, Pappas NR, Geliebter A, Fowler JS. NeuroImage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 32.Kringelbach ML, O'Doherty J, Rolls ET, Andrews C. Cereb Cortex. 2003;13:1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 33.Berridge KC, Robinson TE. Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang G-J, Fowler JS, Logan J, Jayne M, Franceschi D, Wong CT, Gatley SJ, Gifford AN, Ding YS, Pappas N. Synapse. 2002;44:175–180. doi: 10.1002/syn.10075. [DOI] [PubMed] [Google Scholar]
- 35.Hollerman JR, Tremblay L, Schultz W. Prog Brain Res. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Z, Dietrichs E, Walberg F. Neurosci Lett. 1982;32:113–118. doi: 10.1016/0304-3940(82)90259-2. [DOI] [PubMed] [Google Scholar]
- 37.Ladabaum U, Minoshima S, Owyang C. Am J Physiol. 2000;279:G1–G6. doi: 10.1152/ajpgi.2000.279.1.G1. [DOI] [PubMed] [Google Scholar]
- 38.Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Proc Natl Acad Sci USA. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gautier JF, Chen K, Salbe AD, Bandy D, Pratley RE, Heiman M, Ravussin E, Reiman EM, Tataranni PA. Diabetes. 2000;49:838–846. doi: 10.2337/diabetes.49.5.838. [DOI] [PubMed] [Google Scholar]
- 40.Volkow ND, Fowler JS, Wang G-J. Behav Pharmacol. 2002;13:355–366. doi: 10.1097/00008877-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Parolaro D, Vigano D, Rubino T. Curr Drug Targets CNS Neurol Disord. 2005;4:643–655. doi: 10.2174/156800705774933014. [DOI] [PubMed] [Google Scholar]
- 42.Bartlett EJ, Brodie JD, Wolf AP, Christman DR, Laska E, Meissner M. J Cereb Blood Flow Metab. 1988;8:502–512. doi: 10.1038/jcbfm.1988.91. [DOI] [PubMed] [Google Scholar]
- 43.Wang G-J, Volkow ND, Fowler JS, Franceschi D, Wong CT, Pappas NR, Netusil N, Zhu W, Felder C, Ma Y. Alcohol Clin Exp Res. 2003;27:909–917. doi: 10.1097/01.ALC.0000071740.56375.BA. [DOI] [PubMed] [Google Scholar]
- 44.Stunkard AJ, Messick S. Handbook of Psychiatric Measures. Washington, DC: Am Psychiatr Assoc; 2000. pp. 657–659. [Google Scholar]
- 45.MRC Cyclotron Unit. London: Hammersmith Hospital; 1999. SPM99 Software. [Google Scholar]