Abstract
The maize Activator/Dissociation (Ac/Ds) elements are members of the hAT (hobo, Ac, and Tam3) superfamily of type II (DNA) transposons that transpose through a “cut-and-paste” mechanism. Previously, we reported that a pair of Ac ends in reversed orientation is capable of undergoing alternative transposition reactions that can generate large-scale chromosomal rearrangements, including deletions and inversions. We show here that rearrangements induced by reversed Ac ends transposition can join the coding and regulatory sequences of two linked paralogous genes to generate a series of chimeric genes, some of which are functional. To our knowledge, this is the first report demonstrating that alternative transposition reactions can recombine gene segments, leading to the creation of new genes.
Synopsis
Transposable elements, or “jumping genes,” are DNA segments that can move to new sites in the genome. One type of transposable element from maize, called Ac/Ds, moves by a reaction known as “cut-and-paste.” In this mechanism, a transposase enzyme cleaves at both ends of a single Ac/Ds element, releasing the element from one site and inserting it at another location. However, if two Ac/Ds elements are situated near each other, the transposase may sometimes cut at the ends of two different elements. When these two Ac/Ds ends insert at a new location, a large rearrangement of the genome can occur; this process is termed alternative transposition. In this work, the authors studied alternative transposition events that affect the structure and expression of two genes that control maize kernel color. Alternative Ac transposition can cause fusions of the coding sequences of the two genes, generating a new functional chimeric gene that specifies a new maize kernel color. This mechanism of gene creation through alternative transposition is similar to the way that functional antibody genes are generated in the vertebrate immune system. These results show how the actions of transposable elements can reshuffle the genome to generate new functional genes.
Introduction
The maize Ac element is 4,565 base pairs (bp) in length and encodes an 807–amino acid transposase that catalyzes Ac/Ds transposition. The Ac/Ds element ends are delineated by complementary 11-bp terminal inverted repeat sequences, while the sub-terminal sequences are distinct from each other [1]. The individual Ac termini are designated as 5′ or 3′ according to their proximity to the beginning and end of the Ac transcript. Transposition requires one Ac 5′ end and one Ac 3′ end [2]. In standard transposition, the Ac 5′ and 3′ ends are part of a single transposon, and the outcome of transposition is the excision of the element from a donor site and insertion into a target site. However, transposition reactions can also involve the 5′ and 3′ ends of different Ac/Ds elements, which can be in either a direct or reversed orientation with respect to each other [3,4]. These alternative transposition events can generate deletions, duplications, inversions, and other sequence rearrangements. Because Ac/Ds preferentially transposes into genic regions, the rearrangements induced by alternative Ac/Ds transposition would be predicted to shuffle coding and regulatory sequences, and thereby generate new genes. We searched for such events in maize stocks containing a pair of reversed Ac ends in the p1 gene, which regulates kernel pericarp pigmentation. We obtained four chimeric alleles in which the promoter, exon 1 and exon 2 of the p2 gene (a paralog of p1) [5] is joined with exon 3 of the p1 gene. Because the p1 and p2 coding sequences are very similar, the new chimeric genes would encode proteins nearly identical to that encoded by the p1 gene. The p2 promoter is inactive in pericarp in the progenitor allele; however, these four new alleles show significant expression in kernel pericarp, and specify a novel orange pericarp phenotype. We propose that this new phenotype is largely caused by an altered expression pattern resulting from the chromosomal rearrangement. These results demonstrate that alternative transposition reactions can generate gene fusions and therefore may have been an important force in gene and genome evolution.
Results
Structures of Novel Chimeric Alleles
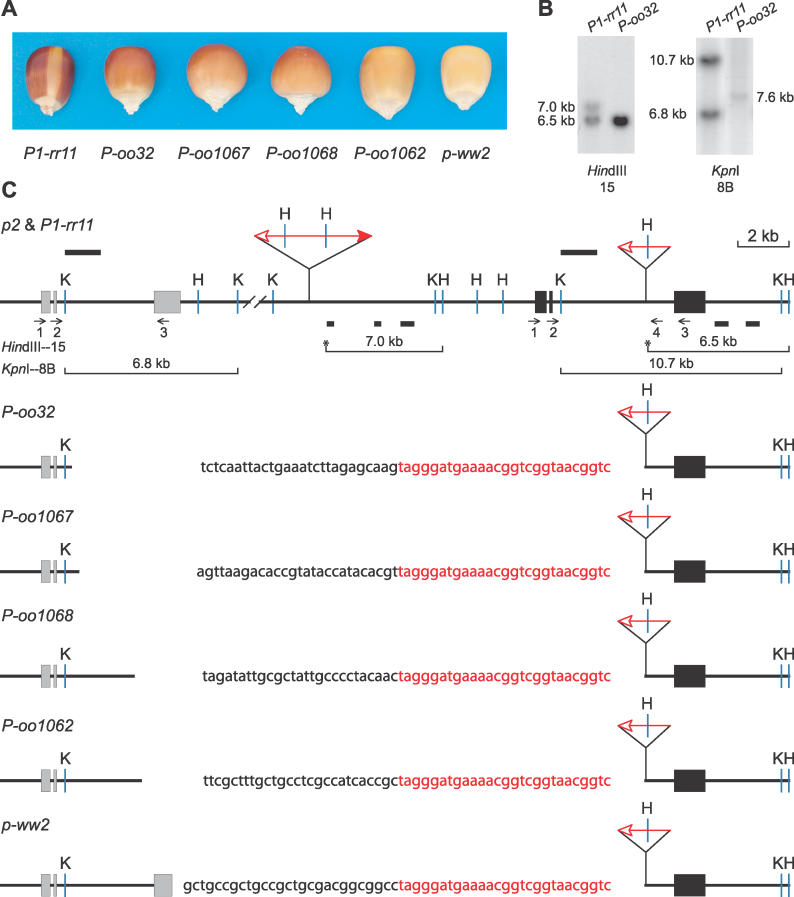
The maize p1 gene encodes a Myb-homologous transcriptional regulator required for synthesis of red pigments in kernel pericarp (Figure 1) and cob glumes [6]. The P1-rr11 allele (red pericarp, red cob) contains a truncated Ac element (fAc, fractured Ac) inserted in the second intron of p1, and a full-length Ac element inserted 13,175 bp upstream of the fAc element; the 5′ end of Ac and the 3′ end of fAc in P1-rr11 are oriented towards each other (Figure 1C). A paralog of p1, termed p2, is located approximately 60 kilobases (kb) upstream of the p1 gene in the chromosome containing the P1-rr11 allele [7] (Figure 1C). The p2 gene is not expressed in kernel pericarp and hence does not contribute to pericarp color [5,8]. Reversed Ac ends transposition in P1-rr11 would eliminate p1 gene function, and most mutants derived from P1-rr11 have colorless kernel pericarp and cob. However, we did isolate four alleles with orange pericarp and orange cob, and these were designated as P-oo32, P-oo1062, P-oo1067, and P-oo1068 (Figure 1A).
Figure 1. Phenotypes and Gene Structures of P-oo Alleles.
(A) The kernel pericarp pigmentation phenotypes specified by the indicated alleles.
(B) Genomic Southern blot. Genomic DNA from plants homozygous for the indicated alleles was cut with KpnI and HindIII, and hybridized with probes 15 or 8B from the p1 gene. Lanes marked P-oo32 contain approximately twice as much DNA as lanes marked P1-rr11; this DNA overloading enables the detection of the 7.6-kb band in the KpnI 8B blot, but also results in the intense 6.5-kb band in the HindIII 15 blot.
(C) Restriction map. The solid and gray boxes are exons 1, 2, and 3 (left to right) of p1 and p2, respectively. Red triangles indicate Ac or fAc insertions, and the open and solid arrowheads indicate the 3′ and 5′ ends, respectively, of Ac/fAc. Sequences hybridizing with Southern blot probes are indicated by the solid bars above (probe 8B) and below (probe 15) the map. The short horizontal arrows indicate the orientations and approximate position of PCR primers. Primers are identified by numbers below the arrows. The sequence of the junction of each fusion allele is shown here; the black letters indicate p2 sequence, while the red letters indicate fAc sequence. K, KpnI; H, HindIII. Lines below the map indicate the restriction fragments produced by digestion with KpnI or HindIII and hybridizing with the indicated probe; asterisks indicate HindIII restriction sites located within Ac or fAc sequences.
Genetic tests indicate that there is no Ac activity in the genome of the P-oo alleles. We characterized the structural rearrangements in the P-oo alleles by genomic DNA gel blot and PCR. Genomic DNA from plants carrying the P-oo32 allele was cut with HindIII and KpnI, and hybridized with maize p1 genomic probe fragments 15 or 8B (Figure 1B). In comparison to P1-rr11, the P-oo32 allele lacks the 7.0-kb HindIII fragment (from p1), the 10.7-kb KpnI fragment (from p1), and the 6.8-kb KpnI fragment (from p2). The absence of these fragments and the lack of Ac activity in the genome suggest that P-oo32 has a deletion that includes both p1 and p2 sequences. On the other hand, the presence of the 6.5-kb HindIII fragment detected by p1 fragment 15 indicates that the 3′ portion of the p1 gene and at least a part of fAc are intact. The faint 7.6-kb fragment in the KpnI-8B blot suggests that the upstream deletion end point is within the 8B-homologous fragment in p2. To test this, we performed PCR analysis using oligonucleotide primers 2 and 4, which flank the fAc insertion in p1 and are complementary to corresponding sites in the p2 gene. A ~2.4-kb product was amplified from P-oo32 DNA and sequenced. The results indicate that the 3′ end of fAc is inserted into a site in intron 2 of p2 (position 5619 in GenBank sequence AF210616), while the sequence downstream of fAc is from intron 2 of p1 (the p1 and p2 sequences are highly homologous, but sufficient sequence polymorphisms exist to distinguish the origin of PCR products). This result, together with the DNA gel blot results, indicates that P-oo32 is a gene fusion containing exon 1 and exon 2 of the p2 gene, the fAc sequence, and exon 3 of the p1 gene.
We characterized three additional P-oo alleles derived from P1-rr11: P-oo1062, P-oo1067, and P-oo1068. We performed PCR using primer 4 of the p1 gene, and a series of primers complementary to intron 2 of p2. Sequencing of the PCR products revealed that these three alleles have structures resembling that of P-oo32: each has exons 1 and 2 from p2, and exon 3 from p1. However, each allele exhibits a distinct site of fAc insertion in p2 intron 2: nucleotides 5912, 8088, and 8365 of AF210616 in P-oo1067, P-oo1068, and P-oo1062, respectively (Figure 1C). Importantly, the rearrangements show precise junctions of the p2 sequence with the fAc terminus. In contrast, transposition of Ds elements in Arabidopsis is reported to generate large deletions, but the deleted sequences extend into the Ds termini, indicating the involvement of cellular DNA repair mechanisms in deletion formation [9]. The precise junctions observed in the P-oo alleles are consistent with their formation through a single transposase-mediated insertion event.
The P-oo Alleles Are Generated by Reversed Ac Ends Transposition
As mentioned above, the p2 gene is located approximately 60 kb proximal to p1, and in the same transcriptional orientation [7]. The P1-rr11 allele contains reverse-oriented Ac 5′ and fAc 3′ ends whose transposition can generate a variety of chromosomal rearrangements [3]. If the excised Ac/fAc ends insert into a site in intron 2 of p2, the fAc in p1 intron 2 will be precisely joined to the insertion point in intron 2 of p2, and the ~60 kb of DNA between them will be deleted (Video S1). The resulting chromosome will carry a new fusion gene, composed of the promoter, exon 1 and exon 2 of p2, joined through fAc to exon 3 of p1 (Figure 2). The structures of P-oo32, P-oo1062, P-oo1067, and P-oo1068 are consistent with their origin via this reversed Ac ends transposition mechanism.
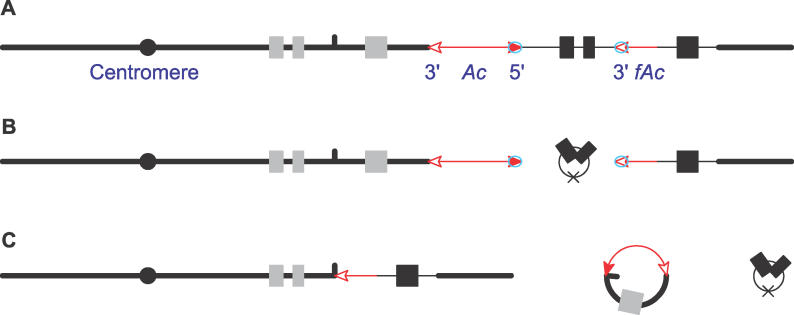
Figure 2. Deletions by Reversed Ac Ends Transposition Generate Chimerical Genes.
The solid circle indicates the centromere, the short vertical line indicates the target site, and the other symbols have the same meaning as those in Figure 1. (For animated version, see Video S1).
(A) Ac transposase (blue oval) binds to the 5′ end of Ac and 3′ end of fAc.
(B) As in ordinary transposition, the Ac 5′ end and the fAc 3′ end are excised by transposase cleavage, and the sequences flanking the Ac/fAc ends join together to form a ~13-kb circle. The X mark at the junction indicates the transposon footprint.
(C) The excised transposon ends insert into a site in intron 2 of p2. The Ac 5′ end joins to the distal side of the insertion site to form a circle, and the fAc 3′ end joins to the proximal side of the insertion site to generate a chimeric gene containing exon 1 and exon 2 of p2 and exon 3 of p1.
This study reports the isolation of the progenitor (A) and deletion products (C). Note that the hypothetical structures shown in (B) are transient in nature and would not be amenable to physical isolation.
We considered an alternative mechanism for generation of the fusion alleles via transposon-induced homologous recombination. Transposition of Ac/Ds elements is known to induce recombination between flanking homologous sequences [10,11]. The second introns of the p1 and p2 genes are 4.6 and 3.8 kb, respectively, and are 84% identical over their common lengths. Alleles formed by homologous recombination should have crossover sites at homologous sequences. However, the P-oo alleles have breakpoints at various sites within the p2 intron, and each junction occurs precisely at the fAc 3′ end. Moreover, all alleles retain the fAc sequence, with the sequences upstream of fAc resembling p2 and the sequences downstream of fAc resembling p1. This structure would not be expected from homologous recombination, but is consistent with transposition-induced rearrangement.
Expression of the P-oo Alleles in Pericarp
In addition to the P-oo alleles described above, we isolated an additional allele, termed p-ww2, that specifies colorless kernel pericarp and cob (Figure 1A). The p-ww2 allele was derived via an alternative transposition reaction involving fAc and a nearby, directly oriented Ac element inserted 3′ of fAc [4], followed by excision of the Ac element. The structure of p-ww2 is very similar to that of the four fusion alleles, except that fAc is joined to a site in exon 3 of p2, instead of intron 2 of p2 (Figure 1C). Although the deletion in p-ww2 is slightly smaller than those of the P-oo alleles, the colorless kernel pericarp and cob phenotype indicates that the p gene is not functional in p-ww2, whereas the four P-oo alleles that specify orange pericarp color indicate that the p2/ p1 fusion genes are functional.
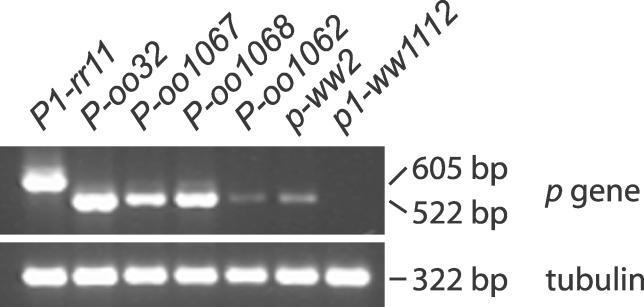
To test for expression of the fusion alleles, we performed RT-PCR on RNA extracted from developing kernel pericarp. Previous studies showed that p1 is expressed in various floral organs including kernel pericarp, while the p2 gene is expressed in other tissues including maize silk, but not in pericarp. The PCR primers 1 and 3 amplify a product of 605 bp from the p1 gene, and 522 bp from p2 gene, due to different lengths of the 5′ UTR of each gene [5]. The progenitor allele P1-rr11 has both p1 and p2 genes intact, and was used as a positive control. It generates a product of 605 bp as expected for p1 expression in kernel pericarp. The p1-ww1112 allele was used as a negative control; it has a deletion of the p1 coding sequence, but retains the sequences upstream of p1, including the p2 gene [10]. As expected, no products were amplified from this allele. The P-oo alleles generated RT-PCR products of 522 bp, which is consistent with expression of the fusion genes that include a 5′ UTR derived from the p2 gene (Figure 3). Sequencing of the RT-PCR products confirmed that the P-oo transcripts contained exon 1 and exon 2 of p2, and exon 3 of p1, as predicted by the gene structures. The chimeric P-oo genes would encode a protein identical to that encoded by the p1 gene except for a change in the fourth amino acid residue [5,12].
Figure 3. RT-PCR Analysis of P-oo Transcripts.
RNA was extracted from kernel pericarp (20 DAP), reverse transcribed, and PCR-amplified using primers complementary to both p1 and p2 transcripts. The progenitor allele (P1-rr11) shows amplification of a 605-bp band from p1. The p-ww2 and P-oo alleles show amplification of a 522-bp band characteristic of the 5′ region of the p2 gene. The p1-ww1112 allele has a deletion of p1; the native p2 gene is intact in this allele, but is not expressed in kernel pericarp.
No expression of the unrearranged p2 gene was detected in either p1-ww1112 or P1-rr11. This is consistent with previous reports, and supports the conclusion that the native p2 gene is not expressed in kernel pericarp [5]. It is somewhat surprising that p-ww2 and the P-oo genes, each of which contain the p2 promoter, generate transcripts in kernel pericarp. It has previously been shown that sequences nearly identical to genomic fragment 15 of the p1 gene form part of an enhancer located approximately 5 kb upstream of the p1 transcription start site [13]. In p-ww2 and the P-oo alleles, the p1 fragment 15 is located at new positions ranging from 6.2 kb to 14.4 kb 3′ of the p2 transcription start site. At these new sites, the fragment 15 sequence may enhance expression of the fusion genes in pericarp. This idea is consistent with the observation that the intensity of pericarp pigment specified by each P-oo allele is approximately correlated with the size of the deletion; i.e., alleles in which the fragment 15 sequence is located closer to the p2 promoter produce more intense pericarp color. Further analysis will be required to test this model.
Discussion
Our results document four cases of exon shuffling induced by members of the hAT superfamily of DNA transposons. hAT elements are widespread in plants, animals, and fungi. The somatic rearrangement of vertebrate immunoglobulin genes through V(D)J recombination is catalyzed by proteins (Rag1/Rag2) that are functionally related to hAT family transposases [14,15]. Indeed, the formation of the P-oo alleles described here through transposase-induced intra-chromosomal deletion is analogous to the mechanism of vertebrate antibody gene rearrangement [16,17]. In contrast to the situation in vertebrates in which the immunoglobulin rearrangements are limited to somatic cells, the genome rearrangements detected in maize can be inherited because of the late recruitment of gametophytic cells during plant development [18].
Recent sequence analysis of the rice and maize genomes have shown that the Mutator and Helitron transposon families are involved in large-scale duplication and shuffling of coding sequences [19–21]. Although it is not yet known whether the resulting chimeric genes are functional, their sheer abundance suggests that these transposon-induced rearrangements could be an additional large potential source of chimeric genes.
Previous reports of exon shuffling in cultured human cells have been associated with illegitimate recombination, or retrotransposition of long interspersed nuclear elements [22,23]. Exon shuffling via retrotransposition can occur only when retroelements are inserted in or near exon sequences. In rice, the Tos17 retrotransposon inserts preferentially into low-copy-number sequences [24]. In contrast, the vast majority of retroelement sequences in the maize genome are located predominantly in intergenic regions [25] and hence would not be expected to contribute to exon shuffling, whereas the tendency of Ac to insert preferentially into genic regions [26] greatly enhances its potential role in mediating exon shuffling reactions. Some cases of exon shuffling may confer a positive selective advantage that could promote fixation of variant chromosomal structures, such as inversions or reciprocal translocations, in sympatric populations [27,28].
Chromosomal rearrangements have been reported for other, non-hAT, transposon systems. In the fungus Fusarium, transposition involving termini of different Tc1-mariner elements can generate deletions and inversions that also may shuffle coding and regulatory sequences [29]. In Drosophila, transposition of Foldback elements and an associated white gene can result in activation of white gene expression, although little is known about the mechanism of Foldback transposition [30]. Also in Drosophila, transposition involving the termini of different P elements can induce various chromosomal rearrangements including deletions and inversions [31,32]. It seems likely that alternative transposition reactions of the type we report here are not unique to the hAT transposon superfamily, but may be a common feature of “cut-and-paste” eukaryotic transposons. Some transposable elements, such as Ac/Ds and Sleeping Beauty, tend to transpose to linked sites [33,34], leading to transposon clusters in which the termini of the linked transposons could be in either direct or reversed orientation. Alternative transposition reactions may then act upon these clustered transposon termini to generate large-scale chromosomal rearrangements. In support of this idea, a recent report has demonstrated that transgenic mice containing clusters of Sleeping Beauty transposon ends exhibit a high frequency of chromosomal aberrations [35]. Given the abundance of tandemly duplicated segments in plant and animal genomes, our results suggest that the alternative transposition events could represent an important evolutionary mechanism for the generation of new genes.
Materials and Methods
Genetic stocks.
Alleles of the maize p1 gene are identified by a two-letter suffix that indicates their expression pattern in pericarp and cob: e.g., P1-rr (red pericarp and red cob); and p1-ww (white pericarp and white cob). The P-oo (orange pericarp and orange cob) alleles described here were derived from P1-rr11 [3]; p-ww2 was derived from p1-vv9D9A [4].
Genomic DNA extractions and Southern blot hybridization.
Total genomic DNA was prepared from leaf tissue using a modified cetyltrimethylammonium bromide (CTAB) extraction protocol [36]. Agarose gel electrophoresis and Southern hybridizations were performed as described [37], except hybridization buffers contained 250 mM NaHPO4 (pH 7.2), 7% SDS, and wash buffers contained 20 mM NaHPO4 (pH 7.2), 1% SDS.
PCR amplifications.
PCR amplifications were performed as described [38] using the following oligonucleotide primers: CGCGACCAGCTGCTARCCGTG, CCAAGGAGGAAGAAGA CATCATCATCAAG, GCAGCTTGCTCATGTCGATGGC, and GCAGCTTGCTCATGTCG ATGGC. HotMaster Taq polymerase from Eppendorf (Hamburg, Germany) was used in the PCR reaction. Reactions were heated at 94 °C for 3 min, and then cycled 35 times at 94 °C for 20 s, 63 °C for 30 s, and 65 °C for 1 min per 1 kb length of expected PCR product, then 65 °C for 8 min. In most of the PCR reactions 2 M betaine and 4%–8% DMSO were added. The band amplified was purified from an agarose gel and sequenced directly. Sequencing was done by the DNA Synthesis and Sequencing Facility, Iowa State University, Ames, Iowa, United States.
For RT-PCR, total RNA was extracted from 20 DAP (days after pollination) pericarp using the RNeasy plant mini kit by Qiagen (Valencia, California, United States of America) and treated with DNase (Qiagen) to remove residual genomic DNA. Using StrataScript Reverse Transcriptase by Stratagene (La Jolla, California, United States of America) with oligo(dT) at 42 °C, 1 μg of total RNA was reverse-transcribed, while 3 μl of cDNA was subject to PCR amplification.
Oligonucleotide primers used for screening the P-oo alleles by genomic PCR were: p2-5044f: CCAAGGAGGAAGAAGACATCATCATCAAG, p2-5802f: ATAATGTTCCTTACTTACAACCAGCGG, p2-6841f: AACAGTCCCGATGATGCCCGCCAC, p2-7438f: TACACACAAACACCTTCCACTCCATAAAAT, p2-7951f: CTGAAGAAATCCTCTAACAAAACTGGCG, p2-8208f: TCTGGTCCAACACTCCCTCTTCATTC, and p2-8658f: GCGATCAATGAGTGAGCTAGTTTGTTGC.
Supporting Information
Press buttons to play animation. For details, see Figure 2 legend.
(34 KB MOV)
Accession Numbers
The GenBank (http://www.ncbi.nlm.nih.gov/Genbank) accession numbers for p1 and p2 are z11879 and af210616, respectively.
Acknowledgments
We thank ISU undergraduate students Lisa Coffey, Aaron Newell, and Laura Schmitt for assistance in various aspects of this study.
Abbreviations
- bp
base pair
- kb
kilobase
Footnotes
Competing interests. The authors have declared that no competing interests exist.
A previous version of this article appeared as an Early Online Release on August 11, 2006 (DOI: 10.1371/journal.pgen.0020164.eor).
Author contributions. JZ, FZ, and TP conceived and designed the experiments. JZ, FZ, and TP performed the experiments. JZ and TP analyzed the data. JZ and TP contributed reagents/materials/analysis tools. JZ and TP wrote the paper.
Funding. This research was supported by National Science Foundation grant 0450243 to TP and JZ.
References
- Kunze R, Weil CF. The hAT and CACTA superfamilies of plant transposons. In: Craig NL, Craigie R, Gellert M, Lambowitz AM, editors. Mobile DNA II. Washington (D. C.): ASM Press; 2002. pp. 565–610. [Google Scholar]
- Coupland G, Plum C, Chatterjee S, Post A, Starlinger P. Sequences near the termini are required for transposition of the maize transposon Ac in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989;86:9385–9388. doi: 10.1073/pnas.86.23.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Peterson T. Transposition of reversed Ac element ends generates chromosome rearrangements in maize. Genetics. 2004;167:1929–1937. doi: 10.1534/genetics.103.026229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Peterson T. Genome rearrangements by nonlinear transposons in maize. Genetics. 1999;153:1403–1410. doi: 10.1093/genetics/153.3.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Chopra S, Peterson T. A segmental duplication generated differentially expressed Myb-homologous genes in maize. The Plant Cell. 2000;12:1–12. doi: 10.1105/tpc.12.12.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotewold E, Athma P, Peterson T. Alternatively spliced products of the maize P gene encode proteins with homology to the DNA binding domain of Myb-like transcription factors. Proc Natl Acad Sci U S A. 1991;88:4587–4591. doi: 10.1073/pnas.88.11.4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Peterson T. A segmental deletion series generated by sister-chromatid transposition of Ac transposable elements in maize. Genetics. 2005;171:333–344. doi: 10.1534/genetics.104.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Wang Y, Zhang J, Snook M, Peterson T. A maize QTL for silk maysin levels contains duplicated myb-homologus genes which jointly regulate flavone biosynthesis. Plant Mol Biol. 2003;52:1–15. doi: 10.1023/a:1023942819106. [DOI] [PubMed] [Google Scholar]
- Page DR, Kohler C, da Costa-Nunes JA, Baroux C, Moore JM, et al. Intrachromosomal excision of a hybrid Ds element induces large genomic deletions in Arabidopsis . Proc Natl Acad Sci U S A. 2004;101:2969–2974. doi: 10.1073/pnas.0400089101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athma P, Peterson T. Ac induces homologous recombination at the maize P locus. Genetics. 1991;128:163–173. doi: 10.1093/genetics/128.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao YL, Li X, Peterson T. Ac insertion site affects the frequency of transposon-induced homologous recombination at the maize p1 locus. Genetics. 2000;156:2007–2017. doi: 10.1093/genetics/156.4.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athma P, Grotewold E, Peterson T. Insertional mutagenesis of the maize P gene by intragenic transposition of Ac . Genetics. 1992;131:199–209. doi: 10.1093/genetics/131.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorenko L, Li X, Tagliani L, Bowen B, Peterson T. Characterization of the regulatory elements of the maize P-rr gene by transient expression assays. Plant Mol Biol. 1999;39:11–19. doi: 10.1023/a:1006172815663. [DOI] [PubMed] [Google Scholar]
- Jones JM, Gellert M. The taming of a transposon: V(D)J recombination and the immune system. Immunol Rev. 2004;200:233–248. doi: 10.1111/j.0105-2896.2004.00168.x. [DOI] [PubMed] [Google Scholar]
- Zhou L, Mitra R, Atkinson PW, Hickman AB, Dyda F, et al. Transposition of hAT elements links transposable elements and V(D)J recombination. Nature. 2004;432:995–1001. doi: 10.1038/nature03157. [DOI] [PubMed] [Google Scholar]
- Hiom K, Melek M, Gellert M. DNA transposition by the RAG1 and RAG2 proteins: A possible source of oncogenic translocations. Cell. 1998;94:463–470. doi: 10.1016/s0092-8674(00)81587-1. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Eastman QM, Schatz DG. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature. 1998;394:744–751. doi: 10.1038/29457. [DOI] [PubMed] [Google Scholar]
- Walbot V, Evans MM. Unique features of the plant life cycle and their consequences. Nat Rev Genet. 2003;4:369–379. doi: 10.1038/nrg1064. [DOI] [PubMed] [Google Scholar]
- Jiang N, Bao Z, Zhang X, Eddy SR, Wessler SR. Pack-MULE transposable elements mediate gene evolution in plants. Nature. 2004;431:569–573. doi: 10.1038/nature02953. [DOI] [PubMed] [Google Scholar]
- Morgante M, Brunner S, Pea G, Fengler K, Zuccolo A, et al. Gene duplication and exon shuffling by helitron-like transposons generate intraspecies diversity in maize. Nat Genet. 2005;37:997–1002. doi: 10.1038/ng1615. [DOI] [PubMed] [Google Scholar]
- Bennetzen JL. Transposable elements, gene creation and genome rearrangement in flowering plants. Curr Opin Genet Dev. 2005;15:621–627. doi: 10.1016/j.gde.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Moran JV, DeBerardinis RJ, Kazazian HH., Jr Exon shuffling by L1 retrotransposition. Science. 1999;283:1530–1534. doi: 10.1126/science.283.5407.1530. [DOI] [PubMed] [Google Scholar]
- van Rijk AA, de Jong WW, Bloemendal H. Exon shuffling mimicked in cell culture. Proc Natl Acad Sci U S A. 1999;96:8074–8079. doi: 10.1073/pnas.96.14.8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Tsugawa H, Miyao A, Yano M, Wu J, et al. The rice retrotransposon Tos17 prefers low-copy-number sequences as integration targets. Mol Genet Genomics. 2001;265:336–344. doi: 10.1007/s004380000421. [DOI] [PubMed] [Google Scholar]
- SanMiguel P, Tikhonov A, Jin YK, Motchoulskaia N, Zakharov D, et al. Nested retrotransposons in the intergenic regions of the maize genome. Science. 1996;274:765–768. doi: 10.1126/science.274.5288.765. [DOI] [PubMed] [Google Scholar]
- Cowperthwaite M, Park W, Xu Z, Yan X, Maurais SC, et al. Use of the transposon Ac as a gene-searching engine in the maize genome. Plant Cell. 2002;14:713–726. doi: 10.1105/tpc.010468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieseberg LH. Chromosomal rearrangements and speciation. Trends Ecol Evol. 2001;16:351–358. doi: 10.1016/s0169-5347(01)02187-5. [DOI] [PubMed] [Google Scholar]
- Turelli M, Barton NH, Coyne JA. Theory and speciation. Trends Ecol Evol. 2001;16:330–343. doi: 10.1016/s0169-5347(01)02177-2. [DOI] [PubMed] [Google Scholar]
- Hua-Van A, Langin T, Daboussi MJ. Aberrant transposition of a Tc1-mariner element, impala, in the fungus Fusarium oxysporum . Mol Genet Genomics. 2002;267:79–87. doi: 10.1007/s00438-002-0638-9. [DOI] [PubMed] [Google Scholar]
- Moschetti R, Marsano RM, Barsanti P, Caggese C, Caizzi R. FB elements can promote exon shuffling: A promoter-less white allele can be reactivated by FB mediated transposition in Drosophila melanogaster . Mol Genet Genomics. 2004;271:394–401. doi: 10.1007/s00438-004-1007-7. [DOI] [PubMed] [Google Scholar]
- Gray YH, Tanaka MM, Sved JA. P-element-induced recombination in Drosophila melanogaster: Hybrid element insertion. Genetics. 1996;144:1601–1610. doi: 10.1093/genetics/144.4.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CR, Sved JA, Engels WR. P-element-induced male recombination and gene conversion in Drosophila . Genetics. 1996;144:1611–1622. doi: 10.1093/genetics/144.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy AJ, Akagi K, Largaespada DA, Copeland NG, Jenkins NA. Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature. 2005;436:221–226. doi: 10.1038/nature03691. [DOI] [PubMed] [Google Scholar]
- Fischer SE, Wienholds E, Plasterk RH. Regulated transposition of a fish transposon in the mouse germ line. Proc Natl Acad Sci U S A. 2001;98:6759–6764. doi: 10.1073/pnas.121569298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts AM, Collier LS, Geurts JL, Oseth LL, Bell ML, et al. Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. PLoS Genet. 2006;2:e156. doi: 10.1371/journal.pgen.0020156. DOI: 10.1371/journal.pgen.0020156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984;81:8014–8018. doi: 10.1073/pnas.81.24.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: A laboratory manual. Cold Spring Harbor (New York): Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Saiki RK. The design and optimization of the PCR. In: Erlich HA, editor. PCR technology. New York: Stockton Press; 1989. pp. 7–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Press buttons to play animation. For details, see Figure 2 legend.
(34 KB MOV)