Abstract
The clinical diagnostic criteria for frontotemporal degeneration (FTD) include relative preservation of memory and visuospatial function, in contradistinction to characteristics of Alzheimer’s disease (AD). The Mini-Mental State Examination (MMSE) contains items to assess these areas of cognition. In a retrospective case-control study of participants at two institutionally-based AD centers, we determined whether total MMSE and MMSE subscores would reflect the disease progression projected by the clinical criteria of FTD vs. AD. Participants were 44 subjects with FTD (7 pathologically confirmed) and 45 with pathologically confirmed AD. Each subject had at least two MMSEs with minimum inter-test intervals of 9 months. We compared annualized rates of change for total MMSE scores and cognitive domain subscores over time and between groups by two independent samples t-tests and proportion tests. The total MMSE score (p = 0.03) and language subscore (p = 0.02) showed a greater rate of decline for the FTD group than the AD group, although the constructional praxis item declined less rapidly in the FTD group (p = 0.018). Changes in MMSE subscores paralleled the clinical diagnostic criteria for FTD. The more rapid progression on the language subscore was observed in both language and behavioral variants of FTD.
Keywords: Alzheimer’sdisease, Frontotemporal dementia, Frontotemporal degeneration, Mini-Mental State Examination
Frontotemporal degeneration (FTD) differs from Alzheimer’s disease (AD) in terms of onset age, clinical presentation, disease duration to mortality, and neuropathology. The clinical consensus criteria for FTD include onset age earlier than 65 years and early preservation of both memory and constructional praxis [1]. Patients may have either the language presentation (FTD-L) causing aphasia, or a behavioral presentation (FTD-B, also known as frontotemporal dementia), which features impulsivity and poor judgment [2]. These initial presentations tend to converge over the course of illness [3]. The FTD-L group consists of non-fluent progressive aphasia and semantic dementia [1, 2]. The disease duration to mortality is shorter in FTD than AD [4, 5]. Given the differences between FTD and AD, one would expect differential rates in decline on cognitive tests such as the Folstein Mini-Mental State Examination [6].
Formal neuropsychological testing shows a difference in patterns of deficits early in illness [6-22], but the rate of progression for FTD has not been widely studied.
The MMSE is widely used as a measure of cognitive decline for patients with different types of dementia. Pasquier et al. [23] reported a total MMSE score decline from 21.7 to 19.4 over 2 years in 48 FTD subjects. In a subsequent study, Pasquier et al. [24] reported a slower mean annual total MMSE score decline in FTD than in AD, while Rascovsky et al. [25] showed a significantly more rapid decline in autopsy-confirmed FTD than in AD. None of these studies analyzed MMSE scores by cognitive domain.
In contrast to Pasquier’s findings, our clinical experience led us to hypothesize that the total MMSE scores of patients with FTD decline more rapidly than those of patients with AD. We also predicted that patients with FTD would show differences in progression on MMSE items in specific cognitive domains, compared with patients with AD. Corresponding with the clinical criteria for FTD [1] and previous results from more formal neuropsychological evaluations of patients [26, 27], we posited that subjects with FTD would show slower decline on MMSE subscores of orientation, memory, and figure copy. Due to involvement of the frontal lobes, we anticipated that subjects with FTD would show more rapid decline on attention than those with AD. We also expected FTD-L subjects to decline more rapidly on language, and that this might explain their more rapid decline on the MMSE overall.
Methods
We conducted a retrospective chart review and database search for MMSE data on subjects with either FTD or AD. Subjects were evaluated at the University of Texas Southwestern Alzheimer’s Disease Center (UTSW) and the University of Southern California Alzheimer’s Disease Research Center (USC). All of the subjects gave consent for participation in research on initial enrollment in longitudinal study at the centers, and the protocols for collecting the data were approved by the IRB at each institution.
To answer our study question, we sought subjects with two or more MMSE scores with a minimum inter-test interval of at least 9 months. At USC, subjects responded to the MMSE annually (within 1 month of first visit anniversary); at UTSW, subjects are followed annually but sometimes have additional testing as part of a parallel research protocol, such that MMSE scores may be measured less than a year apart.
While subjects were not excluded on the basis of lower limits on the MMSE, there may have been cases where the subject stopped visiting the clinic due to nursing home placement or disability so severe that the caregiver could not present the subject for further evaluation at the AD center.
Additional inclusion criteria were: known onset age of dementia, known educational level, and diagnosis of either FTD (n = 44) by published consensus criteria [1] or autopsy-proven AD (n = 45, either by Reagan/NIA [28] or Braak and Braak criteria [29]. Cases with AD were excluded if they also had a secondary neuropathological diagnosis, such as infarct. We further divided the FTD sample into two subgroups: those who presented initially with marked behavioral disturbance (FTD-B) vs. with primary progressive aphasia (FTD-L) [2] .
We excluded those subjects with significant visual impairment whose MMSE scores would have had to be adjusted for impossible tasks, as well as those subjects who could not speak English.
Variables reflecting the annualized rate of change were created using the first and last scores for each patient. Annualized change scores for total MMSE and for each subscore < 1 point (figure copy item excepted) were calculated by first determining the change score (last minus first) and then dividing by the time in years (number of days between visits divided by 365.25 to account for leap years) between these two tests. A minimum inter-test interval of 9 months maximized the available raw scores for analysis with an average of 2.83 data points (range 2-8) for each subject. The majority of subjects, 60.7%, in the total group had more than two MMSE scores and only 22.5% had more than three MMSE scores with inter-test time intervals exceeding 9 months. Individual subscores were available for MMSEs from 43 FTD and 40 AD subjects.
MMSE subscores were calculated by grouping various items of the MMSE by domain: orientation to time (0-5 points possible), orientation to place (0-5), registration (0-3), recall (0-3 points), attention/concentration (0-5 points), language (0-8 points), and figure copy representing constructional praxis (0 or 1 point).
Statistical Analyses: Two independent samples t tests (shown as means and ± standard errors in table 1) were performed to compare the two groups (FTD and AD) on demographic characteristics and initial clinical measures. Analysis of covariance (ANCOVA) was used to examine group differences on the initial total MMSE score. Covariates included in the model (if p< 0.05) were education, disease duration (from onset of illness to initial MMSE), and age at initial MMSE. For the dichotomous variable (gender), a two-sample proportions test was used to compare groups.
Table 1.
Demographics and MMSE scores of frontotemporal degeneration (FTD) and Alzheimer’s disease (AD) groups
| FTD n = 44 | AD n = 45 | FTD vs. AD p value | FTD-B n = 25 | FTD-L n = 19 | FTD-B vs. FTD-L vs. AD, p value | |
|---|---|---|---|---|---|---|
| Women | 43.2% | 57.8% | 0.169 | 44.0% | 42.1% | 0.385 |
| Mean educational level, years, SE | 13.64, 0.53 | 13.91, 0.31 | 0.657 | 13.24, 0.72 | 14.16, 0.79 | 0.527 |
| Mean dementia onset age, years, SE | 61.75, 1.34 | 66.40, 1.30 | 0.015 | 61.72, 1.73 | 61.79, 2.16 | 0.052 |
| Mean age at initial visit, years, SE | 65.82, 1.22 | 70.40, 1.26 | 0.011 | 66.60, 1.41 | 64.79, 2.16 | 0.030 |
| Mean duration of illness at initial MMSE, years, SE | 4.34, 0.60 | 3.91, 0.43 | 0.557 | 5.14, 0.99 | 3.30, 0.39 | 0.182 |
| Mean initial total MMSE score, SE | 21.98, 1.02 | 18.78, 0.78 | 0.014 | 22.12, 1.34 | 21.79, 1.62 | 0.050 |
| Mean initial total MMSE score, SE with education as a covariate | 22.06, 0.87 | 18.69, 0.86 | 0.007 | 22.46, 1.17 | 21.55, 1.34 | 0.025 |
| Mean group inter-test interval, years, SE | 2.34, 0.20 | 3.03, 0.29 | 0.056 | 2.36, 0.27 | 2.31, 0.30 | 0.161 |
p values shown are for t tests or ANOVA/ANCOVA, except gender comparison used two independent samples proportion tests.
Comparisons of the two groups on the annualized rate of change measures were performed using ANCOVA. Possible covariates included in each model were education, baseline measurement of the variable, and disease duration (time from onset to the last measure). Covariates were included in the final model if p< 0.05; if no covariates were found significant, t tests were performed. ANCOVA models also compared the FTD-B, FTD-L, and AD groups on all annualized rate of change measures.
To explore the hypothesized slower rate of decline on constructional praxis, groups of patients with perfect performance (score of 1) at the first testing on the figure copy/praxis item of the MMSE were compared with the results at the last testing (0 or 1) using a two independent samples proportion test. Random regression modeling [30] was performed on the all of the consecutive raw score measurements of the MMSE variables, with duration of illness, level of education, onset age, and an indicator for diagnostic group membership as possible covariates. We report the estimates of the rate of change in groups for this exploratory comparison study, as opposed to having set a critical threshold of decline.
Two-tailed analyses were chosen as a more conservative basis for group comparisons and the p value for significance was 0.05. We performed the analyses using both Statistical Package for the Social Sciences (SPSS) software, version 13.0, and SAS version 9.13.
Results
The FTD sample consisted of FTD-B (n = 25) and FTD-L (n = 19). The FTD-L group had 11 progressive non-fluent aphasia and 8 semantic dementia subjects. Seven subjects in the FTD group (n = 44) had autopsy-confirmed diagnoses. Two had dementia lacking distinct histopathologic features; 3 had FTD-motor neuron disease type (FTD-MND, also called FTD with ubiquitinated inclusions and FTD-motor neuron inclusion disease) [2]; 1 had sporadic multiple system tauopathy with dementia [31], and 1 had corticobasal degeneration. One of the FTD-MND cases also had age-associated neurofibrillary changes.
Demographic characteristics of the FTD group (n = 44) and AD group (n = 45) appear in table 1. Consistent with the clinical diagnostic criteria for FTD, onset age (p = 0.015) and age at initial evaluation (p = 0.011) were significantly younger for this group than for AD. The mean delay between onset of symptoms and the initial MMSE was 4.3 ± 0.6 years (range 0.1-20.0) for the FTD group and 3.9 ± 0.4 (range 0.8-18.2) for the AD group, with no significant difference between groups (p = 0.557). The FTD group had significantly higher MMSE scores at initial evaluation (p = 0.014).
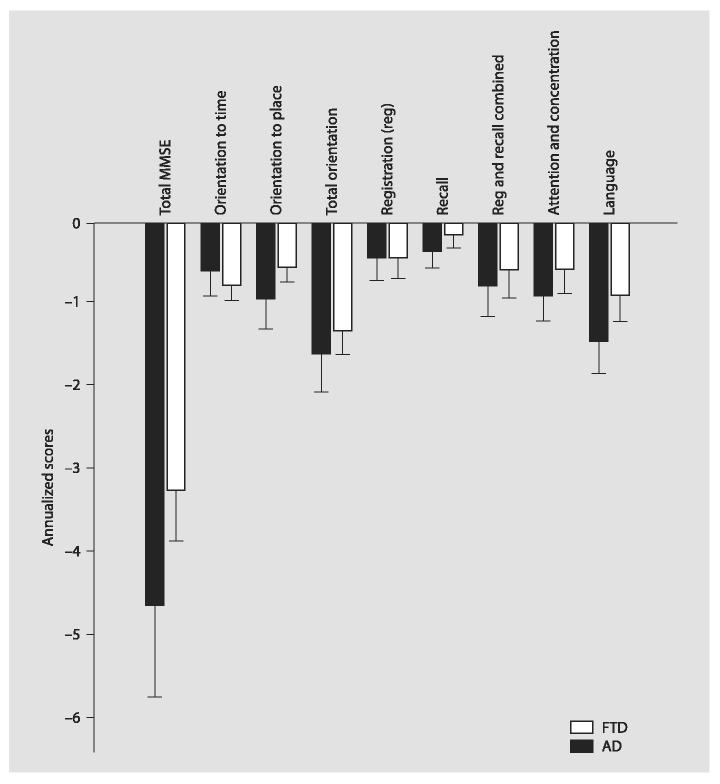
The FTD group had greater average annualized decline on total MMSE (-4.7 ± 0.4 points per year vs. AD -3.2 ± 0.4, p = 0.035, with education as a significant covariate; see fig. 1). The FTD subject group showed a larger annualized decline (-1.5 ± 0.2) on the MMSE language subscore compared to AD subjects (-0.9 ± 0.25, p = 0.020, no significant covariates). Orientation to place showed a significantly more rapid decline in FTD (p = 0.043); with education a significant covariate, while subjects with AD had a larger annualized decline in orientation to time (p = 0.037, with the baseline orientation to time score as a significant covariate), but the difference in mean annualized change for all 10 orientation items combined was 0.4 points or less between both groups. Memory (registration and/or recall subscores) did not decline more rapidly in AD than in FTD.
Fig. 1.
Annualized change scores for FTD vs. AD subjects show more rapid decline on the total MMSE and language subscore. Scores calculated as (last MMSE-initial MMSE)/time between the two scores. Error bars indicate 2 SEM.
At the first testing, 29 FTD patients and 18 AD patients had perfect performance (score of 1) on the figure copy item. The two independent samples proportion test showed that a lower percentage of FTD subjects (11/29, 37.9%) lost constructional praxis as tested by this MMSE item than the AD group (14/18, 77.8%, p = 0.018).
Random regression models to examine the consecutive raw scores for total MMSE and the MMSE subscales (except figure copy) revealed disease duration to be a consistently significant covariate in all models. When comparing FTD (n = 43) versus AD (n = 40), the decline in the orientation to time task began earlier for the AD group than for the FTD group by 0.7 years (SE = 0.3) with significant covariates of disease duration (p< 0.001) and onset age (p = 0.033). For the combined memory score of registration and recall, the decline was significantly more rapid for the AD group than for the FTD group by 0.6 years (SE = 0.2), also with significant covariate of disease duration (p = 0.013). All other models comparing either two groups (FTD and AD) or three groups (FTD-L, FTD-B, and AD) were non-significant.
FTD-L vs. FTD-B: FTD subgroup comparisons were performed to evaluate whether: (1) the rapid decline in language subscore for the FTD group was attributable mainly to the FTD-L subjects and (2) if the loss of language subscore resulted in a more rapid decline on the total MMSE score for FTD-L subjects relative to the FTD-B group. No statistically significant differences in total MMSE scores or language subscores were observed in a comparison of the FTD-L vs. FTD-B vs. AD groups.
Discussion
In contrast to one prior report [24] and as a replication of another [25], the FTD group in the present study demonstrated greater decline on total MMSE scores compared with a group of AD subjects. Although the difference in annual rate of change between FTD and AD was not as marked in our study as reported by Rascovsky et al. [25], we used the same methods for calculating the rate of decline on the MMSE. Our results confirmed their report that the FTD group shows more rapid decline on the MMSE, as well as our hypothesis that a greater proportion of the FTD group would have better constructional praxis over time relative to the AD group. The study also showed that all FTD subjects, and not just those with a language presentation, declined more rapidly on the language items of the MMSE. Performance on the attention subscore of the MMSE did not appear to differentiate FTD and AD. In general, these results indicate that MMSE scores for patients with FTD reflect the consensus clinical criteria for FTD, including language dysfunction and relative preservation of constructional praxis.
Differences between our group of FTD subjects and those of Pasquier et al. [24] may account for the discrepancy between the study’s conclusions. Our FTD group had a shorter delay between onset of symptoms and presentation to a clinic (4 vs. 5.9 years) and lower mean MMSE score at initial presentation (22 vs. 24.5) than the Pasquier group. This indicates that our FTD subjects may have been progressing at a faster rate than the Pasquier group, therefore creating more incentive to seek medical attention earlier in illness and a different comparison to a group of subjects with AD. We followed our subjects from initial presentation to our clinic, regardless of MMSE score, so that we could start with a score as close in time to disease onset as possible.
In this study, we also examined the rates of progression for FTD-B vs. FTD-L subgroups. Although our combined FTD group included more behavioral than language presentations of FTD, the language subscores of the FTD group as a whole declined significantly more than those of the AD group. Aphasia in FTD may affect MMSE scores more adversely than the aphasia of AD, which is often characterized by dysnomia followed by cortical sensory aphasia. In contrast, the typical aphasic profiles of FTD include difficulty with letter fluency, progressive non-fluent aphasia, or loss of word meaning with disabled semantic processing. The FTD-B group’s decline on the language subscore lends support to convergence of the two FTD phenotypes over time [32-34]. Random regression models indicated the significance of duration of illness as a covariate and pointed to orientation to time and combined scores for registration and memory as significant weaknesses of the AD group, relative to the FTD group.
Several factors limit the conclusions of this study. The analysis assumes a constant rate of decline. A prospective longitudinal study would be necessary to determine whether there are plateau phases of stabilization on the MMSE or its subscores for either dementia etiology. The number of within-subject data points varies among subjects in this study; with 39% of subjects having just two evaluations, limiting conclusions from the random regression models, which are not optimal for such a data-set, yet the results of this type of analysis were consistent with the findings from annualized rates of change analyses. In addition, all of the AD subjects were pathologically proven, but few of the FTD cases had autopsy confirmation. Rascovsky’s study [25] using only those FTD subjects with autopsy confirmation implies that doing so may enhance the difference in findings between the two dementia groups.
Despite these limitations, the results support our hypotheses and the consensus clinical criteria for FTD. Gregory et al.’s [9] cross-sectional study compared neuropsychological test findings on pairs of FTD and AD subjects matched by MMSE score (mean 24-25); category fluency and not letter fluency was the only cognitive test item with high specificity and sensitivity for differentiation between the two dementias. Because the MMSE does not include this type of testing, it is noteworthy that we were able to find an MMSE subscore on which FTD differed from AD.
This study confirms differences between FTD and AD that are concerns for clinicians, patients, and families dealing with dementia. First, it adds to the information available regarding progression and prognosis, supporting reports that patients with FTD decline more rapidly than patients with AD. Secondly, language performance declined for both phenotypes of FTD, such that compensation for loss of communicative skills should be addressed for all patients with FTD, not only those with FTD-L. Despite our findings, we do agree with other authors that behavioral inventories form a more important component of the clinical diagnosis of FTD than a cognitive screen such as the MMSE [35-37]. However, primary care physicians who may only administer the MMSE on a regular basis might be better informed of this type of profile to recognize non-AD.
Acknowledgments
Without the generous participation of our subjects, this work would not be possible. We also thank Ms. Maureen Wunderlich for her assistance in assembling the dataset. This study was supported in part by NIA grant F32 AG022802 (T.W.C.), ADRC grant AG AG10123 to the University of Southern California, California Department of Health Services Alzheimer’s Disease Research Center of California grants to USC and the Rancho Los Amigos National Rehabilitation Center, and NIA ADC grant P30 AG12300 to University of Texas-Southwestern Medical Center at Dallas.
References
- 1.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.McKhann GM, Albert MS, Grossman M, Miller B, Dickson D, Trojanowski JQ. Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol. 2001;58:1803–1809. doi: 10.1001/archneur.58.11.1803. [DOI] [PubMed] [Google Scholar]
- 3.Chow TW, Miller BL, Boone K, Mishkin F, Cummings J. Frontotemporal dementia classification and neuropsychiatry. Neurologist. 2002;8:263–269. doi: 10.1097/00127893-200207000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipton AM, Benavides R, Hynan LS. Disease duration is shorter in frontotemporal dementia than in Alzheimer’s disease. Neurology. 2003;60(suppl 1):A377. [Google Scholar]
- 5.Hodges JR, Davies R, Xuereb J, Kril J, Halliday G. Survival in frontotemporal dementia. Neurology. 2003;61:349–354. doi: 10.1212/01.wnl.0000078928.20107.52. [DOI] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Mendez MF, Cherrier M, Perryman KM, Pachana N, Miller BL, Cummings JL. Frontotemporal dementia versus Alzheimer’s disease: differential cognitive features. Neurology. 1996;47:1189–1194. doi: 10.1212/wnl.47.5.1189. [DOI] [PubMed] [Google Scholar]
- 8.Miller BL, Cummings JL, Boone K, et al. Clinical and neurobehavioral characteristics of fronto-temporal dementia and Alzheimer disease. Neurology. 1995;45:A318. [Google Scholar]
- 9.Gregory CA, Orrell M, Sahakian B, Hodges JR. Can frontotemporal dementia and Alzheimer’s disease be differentiated using a brief battery of tests? Int J Geriatr Psychiatry. 1997;12:375–383. doi: 10.1002/(sici)1099-1166(199703)12:3<375::aid-gps518>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Mathuranath PS, Nestor PJ, Berrios GE, Rakowicz W, Hodges JR. A brief cognitive test battery to differentiate Alzheimer’s disease and frontotemporal dementia. Neurology. 2000;55:1613–1620. doi: 10.1212/01.wnl.0000434309.85312.19. [DOI] [PubMed] [Google Scholar]
- 11.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–2284. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 12.Hodges JR, Patterson K, Ward R, Garrard P, Bak T, Perry R, Gregory C. The differentiation of semantic dementia and frontal lobe dementia (temporal and frontal variants of frontotemporal dementia) from early Alzheimer’s disease: a comparative neuropsychological study. Neuropsychology. 1999;13:31–40. doi: 10.1037//0894-4105.13.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Lindau M, Jelic V, Johansson SE, Andersen C, Wahlund LO, Almkvist O. Quantitative EEG abnormalities and cognitive dysfunctions in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2003;15:106–114. doi: 10.1159/000067973. [DOI] [PubMed] [Google Scholar]
- 14.Kramer JH, Jurik J, Sha SJ, Rankin KP, Rosen HJ, Johnson JK, Miller BL. Distinctive neuropsychological patterns in frontotemporal dementia, semantic dementia, and Alzheimer disease. Cogn Behav Neurol. 2003;16:211–218. doi: 10.1097/00146965-200312000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Razani J, Boone KB, Miller BL, Lee A, Sherman D. Neuropsychological performance of right- and lef t-frontotemporal dementia compared to Alzheimer’s disease. J Int Neuropsychol Soc. 2001;7:468–480. doi: 10.1017/s1355617701744037. [DOI] [PubMed] [Google Scholar]
- 16.Siri S, Benaglio I, Frigerio A, Binetti G, Cappa SF. A brief neuropsychological assessment for the differential diagnosis between frontotemporal dementia and Alzheimer’s disease. Eur J Neurol. 2001;8:125–132. doi: 10.1046/j.1468-1331.2001.00179.x. [DOI] [PubMed] [Google Scholar]
- 17.Weintraub S, Mesulam MM. Four neuropsychological profiles in dementia; in Boller F, Spinnler H (eds): Handbook of Neuropsychology. Elsevier; Amsterdam: 1985. pp. 253–282. [Google Scholar]
- 18.Perry RJ, Hodges JR. Differentiating frontal and temporal variant frontotemporal dementia from Alzheimer’s disease. Neurology. 2000;54:2277–2284. doi: 10.1212/wnl.54.12.2277. [DOI] [PubMed] [Google Scholar]
- 19.Pachana N, Boone KB, Miller BL, Cummings JL, Berman N. Comparison of neuropsychological functioning in Alzheimer’s disease and frontotemporal dementia. J Int Neuropsychol Soc. 1996;2:505–510. doi: 10.1017/s1355617700001673. [DOI] [PubMed] [Google Scholar]
- 20.Glosser G, Gallo JL, Clark CM, Grossman M. Memory encoding and retrieval in fronto-temporal dementia and Alzheimer’s disease. Neuropsychology. 2002;16:190–196. [PubMed] [Google Scholar]
- 21.Lipton A, Chow TW, Hynan LS. MMSE scores decline at a greater rate in frontotemporal lobar degeneration than in Alzhei mer’s disease. Neurology. 2003;60(suppl 1):A375. doi: 10.1159/000094870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rascovsky K, Salmon DP, Leverenz JB, De-Carli C, Clark CM, Mendez MF, Graff-Rad-ford NR, Galasko D. Rate of progression differs in frontotemporal dementia and Alzheimer’s disease. Neurology. 2004;62:A344. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 23.Pasquier F, Lebert F, Lavenu I, Guillaume B. The clinical picture of frontotemporal dementia: diagnosis and follow-up. Dement Geriatr Cogn Disord. 1999;10(suppl 1):10–14. doi: 10.1159/000051206. [DOI] [PubMed] [Google Scholar]
- 24.Pasquier F, Richard F, Lebert F. Natural history of frontotemporal dementia: comparison with Alzheimer’s disease. Dement Geriatr Cogn Disord. 2004;17:253–257. doi: 10.1159/000077148. [DOI] [PubMed] [Google Scholar]
- 25.Rascovsky K, Salmon DP, Lipton AM, Leverenz JB, DeCarli C, Jagust WJ, Clark CM, Mendez MF, Tang-Wai DF, Graff-Rad-ford NR, Galasko D. Rate of progression differs in frontotemporal dementia and Alzheimer’s disease. Neurology. 2005;65:397–403. doi: 10.1212/01.wnl.0000171343.43314.6e. [DOI] [PubMed] [Google Scholar]
- 26.Rascovsky K, Salmon DP, Ho GJ, Galasko D, Peavy GM, Hansen LA, Thal LJ. Cognitive profiles differ in autopsy-confirmed fronto-temporal dementia and AD. Neurolog y. 2002;58:1801–1808. doi: 10.1212/wnl.58.12.1801. [DOI] [PubMed] [Google Scholar]
- 27.Bier JC, Ventura M, Donckels V, Van Eyll E, Claes T, Slama H, Fery P, Vokaer M, Pandolfo M. Is the Addenbrooke’s cognitive examination effective to detect frontotemporal dementia? J Neurol. 2004;251:428–431. doi: 10.1007/s00415-004-0345-z. [DOI] [PubMed] [Google Scholar]
- 28.Newell KL, Hyman BT, Growdon JH, Hed-ley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer disease. J Neuropathol Exp Neurol. 1999;58:1147–1155. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Mirra SS, Heyman A, McKeel D, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD). II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;40:1–8. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 30.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, SAS Institute Inc; 1996. [Google Scholar]
- 31.Bigio EH, Lipton AM, Yen SH, Hutton ML, Baker M, Nacharaju P, White CL, Davies P, Lin W, Dickson DW. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol. 2001;60:328–341. doi: 10.1093/jnen/60.4.328. [DOI] [PubMed] [Google Scholar]
- 32.Chow TW, Boone K, Mishkin F, Miller BL. Behavioral changes in subtypes of primary progressive aphasia and other frontotemporal dementias; Rotman Institute Frontal Lobes Meeting, 2000; Toronto, Brain and Cognition. 2000. [Google Scholar]
- 33.Kertesz A, Davidson W, McCabe P, Takagi K, Munoz D. Primary progressive aphasia: diagnosis, varieties, evolution. J Int Neuropsychol Soc. 2003;9:710–719. doi: 10.1017/S1355617703950041. [DOI] [PubMed] [Google Scholar]
- 34.Seeley WW, Bauer AM, Miller BL, Gorno-Tempini ML, Kramer JH, Weiner M, Rosen HJ. The natural history of temporal variant frontotemporal dementia. Neurology. 2005;64:1384–1390. doi: 10.1212/01.WNL.0000158425.46019.5C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pijnenburg YA, Gillissen F, Jonker C, Scheltens P. Initial complaints in frontotemporal lobar degeneration. Dement Geriatr Cogn Disord. 2004;17:302–306. doi: 10.1159/000077159. [DOI] [PubMed] [Google Scholar]
- 36.Kertesz A, Davidson W, McCabe P, Munoz D. Behavioral quantitation is more sensitive than cognitive testing in frontotemporal dementia. Alzheimer Dis Assoc Disord. 2003;17:223–229. doi: 10.1097/00002093-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Diehl J, Kurz A. Frontotemporal dementia: patient characteristics, cognition, and behaviour. Int J Geriatr Psychiatry. 2002;17:914–918. doi: 10.1002/gps.709. [DOI] [PubMed] [Google Scholar]