Abstract
Aneurysms of the left main coronary artery are exceedingly rare clinical entities, encountered incidentally in approximately 0.1% of patients who undergo routine angiography. Thrombosis within the aneurysm can lead to distal embolization and myocardial infarction. These lesions can extend into adjacent coronary branches and can occur in the presence or absence of obstructive coronary disease. Depending on the severity of coexistent coronary stenoses, patients with left main coronary artery aneurysms can be effectively managed either operatively or medically. We report the cases of 2 patients who were treated medically for large left main coronary aneurysms and concomitant right coronary artery ectasia.
Key words: Coronary aneurysm, coronary angiography
Aneurysms of the left main coronary artery are exceedingly rare clinical entities, encountered incidentally in approximately 0.1% of patients who undergo routine angiography. Thrombosis within the aneurysm can lead to distal embolization and myocardial infarction. These lesions can extend into adjacent coronary branches and can occur in the presence or absence of obstructive coronary disease. Depending on the severity of coexistent coronary stenoses, patients with left main coronary artery aneurysms can be effectively managed either operatively or medically with adequate anticoagulation and close follow-up. We report the cases of 2 patients who were treated medically for large left main coronary aneurysms and concomitant right coronary artery ectasia.
Case Reports
Patient 1
Patient 1 was an obese 43-year-old man with a medical history notable for type 2 diabetes mellitus and hyperlipidemia, who presented in July 2004 with acute onset of chest pain at rest that radiated to his jaw and left arm. He denied any history of dyspnea, orthopnea, tobacco use, or any noteworthy febrile illnesses during childhood. After the administration of sublingual nitroglycerin, his chest pain was substantially reduced, and he remained hemodynamically stable.
The physical examination was unexceptional; electrocardiography revealed inverted T waves in lead III, with no ST-segment elevation. Troponin T levels were initially positive at 0.686, reaching a maximum value of 5.24 during the course of the patient's hospitalization. The rest of his laboratory results were normal. Echocardiography showed an ejection fraction of 0.60, with no valvular abnormalities.
Subsequent cardiac catheterization showed a large, 3-cm fusiform aneurysm that involved the distal portion of the left main (LM) coronary artery and extended into the proximal left anterior descending (LAD) and left circumflex (LCx) coronary arteries (Fig. 1A). Except for a 99% stenosis of the 1st diagonal branch of the LAD, no other significant stenoses were seen. Another notable feature of this patient's angiogram was a diffuse yet mild ectasia of the right coronary artery (RCA), also with an absence of flow-limiting stenoses (Fig. 1B). Left ventriculography revealed an ejection fraction of 0.55 with no regional wall motion abnormalities. An abdominal runoff aortogram showed a normal abdominal aorta and normal renal and iliac arteries. The patient had an uneventful hospitalization and was discharged on aspirin and warfarin therapy. At 6 months' follow-up, he remained asymptomatic.
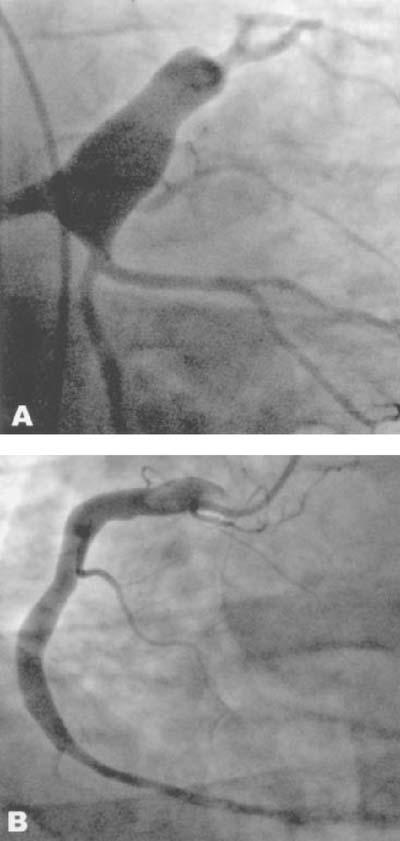
Fig. 1 Patient 1. Coronary arteriogram shows A) a large left main coronary aneurysm extending into the proximal left anterior descending and left circumflex coronary arteries, and B) a mild degree of right coronary artery ectasia.
Patient 2
Patient 2 was an obese 60-year-old man with a medical history notable for type II diabetes mellitus and hyperlipidemia, who presented in September 2004 with progressive exertional angina and dyspnea, paroxysmal nocturnal dyspnea, and orthopnea. He had no history of myocardial infarction, transient ischemic attacks, or smoking. His physical examination was unremarkable, and the electrocardiogram showed T-wave inversion in lead II, without ST-segment changes. Results of all laboratory studies, including serial cardiac enzymes, were normal. He underwent an adenosine Cardiolite stress test, which revealed an ejection fraction of 0.71 with a fixed defect in the inferior wall. On subsequent coronary angiography, the patient was noted to have a 1.7-cm LM coronary aneurysm (Fig. 2A) and a diffuse aneurysmal enlargement of the RCA (Fig. 2B), with no significant coronary stenoses. As with Patient 1, he was discharged on warfarin therapy after an uncomplicated hospital course, but aspirin was withheld due to a severe aspirin allergy. When we saw the patient 6 months after discharge, he reported no recurrence of symptoms. He remained asymptomatic at 12 months' follow-up.
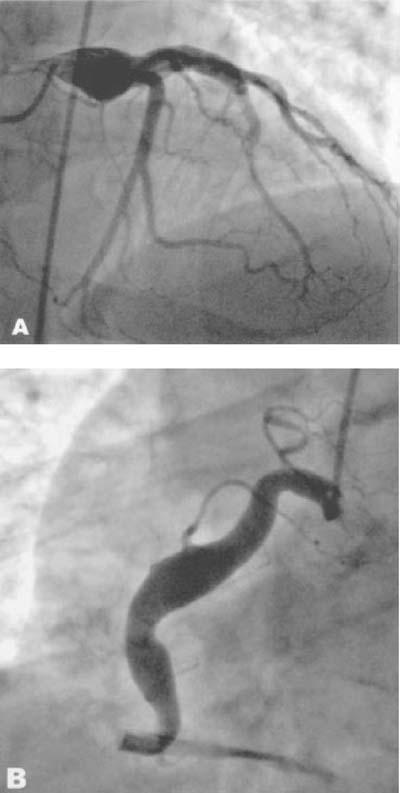
Fig. 2 Patient 2. Coronary angiogram shows A) a left main coronary artery aneurysm and B) mild aneurysmal enlargement of the right coronary artery.
Discussion
Coronary artery aneurysms (CAAs) were originally described by Morgagni in 1761,1 and the 1st actual case report was published by Bougon in 1812.2 Morphologically, these aneurysms may be saccular or fusiform, single or multiple. Such lesions are generally defined as aneurysmal when their diameters exceed by at least 1.5 to 2 times the diameters of adjacent normal vessels.1 Most CAAs occur as a consequence of atherosclerosis, but other causes include congenital malformation, Kawasaki disease, traumatic injury, polyarteritis nodosa, systemic lupus erythematosus, Ehlers-Danlos syndrome, scleroderma, Marfan syndrome, and Takayasu's arteritis.1 No unique cluster of symptoms or risk-factor profile has been ascribed to CAAs, and patients typically present with signs and symptoms indicative of coronary artery disease.1
Before 1967, available data concerning the incidence of CAAs were derived solely from a number of postmortem series, which were thoroughly reviewed and summarized by Daoud and colleagues2 in 1963. A total of 89 cases of CAA were analyzed in this study, with an observed incidence of 1.4% on a prospective series of 694 autopsies performed at Daoud's institution. The site of aneurysmal involvement in the 89 cases reviewed was most commonly the RCA (68%), followed by the LM (39%), the LAD (17%), and the LCx (17%).2
The emergence of angiography as a viable diagnostic tool enabled the diagnosis of CAA in vivo and led to a proliferation of CAA case reports and large angiographic series of CAA patients.3–8 The largest of these series is that of Swaye and coworkers,3 who reviewed the Coronary Artery Surgery Study registry data, which consisted of 20,087 patients, including 978 (4.9%) with CAAs. In that study, aneurysmal disease at any given site was defined as coronary dilatation that was 1.5 times greater than the diameter of normal adjacent segments or the diameter of the patient's largest coronary artery. Swaye's group also noted a greater predilection for RCA aneurysmal involvement in the postmortem analyses.
More recently, the 2nd-largest series of CAA patients was reported by Baman and colleagues4; it included 276 of 32,372 patients (0.9%) who underwent coronary angiography. Of note, this study applied a more stringent criterion for aneurysmal disease: only coronary segments at least 2 times larger than angiographically normal segments were designated aneurysmal. On the basis of these and numerous other angiographic series, the true incidence of CAA lies somewhere within the range of 0.3% to 4.9%.1
In contrast to data derived from postmortem series, findings from angiographic series indicate that the LM coronary artery is the least frequent site of CAA involvement;1 only a few cases have been reported in the world literature.5–7 The largest published series of LM CAAs is that of Topaz and coworkers, who reported 22 cases.5 The incidence of LM involvement in that study was 0.1% among 20,332 adult patients who underwent routine coronary angiography. The width of the largest LM CAA observed by this group was 1.9 cm, considerably smaller than the 3-cm aneurysm described in Patient 1 of our report. Another striking point of dissimilarity between the features observed in our patients and those reported in the Topaz series and in most other case reports is the presence or absence of obstructive coronary stenoses.5–7 Sixteen of the 22 patients (73%) in the Topaz series had severe triple-vessel disease, compared with a relative paucity of coronary stenoses seen in our 2 patients.5 The patients in our report were, however, demographically similar to the patients in Topaz, particularly with regard to medical history and coronary risk factors. As noted with our 1st patient, 12 (55%) of the patients in the Topaz series had extension of the aneurysm into adjacent branches. Concomitant RCA ectasia was present in 6 of those 22 patients (27%) and in both of our patients.
Considerable controversy remains regarding the most appropriate therapeutic strategy for the management of patients with CAAs. Many investigators have hypothesized that abnormal flow within CAAs predisposes patients to thrombus formation and distal embolization, even in the absence of obstructive coronary disease.1,4,8 This phenomenon was substantiated by Rath and colleagues, who described 5 patients with nonstenosed CAAs who later developed acute myocardial infarction and complete occlusion of their aneurysmal vessels.8 One crucial aspect of Rath's study was that these patients did not receive anticoagulation and antiplatelet therapy after the initial discovery of their CAAs. Moreover, the results of several large angiographic series have demonstrated no statistically significant difference in survival rates of CAA patients treated medically or surgically in comparison with control populations of patients who had similar degrees of obstructive coronary disease but no aneurysms.1,3
Most investigators would therefore agree that the presence of CAAs in and of itself does not warrant operative therapy. Rather, the severity of coexistent coronary artery stenoses is the principal factor in deciding whether to proceed with surgical treatment in patients with CAAs. Surgical management is appropriate in symptomatic patients who have evidence of emboli from the aneurysm to the distal coronary bed, leading to myocardial ischemia. Surgery is also indicated in cases of CAA enlargement as documented by serial angiographic measurement. The preferred approach for surgical correction of LM CAAs entails proximal and distal ligation of the aneurysm, with bypass of the LAD by means of the left internal mammary artery. Placement of a saphenous vein graft to bypass the LCx may also be necessary to adequately perfuse the left ventricle.
Surgical and nonsurgical therapeutic options must be discussed extensively with the patient. Both of our patients were hesitant to undergo surgery, and they remained asymptomatic with medical treatment alone. Patients not managed surgically must nevertheless be monitored very closely and treated with antiplatelet and anticoagulation therapy to prevent thrombus formation within the aneurysm. Our standard practice is to follow up with these patients every 3 months, and we instruct them to report any symptoms that they have experienced in the interim.
Footnotes
Address for reprints: James E. Lowe, MD, Division of Cardiovascular & Thoracic Surgery, Department of Surgery, Duke University Medical Center, Box 3954, Durham, NC 27710. E-mail: lowe0004@mc.duke.edu
References
- 1.Robinson FC. Aneurysms of the coronary arteries. Am Heart J 1985;109:129–35. [DOI] [PubMed]
- 2.Daoud AS, Pankin D, Tulgan H, Florentin RA. Aneurysms of the coronary artery. Report of ten cases and review of literature. Am J Cardiol 1963;11:228–37. [DOI] [PubMed]
- 3.Swaye PS, Fisher LD, Litwin P, Vignola PA, Judkins MP, Kemp HG, et al. Aneurysmal coronary artery disease. Circulation 1983;67:134–8. [DOI] [PubMed]
- 4.Baman TS, Cole JH, Devireddy CM, Sperling LS. Risk factors and outcomes in patients with coronary artery aneurysms. Am J Cardiol 2004;93:1549–51. [DOI] [PubMed]
- 5.Topaz O, DiSciascio G, Cowley MJ, Goudreau E, Soffer A, Nath A, et al. Angiographic features of left main coronary artery aneurysms. Am J Cardiol 1991;67:1139–42. [DOI] [PubMed]
- 6.Abbate A, Patti G, Dambrosio A, Di Sciascio G. Left main coronary artery aneurysm: A case report and review of the literature. Ital Heart J 2001;2:711–4. [PubMed]
- 7.Ercan E, Tengiz I, Yakut N, Gurbuz A. Large atherosclerotic left main coronary aneurysm: a case report and review of literature. Int J Cardiol 2003;88:95–8. [DOI] [PubMed]
- 8.Rath S, Har-Zahav Y, Battler A, Agranat O, Rotstein Z, Rabinowitz B, Neufeld HN. Fate of nonobstructive aneurysmatic coronary artery disease: angiographic and clinical follow-up report. Am Heart J 1985;109:785–91. [DOI] [PubMed]


