Abstract
We report 3 cases of a persistent 5th aortic arch. This anomaly is usually associated with other intracardiac defects. Although all 3 patients were found to have similar vascular structures that were best explained by the persistence of the 5th aortic arch into postnatal life, the clinical presentations were quite different. One patient presented with coarctation of the aorta, the 2nd patient with cyanosis, and the 3rd patient with pulmonary overcirculation. The 2nd case is unique in that the 5th aortic arch was found to be sensitive to prostaglandin E1. Understanding the embryologic derivation and differing presentations of a persistent 5th aortic arch aids in diagnosis and management of these patients.
Key words: Aorta, thoracic/abnormalities/embryology; aortic coarctation/complications/diagnosis/surgery; congenital anomalies, multiple/embryology; heart defects, congenital/diagnosis/epidemiology; infant, newborn; persistent 5th aortic arch; pulmonary artery atresia; subclavian artery/abnormalities
Persistent 5th aortic arch is a rare anomaly, first described in 1969 as a double- lumen aortic arch.1 This anomaly usually presents in association with various intracardiac defects. We report the cases of 3 patients with persistent 5th aortic arch. Although all 3 of these patients were found to have similar vascular structures that were best explained by the persistence of the 5th aortic arch into postnatal life, the clinical presentations were quite different. The 2nd case was unique in that the 5th aortic arch was found to be sensitive to prostaglandin E1 (PGE1). We describe the presentation, diagnosis, and treatment of these 3 patients, and discuss the embryologic derivation of a 5th persistent aortic arch.
Patient 1
A 3-day-old female neonate, born to a diabetic mother, was hospitalized for evaluation of a heart murmur. An echocardiogram showed a patent foramen ovale, mild tricuspid regurgitation, mild pulmonary stenosis, mild bilateral peripheral pulmonary stenosis, a patent ductus arteriosus, and a small ventricular septal defect; there was mild biventricular hypertrophy with moderate hypertrophy of the ventricular septum. At 8 months of age, she was hospitalized due to increasing dyspnea, stridor, cyanosis, and hypertension, with a blood pressure in the right arm of 140/95 mmHg. On examination, the patient was noted to have diminished femoral pulses, which led to a suspicion of coarctation of the aorta. On repeat echocardiography, a double aortic arch could not be ruled out. Magnetic resonance imaging showed a persistent 5th aortic arch with patent lumens of both left aortic arches. The upper arch was slightly hypoplastic, tapering from 4 mm to 2 mm and giving rise to 4 great vessels. The lower arch was 6 mm in diameter and tapered to 4 mm at the junction. The left subclavian artery arose at the junction, with turbulence and possible localized coarctation. No vascular ring or narrowing of the trachea was found. Cardiac catheterization revealed narrowing at the insertion of the hypoplastic transverse arch at the takeoff of the left subclavian artery, with a gradient of 20 mmHg across the coarctation (Fig. 1). Because the coarctation gradient was mild, no surgical intervention was deemed necessary. The patient is now 3 years old and normotensive, and has been living a healthy and asymptomatic life with regular follow-up.
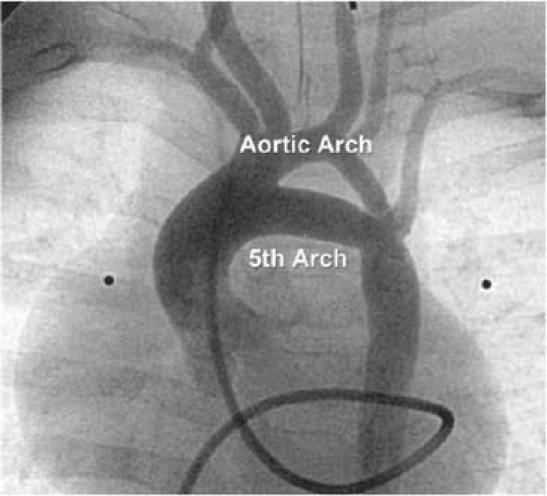
Fig. 1 Patient 1. Anterior projection of an ascending aortogram shows the narrow aortic arch from which the carotid and subclavian arteries arise. There is a persistent 5th aortic arch, which arises proximal to the brachiocephalic artery and inserts opposite the left subclavian artery.
Patient 2
A male infant, born at 32 weeks' gestation, was suspected to have VACTERL syndrome with a tracheoesophageal fistula, duodenal atresia, and cyanosis. He was started on PGE1 and the cyanosis improved. When he was 1 month of age, an echocardiogram showed pulmonary artery atresia, a large ventricular septal defect, an overriding aorta, small pulmonary arteries, a left aortic arch, and a large secundum atrial septal defect. The PGE1 was continued to maintain patency of the ductus and thus provide adequate pulmonary circulation until the patient could undergo a shunt procedure. The systemic oxygen saturation was 91%. Due to the development of pulmonary overcirculation, he underwent cardiac catheterization. The angiograms showed pulmonary atresia, a ventricular septal defect, and the pulmonary arteries arising from the ascending aorta, proximal to the brachiocephalic trunk, via a vessel that was thought to be most consistent with a persistent 5th aortic arch (Fig. 2). From the ascending aorta, via the patent 5th arch, the catheter was inserted into the left pulmonary artery where the pressure was at systemic levels, indicating unobstructed flow from the ascending aorta to the pulmonary arteries. One week later, the patient underwent placement of a modified right Blalock-Taussig shunt and division of the 5th aortic arch. He later showed overcirculation of the right lung and underperfusion of the left lung. At 3 months of age, he underwent another catheterization, which showed isolation of the left pulmonary artery, possibly due to constriction of ductus-like tissue in the 5th arch at the pulmonary bifurcation after the PGE1 was stopped. Reanastamoses of the right and left pulmonary arteries were attempted, but the patient developed ventricular tachycardia and died.
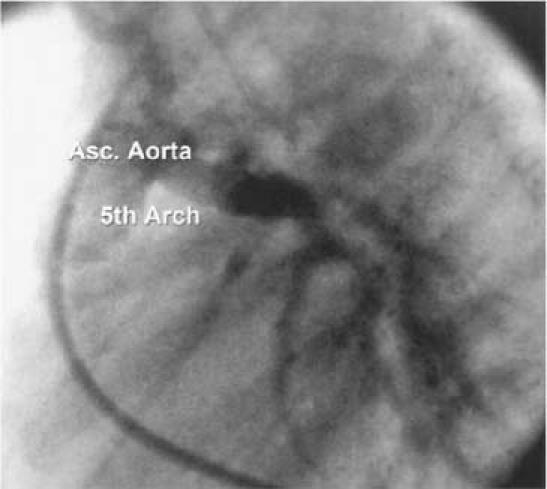
Fig. 2 Patient 2. Lateral projection of an ascending aortogram shows a large vessel arising from the ascending aorta and supplying the pulmonary confluence.
Patient 3
A 2-day-old male neonate was noted to be tachypneic and cyanotic. Echocardiography revealed complex heart disease, including truncus arteriosus with the pulmonary arteries arising from the ascending aorta, patent ductus arteriosus, ventricular septal defect, atrial septal defect, and persistent left superior vena cava to the coronary sinus. At cardiac catheterization, there was a stenotic connection between the ascending aorta and the bifurcation of the pulmonary arteries, which could not be catheterized; this finding was thought to be consistent with a persistent 5th aortic arch associated with main pulmonary artery atresia (Fig. 3). He subsequently underwent placement of an ascending aorta-to-right pulmonary artery shunt at the age of 3 weeks. His anatomy was re-evaluated by cardiac catheterization at 3 months of age. The catheterization revealed a systemic oxygen saturation of 78% to 80%. There was no evidence of any connection of the pulmonary arteries to the aorta apart from the shunt. He was subsequently taken to the operating room for takedown of the Waterston shunt, closure of the ventricular septal defect, and a Rastelli repair. Postoperative catheterization showed right pulmonary artery stenosis, for which he received a stent at 6 years of age, with a good result and reduction in his right ventricular pressure. He is now 10 years old and asymptomatic; however, at some point in the future he may need revision of his homograft conduit, which is calcified.
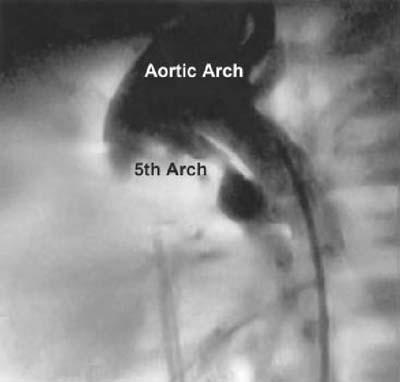
Fig. 3 Patient 3. Lateral projection of an aortic arch angiogram shows a small 5th aortic arch arising from the ascending aorta and supplying the pulmonary confluence.
Discussion
Early in the 4th week of gestation, neural cells migrate into the neck to form the paired branchial arches. Each of these branchial arches is supplied by an artery that arises from the truncus arteriosus or primordial aortic sac, and each ends in 1 of the 2 dorsal aortae.2 These arteries are the so-called aortic arches. In 50% of embryos, the 5th arches are rudimentary vessels that soon degenerate; while in other embryos, these arteries do not develop.3 Because each of these arches is numbered from cranial to caudal, it is reasonable to assume that, were a 5th arch to persist, it would be located below the 4th arch, which forms the right subclavian artery and the definitive aortic arch. It could then have a connection proximal to the future brachiocephalic artery from the posterior aorta or to the left pulmonary artery or ductus, which are derivatives of the 6th arch.
Clinical persistence of a 5th aortic arch presents in 2 main forms, depending on whether the connection is systemic-to-systemic or systemic-to-pulmonary. In the systemic-to-systemic type, the anomalous arch connects the ascending aorta to the descending aorta. The systemic-to-pulmonary type consists of a connection between the ascending aorta and a derivative of the 6th arch, usually the left pulmonary artery. The presentation of this anomaly depends on which of these anatomic connections exists and on the associated cardiovascular anomalies.
Persistent 5th aortic arch is a rare anomaly, with only 21 cases having been reported up until 1989.4 Like ours, many of the cases previously reported had associated intracardiac malformations. Our 1st patient had a secundum atrial septal defect, ventricular septal defect, valvular pulmonary stenosis, and coarctation. Our 2nd and 3rd patients also had a ventricular septal defect, in addition to pulmonary artery atresia.
A ventricular septal defect occurred in 10 of the 21 previously reported cases4 and is the most commonly associated finding. Pulmonary stenosis occurred in 1 of 21. A persistent 5th aortic arch with a systemic-to-pulmonary connection was noted only in the patients with either pulmonary atresia or an interrupted aortic arch. Pulmonary atresia was found in 6 of 21 patients, 4 of whom had a pulmonary-to-systemic connection. Pulmonary-to-systemic connection in association with an interrupted aortic arch was found in 2 of 21 patients. Coarctation occurred in 4 of the 21 previous patients, all of whom had a systemic-to-systemic connection. Both types of connections have potentially favorable hemodynamic consequences,3 by providing an alternative systemic arch in the setting of coarctation or interrupted aortic arch and by acting as a vital systemic-to-pulmonary shunt when associated with pulmonary atresia.
Our 1st patient had a persistent 5th arch, with a complex coarctation at its junction with the hypoplastic transverse arch and the left subclavian arteries. The complex anatomy of the persistent 5th aortic arch made the echocardiographic study difficult to interpret. Therefore, the final diagnosis was made by magnetic resonance angiography and confirmed by cardiac catheterization, which revealed a 20-mm gradient across the coarctation.
Various methods of successful repair for symptomatic coarctation of persistent 5th aortic arches have been reported in the literature.5 Gibbin and coworkers6 described a case of coarctation at the junction of the 5th arch, left subclavian artery, and descending aorta, which was repaired by patch aortoplasty. Konishi's group7 reported a case with coarctation distal to the junction of the 5th arch with the left subclavian artery, which was repaired using a Gore-Tex® tube graft (W.L. Gore & Associates, Inc.; Flagstaff, Ariz).7 In another case, a neonate presented with supraductal coarctation just below the junction of the persistent left 5th arch with the transverse and descending aortas, which was repaired by ligating the 5th arch and using it to repair the 4th arch.8 In our patient, the coarctation was minimal and did not require any surgical repair.
Our 2nd and 3rd cases both involved pulmonary atresia. The persistence of the 5th aortic arch in right ventricular outflow tract obstruction may be an embryonic compensatory measure. Thus, in many such cases, the persistent arch is found to be beneficial, acting as an extra systemic-to-pulmonary shunt, which helps to maintain adequate pulmonary circulation. However, in other cases, such as our 2nd patient, the right ventricular outflow tract obstruction can cause pulmonary overcirculation and pulmonary hypertension.
Our 2nd patient had pulmonary arteries that were previously confluent but became discontinuous after surgical division of the 5th arch. One possible explanation is that the 5th aortic arch included ductal tissue. Some of this tissue might have extended into the proximal left pulmonary artery; after division, the ductal tissue could have contracted, causing the vessels to become discontinuous. There is 1 other report about a PGE1-sensitive persistent 5th aortic arch. Zartner and associates9 described a patient in whom there was a mild narrowing in the persistent 5th aortic arch; when PGE1 was stopped during catheterization, the pressure in the descending aorta decreased from 83/51 mmHg to 49/42 mmHg.9 In both cases, the prostaglandin-sensitive tissue must have extended into the persistent 5th aortic arch from a nearby ductus. This finding is in contrast to the conventional wisdom that the persistent 5th aortic arch is not responsive to PGE1.
It is important to recognize the embryologic derivation of this malformation to keep from missing the diagnosis. After reviewing 2,000 specimens, Gerlis and co-authors concluded that persistence of an embryonic 5th aortic arch may not be as rare as perceived, since it is often misdiagnosed.4 This anomaly is most frequently mistaken for a ductus arteriosus or an aortopulmonary window. In order to prevent this condition from being overlooked, it is important to be familiar with the many different presentations of a persistent 5th aortic arch and to understand its embryologic origins.
Footnotes
Address for reprints: Michael R. Nihill, MB, BS, Section of Pediatric Cardiology, Texas Children's Hospital, MC 19345C, 6221 Fannin Street, Houston, TX 77030. E-mail: nihill@bcm.tmc.edu
References
- 1.Van Praagh R, Van Praagh S. Persistent fifth arterial arch in man. Congenital double-lumen aortic arch. Am J Cardiol 1969;24:279–82. [DOI] [PubMed]
- 2.Moore KL, Persaud TV. The developing human: clinically oriented embryology. 6th ed. Philadelphia: WB Saunders; 1998. p. 387.
- 3.Gerlis LM, Dickinson DF, Wilson N, Gibbs JL. Persistent fifth aortic arch. A report of two new cases and a review of the literature. Int J Cardiol 1987;16:185–92. [DOI] [PubMed]
- 4.Gerlis LM, Ho SY, Anderson RH, Da Costa P. Persistent 5th aortic arch--a great pretender: three new covert cases. Int J Cardiol 1989;23:239–47. [DOI] [PubMed]
- 5.McMahon CJ, Kertesz NJ, Vick GW. Delineation of persistent fifth aortic arch using magnetic resonance angiography. Cardiol Young 2002:12:484–5. [DOI] [PubMed]
- 6.Gibbin CL, Midgley FM, Potter BM, Martin GR. Persistent left fifth aortic arch with complex coarctation. Am J Cardi-ol 1991;67:319–20. [DOI] [PubMed]
- 7.Konishi T, Iizima T, Onai K, Kobayashi G, Kawabe M, Anzai T. Persistent fifth aortic arch complicated by coarctation of the aorta and aneurysm of the left subclavian artery [in Japanese]. Nippon Kyobu Geka Gakkai Zasshi 1981;29: 1243–8. [PubMed]
- 8.Cabrera A, Galdeano J, Lekuona I. Persistent left sided fifth aortic arch in a neonate. Br Heart J 1985;54:105–6. [DOI] [PMC free article] [PubMed]
- 9.Zartner P, Schneider MB, Bein G. Prostaglandin E1 sensitive persistent fifth aortic arch type 2. Heart 2000;84:142. [DOI] [PMC free article] [PubMed]


