Abstract
Iron-deficiency anemia can have deleterious effects on the heart. Herein, we describe the effects of iron deficiency on the heart as corroborated with electrocardiography, radiology, echocardiography, and cardiac catheterization. We review the pathophysiology, clinical features, and management of iron-deficiency–induced cardiomyopathy.
Key words: Anemia, iron-deficiency; cardiomyopathies/epidemiology/physiopathology/therapy; congestive heart failure; high-output heart failure; iron/deficiency; pulmonary edema
Iron-deficiency anemia is the most common form of nutritional anemia in both developed and developing countries. In mild cases, patients are asymptomatic. When the anemia is more significant, dyspnea and fatigue may occur. Severe iron deficiency can produce left ventricular (LV) dysfunction and overt heart failure. Despite numerous published observations regarding the effects of iron-deficiency anemia on the heart, ours is the 1st review of the cardiomyopathy of iron deficiency in the English-language medical literature. We also describe a representative case.
Case Report. In June 2004, a 42-year-old woman presented at the emergency department of our institution with menorrhagia and a 2-month history of fatigue and exertional dyspnea. She smoked tobacco and infrequently drank alcoholic beverages. Physical examination was notable only for conjunctival pallor and a hyperdynamic precordium. Signs of congestive heart failure were absent. A right bundle branch block with borderline LV hypertrophy and multiple atrial ectopic beats were present on the electrocardiogram (ECG). The chest radiograph was normal (Fig. 1). The initial hemoglobin level was 4.6 g/dL, the hematocrit level was 16.9%, the mean corpuscular volume was 71.3 fL, and the red-cell distribution width was 21.3%. The rest of the hemogram results, along with the chemistry and coagulation panels, were normal. Stool guaiac tests were negative for occult blood. The patient received 3 units of packed red blood cells and was admitted for evaluation of anemia. Results of iron studies revealed iron-deficiency anemia, with an iron level of 12 ng/mL (normal range, 40–150 ng/mL), total iron-binding capacity, 428 ng/mL; percent iron saturation, 3% (normal range, 16%–35%); and ferritin, 6 ng/mL (normal range, 13–150 ng/mL).
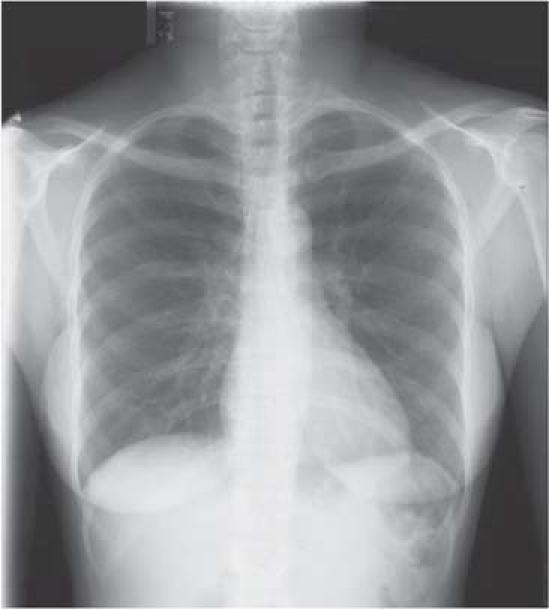
Fig. 1 The chest radiograph was normal at the time of admission
Esophagogastroduodenoscopy was notable only for mild gastritis, with Helicobacter pylori identified by histopathology. A colonoscopy revealed a single inflammatory sigmoid pseudopolyp. After colonoscopy, the patient's pulse oximetry reading dropped to 84% on room air, and she began to have a dry cough. Blood cultures were negative. The chest radiograph showed patchy, bilateral alveolar opacities and small bilateral pleural effusions, consistent with pulmonary congestion (Fig. 2). An ECG showed no change from the one obtained at admission. The serum troponin level was elevated, at 1.4 ng/mL (normal, <0.5 ng/mL), and the serum creatinine phosphokinase level was within the normal range. The B-type natriuretic peptide (BNP) level was elevated, at 1,790 pg/mL.
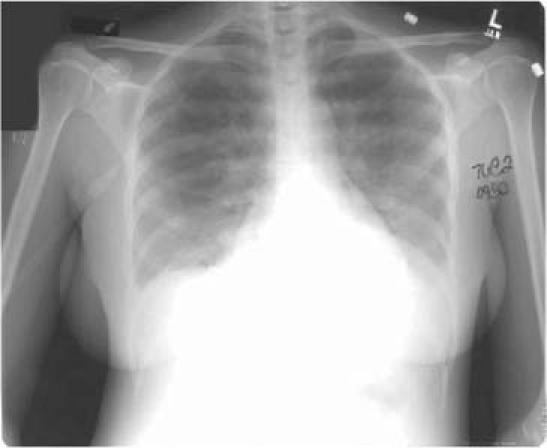
Fig. 2 After colonoscopy, a chest radiograph showed signs of pulmonary congestion: patchy bilateral alveolar opacities and small bilateral pleural effusions.
Transesophageal echocardiography revealed a structurally normal mitral valve. Cardiac pressures were measured: right atrium, 11/10/8 mmHg (normal, 2–7 / 2–7 / 1–5 mmHg); right ventricle, 45/7 mmHg (normal, 15–30 / 1–7 mmHg); pulmonary artery, 83/19 mmHg (normal, 15–30 / 4–12 mmHg) with a mean of 29 mmHg (normal, 9–19 mmHg); and mean pulmonary capillary wedge, 22 mmHg (normal, 4–12 mmHg). Her cardiac output was 3.8 L/min (normal, 4–8 L/min) with a cardiac index of 2.7 L/min/m2 (normal, 2.5–4 L/min/m2). Right and left heart catheterization showed normal coronary arteries, moderate global LV dysfunction with an ejection fraction of 0.30, moderate pulmonary hypertension, and moderate mitral regurgitation. The patient was given furosemide and lisinopril, and she was gradually weaned from supplemental oxygen. She was discharged from the hospital with a prescription for iron sulfate (325 mg, 3 times a day). At discharge, her hemoglobin level was 10.3 g/dL and her hematocrit level was 33.7%. At her 2-week follow-up appointment, she was free of signs and symptoms of heart failure. The furosemide and lisinopril were discontinued. A repeat echocardiogram obtained 10 weeks after the 1st one showed normal systolic function of the left ventricle with only trace (0–+1) mitral regurgitation. Her hemoglobin and hematocrit levels had normalized by the time of the repeat echocardiogram. A myocardial biopsy was not obtained, because suspicion of high-output heart failure was strong and her recovery was rapid. At her last follow-up visit, in November 2004, she remained free of signs and symptoms of cardiac dysfunction.
Discussion
The deleterious effects of anemia on the heart have long been recognized. In 1830, as therapeutic bloodletting was falling out of favor, Marshall Hall commented that “if, instead of one full bleeding, … the person be subjected to repeated blood-lettings, … the action of the heart and arteries is morbidly increased, and there are great palpitation[s], … the pulse varies from 100 to 120 or 130…. The respiration is apt to be frequent and hurried, and attended with alternate panting and sighing.”1 A contemporary of Hall's, George Gregory, warned that the anemia that results from chronic blood loss can cause even more serious cardiac consequences: “All haemorrhagies when long continued are apt to induce a very alarming state of constitutional weakness…. This condition of the fluids is generally known by the name of anemia…. Its symptoms are a pale and bloodless countenance, great weakness, disposition to syncope, loss of appetite, indigestion, swelled legs, and a pulse, weak, tremulous, and intermitting.”2 These descriptions appear to represent the high-output state and then the eventual transition into heart failure due to ongoing anemia.
Iron deficiency is the most common nutritional deficiency in developed and developing regions of the world. Approximately 2% of adult men and 9% of adult women in the United States have iron deficiency, and it is the most common cause of anemia in this country. The prevalence of iron deficiency is greater among infants, pregnant women, and the poor. Much larger proportions of the population are affected in developing countries.3
Roughly one fourth of iron-deficient individuals have consequent anemia. Iron deficiency also impairs intellectual and motor development in children, reduces functional capacity in adults, and may contribute to low birth-weight and preterm delivery.3,4 A more extreme result of iron deficiency is cardiomyopathy. In addition, high-output heart failure has been attributed to a variety of disease states, including anemia, arteriovenous fistulae, hyperthyroidism, Paget's disease, liver disease, and obesity.
The pathogenesis of the cardiomyopathy associated with anemia has not been ascertained. It is, for example, unclear whether the anemia itself contributes to the development of heart failure. Theoretically, severe anemia leads to inadequate oxygen delivery to tissues, which in the heart could cause myocyte dysfunction. Additional disease-specific factors may also contribute, including microinfarctions due to in situ sickling of red blood cells in sickle-cell disease, coronary vascular occlusion by parasites in malaria, iron overload caused by abundant blood transfusions in thalassemia, and reduced myocyte iron stores in iron-deficiency anemia.5–7
The physiologic response to anemia is a compensatory increase in cardiac output in order to maintain adequate oxygen delivery. Exercise capacity falls in correlation to the degree of anemia. This increased cardiac output is made possible by increases in blood volume, preload, heart rate, and stroke volume, along with a decrease in afterload.8 In more severe cases of anemia, the increased blood volume contributes to signs of congestion with peripheral and pulmonary edema.9
It has been shown that the resting human heart can withstand acute severe isovolemic anemia with hemoglobin levels as low as 5 g/dL, without evidence of inadequate tissue oxygenation. Acute isovolemic reduction to 5 g/dL in 33 healthy resting subjects induced neither hypoxia nor lactic acidosis.10 The transition from a high-output (compensated) cardiac state to a state of LV dysfunction (decompensated) appears to begin at a hemoglobin level of approximately 7 g/dL in the iron-deficient patient. As the hemoglobin level drops further, so does the LV function. In a study of iron-deficient children, 24% of those with hemoglobin levels of less than 5 g/dL manifested congestive heart failure (CHF), compared with 6% of the children whose hemoglobin levels were between 5 and 7 g/dL.11 In a study of adolescents and adults with a mean hemoglobin level of 5 g/dL, 27% had CHF.12
In our patient, a very elevated BNP level supported the clinical evidence of LV decompensation. Anemia is associated with elevated plasma BNP in patients with or without CHF.13,14 In patients with iron-deficiency anemia,15 iron supplementation has been reported to raise the average hemoglobin level from 9.0 to 10.5 g/dL, while concomitantly reducing the atrial natriuretic peptide level from 58.3 to 23.5 pg/mL. Among patients with diastolic heart failure, those with anemia have been shown to have significantly higher BNP levels than those without anemia.16 The presence of anemia or elevated BNP is associated with poorer prognosis in patients with CHF, but cause-and-effect relationships have not been established between these 3 entities.
Various ECG abnormalities have been described in the cardiomyopathy of iron deficiency. Sinus tachycardia and LV hypertrophy are common. T wave abnormalities have also been noted. Low voltage on the ECG has been reported by many investigators.17 Supraventricular and ventricular premature contractions are much more frequent in elderly patients than in younger ones with iron-deficiency anemia.15
Between one third and two thirds of patients with severe anemia have cardiomegaly on chest radiography, and the cardiac silhouette reportedly returns to normal within a few weeks of the resolution of anemia.9
Echocardiography has demonstrated some of the hemodynamic changes that accompany iron-deficiency anemia. In cases of mild-to-moderate anemia, hemodynamic adaptations permit adequate cardiovascular compensation. The combination of increased heart rate and stroke volume increases cardiac output, which, in turn, improves oxygen delivery. Diastolic and systolic LV chamber sizes increase to accommodate this greater output.18,19 In a cross-sectional study of 175 patients attending a kidney disease clinic, Levin and colleagues20 evaluated the relationship of hemoglobin levels to echocardiographic findings. The authors found that anemia and systolic blood pressure were the most modifiable risk factors associated with the presence of LV hypertrophy. In multiple logistic regression analysis, each 1-g/ dL decrease in hemoglobin was associated with a 6% increase in risk of LV hypertrophy.20
With severe iron-deficiency anemia, however, subtle echocardiographic indices suggest that LV function deteriorates. Myocardial contractility, as determined by the fractional shortening percentage, decreases as hemoglobin drops below 7 g/dL.18 The ratio of end-systolic wall stress to end-systolic volume index (ESS/ESVI) has also been used to evaluate cardiac contractility. This ratio is reduced—implying functional compromise—in patients with hemoglobin levels of less than 6 g/dL.19
Several hypotheses have been advanced to explain how iron-deficiency anemia causes cardiomyopathy. It has been suggested that, like other types of heart failure, high-output heart failure is driven by ongoing increased sympathetic nervous activity. In order to test this hypothesis, 1 study used β-blockade in an attempt to blunt the deleterious effect of elevated sympathetic tone on the hearts of iron-deficient laboratory rats. β-Blockers, however, failed to prevent cardiac decompensation in those animals.21
As noted earlier, compromised oxygen delivery capacity may result in chronic tissue hypoxemia, which may, in turn, lead to myocyte dysfunction. Because iron moieties also bind to myoglobin, a total-body iron deficit could impair myocytes' ability to extract oxygen from circulating hemoglobin. Indeed, iron replacement has been shown to significantly improve cardiac function even before inducing a substantial rise in hemoglobin.12
The cardiomyopathy of iron deficiency may be completely reversible. Gradual transfusion of packed red blood cells rapidly reverses clinical heart failure, often within hours.11 Rapid transfusion, in contrast, can precipitate pulmonary edema, as in our patient.9,22 Concomitant administration of a loop diuretic is warranted if congestion is already present or develops, or if rapid transfusion is deemed necessary.
With iron replacement alone, clinical and echocardiographic indices have been shown to normalize.12,18 The mean heart rate decreased from 102 to 93 beats/min after iron-dextran total-dose infusion in 30 patients with iron-deficiency anemia in a study by Alvares and colleagues.12 It is not yet known, however, whether complete normalization of LV function occurs in the more severe cases. Rats fed iron-deficient diets developed dilated cardiomyopathy that was histologically associated with atrophic rather than hypertrophic cardiac myocytes. Myocardial iron concentrates were dramatically reduced. Cardiomyopathy also occurred in iron-deficient rats that were not anemic.23 Similarly, skeletal muscle dysfunction has been found in both children and rats without anemia, when fed short-term iron-deficient diets.23
There is growing evidence that anemia contributes to cardiac disease and death. In patients with chronic kidney disease, for example, anemia is an independent risk factor for the development of cardiovascular disease.24 In patients with heart failure, anemia is associated with increased morbidity.25 Anemia has also been correlated with the development of LV hypertrophy.26 When the anemia is corrected, the LV hypertrophy decreases, LV function improves, and hospitalizations decrease.26,27
To our knowledge, our case is the first to demonstrate overt clinical CHF, which was corroborated with laboratory, echocardiographic, and cardiac catheterization evidence of significant LV impairment in a patient with severe iron-deficiency anemia. The case also documents the dramatic clinical and echocardiographic recovery of LV function after iron replacement.
Summary and Recommendations
A review of the literature, in tandem with observations made in our patient, generates several clinical and therapeutic caveats in regard to the cardiomyopathy of iron deficiency.
Left ventricular dysfunction may result from iron deficiency, particularly when the hemoglobin level is less than 5 g/dL.
The cardiomyopathy associated with iron deficiency is reversible.
When transfusions are given, they should be given cautiously to avoid overwhelming a compromised LV with a surge of intravascular volume. Concomitant diuretic administration should be considered in order to avoid pulmonary edema.
A threshold for transfusion therapy has not been determined. It is possible that iron therapy by itself may be adequate. Until this issue has been clarified, it is reasonable to use transfusions when patients are symptomatic, or empirically when transfusions are needed to maintain hemoglobin levels above 5g/dL—the level below which cardiac compromise commonly occurs.
Iron supplementation should be initiated as soon as possible, because the cardiomyopathy may be driven solely by iron deficiency.
Because the cardiomyopathy appears to be rapidly reversible upon iron replacement, with or without transfusion therapy, such traditional CHF medications as angiotensin-converting enzyme inhibitors and β-blockers may be unnecessary.
Afterload reduction by vasodilator therapy is unwarranted, because the systemic vascular resistance may already be very low.
Acknowledgments
The authors thank Kenneth Berkovitz, MD, and Steven Radwany, MD, for their editorial expertise in the preparation of this manuscript.
Footnotes
Address for reprints: Michael W. Rich, MD, 55 Arch Street, Suite 1A, Akron, OH 44304. E-mail: mwrich@neoucom.edu
References
- 1.Hall M. Researches principally relative to the morbid and curative effects of loss of blood. Philadelphia: E.L. Carey and A. Hart; 1830. p. 25–6.
- 2.Gregory G. Elements of the theory and practice of physic: designed for the use of students. New York: M. Sherman; 1830. p. 269.
- 3.Lee GR. Iron deficiency and iron-deficiency anemia. In: Lee GR, Bithell TC, Foerster J, Athens JW, Luken JN, editors. Wintrobe's Clinical Haematology. Philadelphia: Lea & Febiger; 1993. p. 808–39.
- 4.Centers for Disease Control and Prevention (CDC). Iron deficiency--United States, 1999–2000. MMWR Morb Mortal Wkly Rep 2002;51:897–9. [PubMed]
- 5.Martins W, Mesquita ET, Cunha DM, Pinheiro LA, Romeo Filho LJ, Pareto Junior RC. Doppler echocardiographic study in adolescents and young adults with sickle cell anemia [in Portuguese]. Arq Bras Cardiol 1999;73:463–74. [DOI] [PubMed]
- 6.Franzen D, Curtius JM, Heitz W, Hopp HW, Diehl V, Hilger HH. Cardiac involvement during and after malaria. Clin Investig 1992;70:670–3. [DOI] [PubMed]
- 7.Fridlender ZG, Rund D. Myocardial infarction in a patient with beta-thalassemia major: first report. Am J Hematol 2004; 75:52–5. [DOI] [PubMed]
- 8.Pereira AA, Sarnak MJ. Anemia as a risk factor for cardiovascular disease. Kidney Int Suppl 2003;87:S32–9. [DOI] [PubMed]
- 9.Varat MA, Adolph RJ, Fowler NO. Cardiovascular effects of anemia. Am Heart J 1972;83:415–26. [DOI] [PubMed]
- 10.Weiskopf RB, Viele MK, Feiner J, Kelley S, Lieberman J, Noorani M, et al. Human cardiovascular and metabolic response to acute, severe isovolemic anemia [published erratum appears in JAMA 1998;280:1404]. JAMA 1998;279:217–21. [DOI] [PubMed]
- 11.Pegelow C, Powars D, Wingert W. Severe iron deficiency anemia. West J Med 1977;126:190–5. [PMC free article] [PubMed]
- 12.Alvares JF, Oak JL, Pathare AV. Evaluation of cardiac function in iron deficiency anemia before and after total dose iron therapy. J Assoc Physicians India 2000;48:204–6. [PubMed]
- 13.Wold Knudsen C, Vik-Mo H, Omland T. Blood haemoglobin is an independent predictor of B-type natriuretic peptide (BNP). Clin Sci (Lond) 2005;109:69–74. [DOI] [PubMed]
- 14.Tsuji H, Nishino N, Kimura Y, Yamada K, Nukui M, Yamamoto S, et al. Haemoglobin level influences plasma brain natriuretic peptide concentration. Acta Cardiol 2004;59: 527–31. [DOI] [PubMed]
- 15.Kikuchi M, Inagaki T. Atrial natriuretic peptide in aged patients with iron deficiency anemia. Arch Gerontol Geriatr 1999;28:105–15. [DOI] [PubMed]
- 16.Brucks S, Little WC, Chao T, Rideman RL, Upadhya B, Wesley-Farrington D, Sane DC. Relation of anemia to diastolic heart failure and the effect on outcome. Am J Cardiol 2004;93:1055–7. [DOI] [PubMed]
- 17.Mehta BC, Panjwani DD, Jhala DA. Electrophysiologic abnormalities of heart in iron deficiency anemia. Effect of iron therapy. Acta Haematol 1983;70:189–93. [DOI] [PubMed]
- 18.Georgieva Z, Georgieva M. Compensatory and adaptive changes in microcirculation and left ventricular function of patients with chronic iron-deficiency anaemia. Clin Hemorheol Microcirc 1997;17:21–30. [PubMed]
- 19.Hayashi R, Ogawa S, Watanabe Z, Yamamoto M. Cardiovascular function before and after iron therapy by echocardiography in patients with iron deficiency anemia. Pediatr Int 1999;41:13–7. [DOI] [PubMed]
- 20.Levin A, Singer J, Thompson CR, Ross H, Lewis M. Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 1996;27:347–54. [DOI] [PubMed]
- 21.Turner LR, Premo DA, Gibbs BJ, Hearthway ML, Motsko M, Sappington A, et al. Adaptations to iron deficiency: cardiac functional responsiveness to norepinephrine, arterial remodeling, and the effect of beta-blockade on cardiac hypertrophy. BMC Physiol 2002;2:1. [DOI] [PMC free article] [PubMed]
- 22.Sanghvi LM, Kotia KC, Sharma SK, Bordia AK, Jain YP. Circulatory haemodynamics after blood transfusion in chronic severe anaemia. Br Heart J 1968;30:125–9. [DOI] [PMC free article] [PubMed]
- 23.Petering DH, Stemmer KL, Lyman S, Krezoski S, Petering HG. Iron deficiency in growing male rats: a cause of development of cardiomyopathy. Ann Nutr Metab 1990;34:232–43. [DOI] [PubMed]
- 24.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003;108:2154–69. [DOI] [PubMed]
- 25.Anand IS, Kuskowski MA, Rector TS, Florea VG, Glazer RD, Hester A, et al. Anemia and change in hemoglobin over time related to mortality and morbidity in patients with chronic heart failure: results from Val-HeFT. Circulation 2005;112:1121–7. [DOI] [PubMed]
- 26.Portoles J, Torralbo A, Martin P, Rodrigo J, Herrero JA, Barrientos A. Cardiovascular effects of recombinant human erythropoietin in predialysis patients. Am J Kidney Dis 1997; 29:541–8. [DOI] [PubMed]
- 27.Silverberg D, Wexler D, Blum M, Wollman Y, Iaina A. The cardio-renal anaemia syndrome: does it exist? Nephrol Dial Transplant 2003;18 Suppl 8:viii7–12. [DOI] [PubMed]


