Abstract
Endovascular grafts have been widely used for the treatment of aneurysms since the early 1990s. They are preferred especially for use in patients in whom conventional surgical methods carry high risks of death and morbidity. Increasing operator experience and technical refinements in endovascular grafting have enabled these procedures to be performed even in critical segments of the aorta, such as the thoracic and arch levels. In this report, we present the case of a patient who was treated successfully with an endovascular graft for a mycotic saccular aneurysm located just below the left subclavian artery.
Key words: Aortic aneurysm; aneurysm, infected/therapy; blood vessel prosthesis implantation; hoarseness/diagnosis; stents
Endovascular grafts have been used for the treatment of aneurysms since the early 1990s.1 They are especially preferred for those patients in whom conventional surgical methods carry a high risk of morbidity or death, although some operators use endovascular grafting because of the ease of the procedure. Nowadays, increasing experience with graft placement and refinements in graft technology have led to the performance of endovascular procedures for aneurysms of critical segments of the aorta—at, for example, the thoracic and arch levels. In this report, we present the case of a patient who was treated with an endovascular graft for a saccular aortic aneurysm that was just below the left subclavian artery (SCA), an aneurysm that we considered mycotic.
Case Report
In March 2005, a 38-year-old man was referred to our institution with a diagnosis of saccular aneurysm at the distal aortic arch. He had been treated and followed up at our hematology clinic for acute lymphoblastic leukemia (ALL), which had been diagnosed in February 2005. At the end of his 1st dose of chemotherapy—when he had been discharged before his consolidation chemotherapy—the patient was readmitted with a productive cough, chills, and fever. Chest radiography revealed pneumonia, and antibiotic therapy with penicillin–β-lactamase complex was started immediately. After 1 week, the patient developed acute hoarseness. Chest radiography showed an unusual opacity on the left upper margin of the sternum (Fig. 1). Laryngeal examination revealed vocal cord palsy. Spiral computed tomography (CT) showed a 3.8-cm-diameter saccular aneurysm (with 1.8-cm neck) at the distal aortic arch, 1 cm beyond the left SCA (Fig. 2). As an incidental finding, both carotid arteries were seen to arise from the brachiocephalic trunk (Fig. 2B).
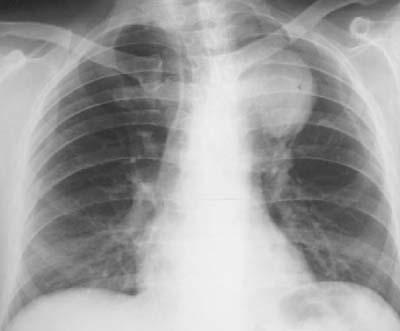
Fig. 1 Chest radiography showed an unusual opacity at the left upper margin of the sternum. This mass, the cause of Ortner's syndrome in this patient, was later diagnosed by contrast computed tomography as a saccular aneurysm at the distal aortic arch.
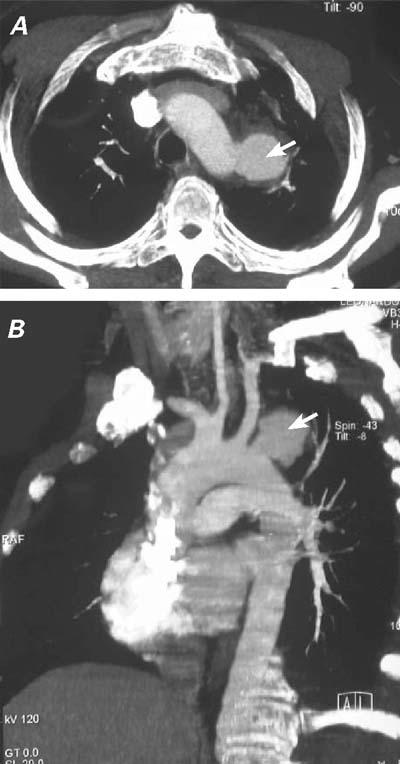
Fig. 2 A) Computed tomographic scan shows the 3.8-cmdiameter aneurysm (arrow) distal to the left subclavian artery. B) Computed tomographic angiography shows the 3.8-cm saccular aneurysm (arrow) with a 1.8-cm neck, located 1 cm below the left subclavian artery. Note that both carotid arteries arise from the brachiocephalic trunk.
The patient had a history of ankylosing spondylitis, chronic hepatitis B infection, and (in the past) cocaine addiction. He had been a soldier but had retired after a gunshot injury to his left arm; there had been no other trauma or surgical intervention.
The aneurysm was thought to be mycotic, even though blood cultures for infective microorganisms were all negative, because a CT scan performed 2 months earlier in order to evaluate the lungs and abdomen had showed no evidence of vascular disease. After risk–benefit evaluation and the informed-consent procedure, we considered endoluminal stenting as an alternative to open surgery. Because the saccular aneurysm was in the vicinity of the left SCA, we decided to occlude the aneurysm's neck completely by extending the stent above the left SCA. Transcranial and cervical Doppler ultrasonographic studies demonstrated patency of the right SCA, both vertebral arteries, and the circle of Willis, which did not necessitate performing an extra-anatomic bypass to the left SCA before the endovascular intervention.
The patient was placed under spinal epidural and local anesthesia, the right femoral artery was surgically accessed, and a longitudinal arteriotomy was performed. From the femoral artery, a Superstiff 0.035-inch guidewire (Back-up Meier, Schneider Co.; Blach, Switzerland) was positioned at the aortic arch. A 5F sheath was inserted percutaneously through the right brachial artery, through which a 5F pigtail catheter was directed for angiographic monitoring of the stent position. After administrating 5,000 units of heparin, we inserted the 24F delivery system (Talent Thoracic Stent Graft, Coil Track TDS, Medtronic AVE; Santa Clara, Calif) through a longitudinal incision in the right common femoral artery and positioned it, with fluoroscopic guidance, approximately at the aortic arch (Fig. 3A). The stent was then expanded, excluding both the saccular aneurysm and the orifice of the left SCA; however, uncovered parts of the stent unfortunately protruded into the brachiocephalic artery (Fig. 3B). The femoral artery was reconstructed with a patch. The patient was put on oral antibiotic therapy for the presumed mycotic aneurysm and on antiaggregant therapy for both the femoral artery patch and for the protrusion of the uncovered part of the stent into the brachiocephalic artery. Postoperatively, the patient developed no notable signs or symptoms of left-upper-extremity ischemia, but his hoarseness was only slightly diminished. Ten days after the treatment, a CT scan showed a type II endoleak (White classification) into the aneurysm. Graft positioning was correct, but there was a minimal leak, with thrombus formation chiefly inside the aneurysmal sac (Fig. 4). We decided to monitor the patient carefully and to perform coil embolization in the event that the aneurysm increased in size. Accordingly, we released him to the hematology clinic for ALL consolidation therapy. Magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) performed at the hematology clinic 2 months after the procedure revealed successful exclusion of the aneurysm with no residual endoleakage and with complete thrombus formation inside the aneurysmal sac; in addition, the size of the aneurysm had decreased to 3 cm in diameter (Fig. 5). The patient has been well for more than 7 postoperative months, under the joint care of our hematology and cardiovascular surgery departments.
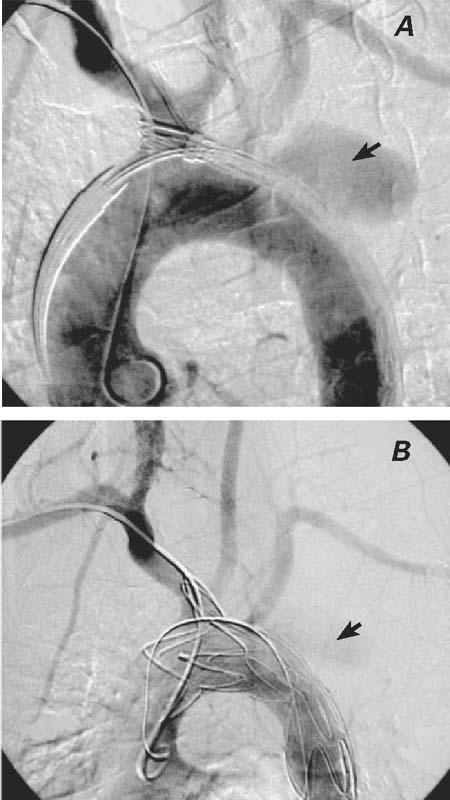
Fig. 3 A) Perioperative angiography shows the positions of the catheters and the unexpanded stent, as well as the brachiocephalic trunk, carotid arteries, subclavian arteries, and the saccular aneurysm (arrow). B) Perioperative angiography shows the successful exclusion of the saccular aneurysm. Left subclavian artery flow from the collateral circulation has not been compromised. There was minor type II endoleakage (arrow) after the stent-graft implantation at the late phase of angiography.
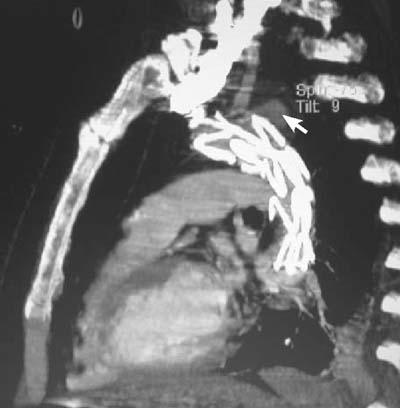
Fig. 4 Ten days after surgery, computed tomographic angiography shows the exclusion of the saccular aneurysm, with blood flow from the left subclavian artery filling the aneurysmal sac (type II endoleak, arrow).
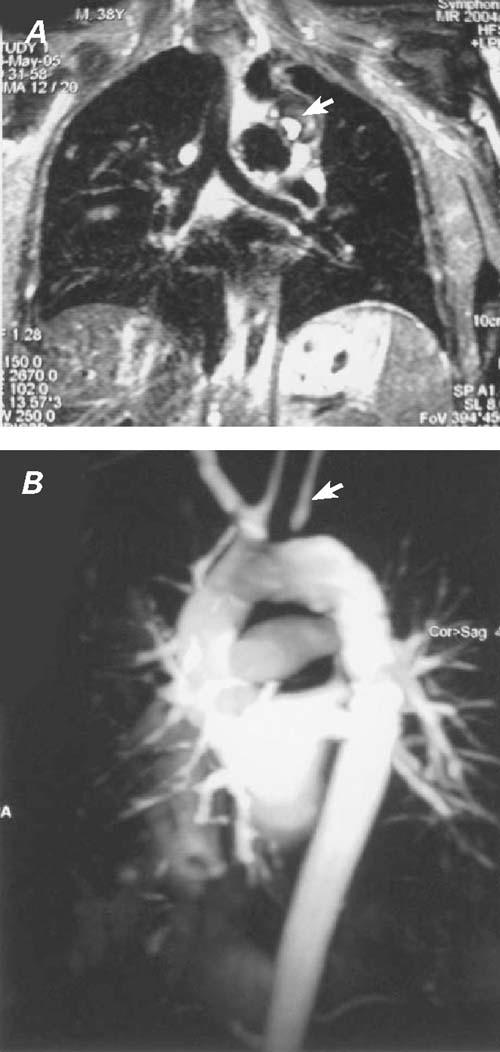
Fig. 5 A) Two months postoperatively, magnetic resonance imaging shows thrombus formation and occlusion of the aneu rysm (arrow), the size of which has decreased to 3 cm in diameter. B) Magnetic resonance angiography shows successful exclusion of the saccular aneurysm with no remaining endoleak. The left subclavian artery is filled by retrograde flow (arrow), through a subclavian steal
Discussion
Endovascular repair of aortic aneurysms was first applied to the abdominal aorta in 1991.1 Since then, increased experience and technological advances have extended the application of endovascular stent-grafts to the thoracic aorta and even to the arch. Stent-grafts are now commonly used for aneurysms and dissections involving the descending aorta, with very good outcomes.2 They are also considered to be a good alternative to open surgical repair because they yield equal mid-term life expectancy, with less perioperative morbidity and lower hospital costs.3,4
The main obstacles to endoluminal treatment at the proximal levels of the aorta are the origins of the arch vessels, especially the brachiocephalic trunk and left common carotid artery. After stenting5 or bypass6of these arteries, endovascular treatment becomes a safe procedure for the treatment of aneurysms at this location. Nowadays, branched and fenestrated endovascular grafts have been produced and marketed, and experiments and trials are evolving rapidly to test the effectiveness of these new products.7–9 On the other hand, the most commonly found and studied endovascular grafts are still the endoluminal tube stent-grafts. When stent-grafts were first applied to the aortic arch, it was thought that revascularization of the left SCA was mandatory after its orifice had been excluded. However, experience has shown that SCA revascularization may not be necessary, provided that appropriate collateral flow to the vessel can be documented. Regardless of the adequacy of collateral blood flow, the main indications for prophylactic left SCA revascularization include the likelihood of vertebral territory stroke after SCA exclusion, the presence of coronary artery bypass grafting with use of the left internal thoracic artery, the presence of an arteria lusoria, and the patient's need for arteriovenous hemodialysis access in the left arm or for precision in the performance of left-handed maneuvers.
In patients under consideration for thoracoabdominal stent-grafting, SCA revascularization may thereby avoid jeopardizing spinal cord blood flow and prevent paraplegia.10 However, SCA revascularization itself carries significant risks of morbidity and death.
If collateral flow is sufficient, the orifice of the left SCA can safely be covered with a graft in order to decrease the morbidity of bypass to the arch vessels and to prevent endoleakage.10 In our patient, transcranial and cervical Doppler ultrasonographic studies documented sufficient collateral blood flow. In addition, both carotid arteries arose from the brachiocephalic trunk, which gave us an even clearer margin of safety for the placement of a stent-graft over the orifice of the left SCA. Even with that clear zone, there was minor type II endoleakage into the aneurysmal sac. In our follow-up on the patient, we found that the aneurysm had thrombosed completely and that no major vascular insufficiency had developed in his left arm.
Mycotic aneurysms, compared with other aneurysms, have a higher risk of rupture before treatment or after unsuccessful treatment. Therefore, major leakage, which can increase aneurysmal diameter, must be managed immediately by such methods as balloon angioplasty or coil embolization. In our patient, the aneurysm decreased in size and substantially thrombosed during the early postoperative period. Both factors were promising for complete thrombosis and occlusion of the aneurysm. Therefore, we decided to monitor our patient periodically with MRA or CT angiography and to consider coil embolization during follow-up, if we observed an increment in the diameter of the aneurysm or blood filling the sac. As it happened, postoperative follow-up MRA showed complete occlusion of the saccular aneurysm in the 2nd month.
Although mycotic aneurysm is a misnomer, these lesions are caused by infective microorganisms, fungal or not.11 They account for fewer than 1% of all aneurysms.12 The most commonly encountered bacterial agents that lead to aneurysm formation are Salmonella, Staphylococcus, and Mycobacterium species.1,12,13 Human immunodeficiency virus is another infective agent that can cause multiple aneurysms at different regions of the aorta.14 The symptoms and blood chemistry values are usually nonspecific.12 Definitive diagnosis is made on the basis of blood or direct tissue cultures from the aneurysm, but both can be negative.13
The conventional treatment of mycotic aneurysms is open aneurysmectomy, together with the débridement of peripheral tissues. Distal revascularization can be achieved either with an in situ prosthesis, which will be at risk from adjacent infection, or with extra-anatomic bypass procedures, which do not have very promising long-term patency rates. In the literature, the mortality rate associated with surgical treatment is reported to reach 36%.12 Mycotic aneurysms still account for high mortality and morbidity rates, notwithstanding improved diagnostic techniques, antibiotics, and surgical interventions.
Our patient had recently been diagnosed with ALL and, after his 1st dose of chemotherapy, pneumonia. He suddenly developed Ortner's syndrome,1 which was found to be due to the saccular aneurysm's compressing the left recurrent laryngeal nerve. Aneurysm formation is a well-known phenomenon in patients with connective tissue disorders15 or a history of drug abuse.16 Even though our patient had ankylosing spondylitis and a history of cocaine addiction, his CT scans only 2 months earlier had been negative for aneurysms, and this led us to consider a mycotic aneurysm even in the presence of negative blood cultures. Those negative results were probably due to the antibiotic therapy that he had been given for pneumonia.
The high mortality and morbidity rates associated with conventional open surgical treatment of mycotic aneurysms direct surgeons to alternative options. Although placement of an artificial material in an infected area is controversial, the low mortality and morbidity rates associated with endovascular grafting have made such grafting an attractive option, even for critical segments of the aorta. Many authors maintain that endovascular stenting is feasible when antibiotic suppression has attained negative blood cultures before the intervention. Moreover, after long-term antibiotic therapy, the results of endovascular grafting have been impressive.1,11–13
To conclude, we believe—in light of our own experience and after reviewing the literature—that endoluminal treatment for mycotic and other aneurysms located in critical segments of the aorta is an attractive alternative to open surgery, especially for high-risk patients. This is a very rare case, in which a mycotic saccular aneurysm of the aortic arch was treated successfully with an endovascular graft. We believe that further information is needed in the form of studies with more patients and with long-term follow-up results.
Footnotes
Address for reprints: Murat Ugurlucan, MD, Bozkurt Caddesi, No:110-112, Benli Apt., Daire: 6, 80250 Kurtulus - Istanbul, Turkey E-mail: muratugurlucan@yahoo.com
References
- 1.Ting AC, Cheng SW, Ho P, Poon JT. Endovascular repair for multiple Salmonella mycotic aneurysms of the thoracic aorta presenting with Cardiovocal syndrome. Eur J Cardiothorac Surg 2004;26:221–4. [DOI] [PubMed]
- 2.Sayed S, Thompson MM. Endovascular repair of the descending thoracic aorta: evidence for the change in clinical practice. Vascular 2005;13:148–57. [DOI] [PubMed]
- 3.Destrieux-Garnier L, Haulon S, Willoteaux S, Decoene C, Mounier-Vehier C, Halna P, et al. Midterm results of endoluminal stent grafting of the thoracic aorta. Vascular 2004;12: 179–85. [DOI] [PubMed]
- 4.Glade GJ, Vahl AC, Wisselink W, Linsen MA, Balm R. Mid-term survival and costs of treatment of patients with descending thoracic aortic aneurysms; endovascular vs. open repair: a case-control study. Eur J Vasc Endovasc Surg 2005;29:28–34. [DOI] [PubMed]
- 5.Larzon T, Gruber G, Friberg O, Geijer H, Norgren L. Experiences of intentional carotid stenting in endovascular repair of aortic arch aneurysms--two case reports. Eur J Vasc Endovasc Surg 2005;30:147–51. [DOI] [PubMed]
- 6.Dambrin C, Marcheix B, Hollington L, Rousseau H. Surgical treatment of an aortic arch aneurysm without cardio-pulmonary bypass: endovascular stent-grafting after extra-anatomic bypass of supra-aortic vessels. Eur J Cardiothorac Surg 2005;27:159–61. [DOI] [PubMed]
- 7.Verhoeven EL, Zeebregts CJ, Kapma MR, Tielliu IF, Prins TR, van den Dungen JJ. Fenestrated and branched endovascular techniques for thoraco-abdominal aneurysm repair. J Cardiovasc Surg (Torino) 2005;46:131–40. [PubMed]
- 8.Saito N, Kimura T, Odashiro K, Toma M, Nobuyoshi M, Ueno K, et al. Feasibility of the Inoue single-branched stent-graft implantation for thoracic aortic aneurysm or dissection involving the left subclavian artery: short- to medium-term results in 17 patients. J Vasc Surg 2005; 41:206–12. [DOI] [PubMed]
- 9.Ishimaru S. Endografting of the aortic arch. J Endovasc Ther 2004;11 Suppl 2:II62–71. [DOI] [PubMed]
- 10.Melissano G, Civilini E, Bertoglio L, Setacci F, Chiesa R. Endovascular treatment of aortic arch aneurysms. Eur J Vasc Endovasc Surg 2005;29:131–8. [DOI] [PubMed]
- 11.Jones KG, Bell RE, Sabharwal T, Aukett M, Reidy JF, Taylor PR. Treatment of mycotic aortic aneurysms with endoluminal grafts. Eur J Vasc Endovasc Surg 2005;29:139–44. [DOI] [PubMed]
- 12.Koeppel TA, Gahlen J, Diehl S, Prosst RL, Dueber C. Mycotic aneurysm of the abdominal aorta with retroperitoneal abscess: successful endovascular repair. J Vasc Surg 2004;40:164–6. [DOI] [PubMed]
- 13.Haulon S, Destrieux-Garnier L, Decoene C, Gaudric J, Halna P, Koussa M. A rare case of mycotic aortic pseudoaneurysm. Eur J Vasc Endovasc Surg 2002;23:272–4. [DOI] [PubMed]
- 14.Heikkinen MA, Dake MD, Alsac JM, Zarins CK. Multiple HIV-related aneurysms: open and endovascular treatment. J Endovasc Ther 2005;12:405–10. [DOI] [PubMed]
- 15.Kasirajan K, Matteson B, Marek JM, Langsfeld M. Covered stents for true subclavian aneurysms in patients with degenerative connective tissue disorders. J Endovasc Ther 2003;10:647–52. [DOI] [PubMed]
- 16.Friedman SG, Pogo GJ, Moccio CG. Mycotic aneurysm of the superior mesenteric artery. J Vasc Surg 1987;6:87–90. [DOI] [PubMed]


