Abstract
Severe hemodilutional anemia on cardiopulmonary bypass increases morbidity and mortality after coronary surgery. The present study focuses on the lowest hematocrit values during extracorporeal circulation and on allogenic blood transfusions as mortality and morbidity risk factors.
The records of 1,766 consecutive adult patients undergoing isolated coronary artery bypass graft surgery at 3 institutions have been analyzed retrospectively for in-hospital mortality and adverse outcomes. Clinical data were from the Italian National Cardioanesthesia Database. Multivariate analysis and analysis of receiver operating characteristic curves were applied.
The lowest hematocrit value on cardiopulmonary bypass was an independent risk factor for postoperative low-output syndrome and renal failure. The hematocrit cutoff values were similar for renal failure (23%) and low-output syndrome (24%). Blood transfusions were significantly associated with both renal failure and low-output syndrome. The risk of renal failure doubled when the nadir-on-cardiopulmonary-bypass hematocrit occurred in transfused patients. Anemia upon cardiopulmonary bypass was not associated with death.
Our findings confirm that both severe anemia and blood transfusions were significantly associated with renal failure and low-output syndrome.
Key words: Anemia/etiology; blood transfusion; cardiopulmonary bypass/adverse effects; extracorporeal circulation/adverse effects; hematocrit; hemodilution; intraoperative care; kidney failure, acute; multivariate analysis; postoperative complications
In cardiac surgery, several factors can cause hemodilutional anemia during cardiopulmonary bypass (CPB). Among them are the patients' characteristics (such as body size, sex, and baseline hematocrit level) and CPB management (transfusion policy, priming volume, and size of the circuit and oxygenator).
From many points of view, hemodilution throughout CPB is considered favorable: 1) the reduction of blood viscosity improves peripheral perfusion and blood flow to tissues and facilitates venous return1–4; 2) in hypothermic procedures, hemodilution counteracts the tendency towards high viscosity at low temperature; and 3) accommodating anemia may decrease the number of blood transfusions.5
However, the suitable limit of hemodilutional anemia is not yet clear, and numerous reports1–12 have pointed out the potential disadvantages of low hematocrit values during CPB.
Unfortunately, the literature lacks studies that fulfill 2 important criteria. First, many existing studies do not consider the fact that some intraoperative factors (CPB duration, volume of fluids administered, and body temperature on bypass) can change either the hematocrit value or the physiological consequences of its reduction. Second, many existing studies do not define the essential role, with respect to outcome, of the relationship between a low hematocrit value during CPB and the need for blood transfusion. Blood transfusion—itself a risk factor in cardiac surgery13,14—should not be underestimated when considering hemodilution as a predictor of poor outcome.
The present study attempts to evaluate the effects of the nadir hematocrit during CPB and of allogenic blood transfusions upon outcome in cardiac surgery.
Methods
Data Collection. This is a multicenter retrospective cohort study that draws on prospectively collected data from 3 Italian cardiothoracic surgical centers (Bologna, Milan, and Siena). Clinical data were from the Italian National Cardioanesthesia Database. We studied the data on 1,766 consecutive patients who underwent isolated coronary artery bypass grafting (CABG) from January 2003 through December 2004. Patients who underwent associated procedures (valve repair or replacement, resection of a ventricular aneurysm with or without remodeling of the left ventricle, or combined carotid artery surgery) or who underwent off-pump CABG were excluded from the analysis.
Perioperative data also included unstable angina, antiaggregant (heparin) therapy, priority of surgery (elective, urgent, or emergent), number of vessels grafted, priming pump volume, and postoperative bleeding. Patients undergoing “redo” CABG operations were also enrolled, because they represent a growing part of the cardiac surgical population.
The lowest hematocrit value on bypass was defined as the nadir hematocrit value throughout the CPB. Allogenic blood transfusions were defined as a binary (yes/no) variable, and they were taken into account only if more than 2 red-blood-cell units were transfused during or after surgery. The outcome variables included reopening (due to postoperative bleeding), low-output syndrome (LOS) (postoperative need for inotropic support for more than 48 hours or the need for an intra-aortic balloon pump or ventricular assist device), perioperative myocardial infarction (new Q-waves and enzymatic criteria according to local definition), cardiac arrhythmias (requiring treatment), stroke (new focal neurologic deficit documented by computed tomography and lasting for at least 72 hours), coma (loss of consciousness lasting more than 24 hours), renal failure (need for dialysis, except for patients on chronic dialysis), lung dysfunction (poor gas exchange that requires more than 48 hours of mechanical ventilation), tracheostomy, mesenteric infarction, wound infection, mediastinitis, and systemic infection. Mortality was defined as in-hospital death.
Statistical Methods. A χ2 test or Fisher's exact test (as appropriate) was used for categorical variables, and a Student's t-test was used for continuous variables. Factors considered for multiple logistic regression included only those found significant by univariate analysis. The logistic regression analysis used a forward stepwise selection procedure, and adjustments for multiple comparisons were performed. A P value ≤0.15 was used as a screening criterion for the selection of candidate variables that potentially correlated with the nadir hematocrit on CPB. Receiver operating characteristic (ROC) curves were generated to identify the predictive lowest hematocrit on CPB threshold (cutoff) for adverse outcomes. Receiver operating characteristic curves graph the sensitivity (SE) of a diagnostic test (true positive proportion) versus 1 minus specificity (SP) (false positive proportion) and provide an improved measure of the overall discriminatory power of a test as they assess all possible threshold or cutoff values. Finally, to determine the role of the lowest hematocrit on CPB in comparison with the role of transfusion, complicated patients were divided into 4 groups according to the hematocrit cutoff value and the need for allogenic blood transfusions; and analyses (a χ2 test or Fisher's exact test, as appropriate) for LOS and for renal failure were completed. The statistical analysis was done with SPSS application software version 11.5 (SPSS Inc.; Chicago, Ill).
Results
Clinical characteristics and the main perioperative data are presented in Table I. Upon univariate analysis, the lowest hematocrit on CPB, together with other covariates, was significantly related to LOS, lung dysfunction, renal failure, and mortality (Table II). After the analysis for those covariates was adjusted, the nadir hematocrit on CPB remained an independent risk factor for LOS and renal failure (Table III). Upon multivariate analysis, the nadir hematocrit on CPB was not a risk factor for mortality (Table IV). The ROC curve analysis performed for LOS identified a predictive value of nadir hematocrit on CPB of 24%, with an area under curve (AUC) of 0.62 (95% confidence interval [CI] = 0.572 to 0.671; SE=0.60, SP=0.57; P <0.01). The threshold hematocrit value on CPB for renal failure was 23%, with an AUC of 0.74 (95% CI = 0.631 to 0.852; SE=0.70, SP=0.68; P <0.01). The ROC curve analysis identified a cutoff value of hematocrit on CPB of 24% for the requirement of blood transfusion, with an AUC of 0.746 (95% CI = 0.722 to 0.770; SE =0.70, SP =0.72; P <0.001).
TABLE I. Demographics, Clinical Characteristics, and Operative Data
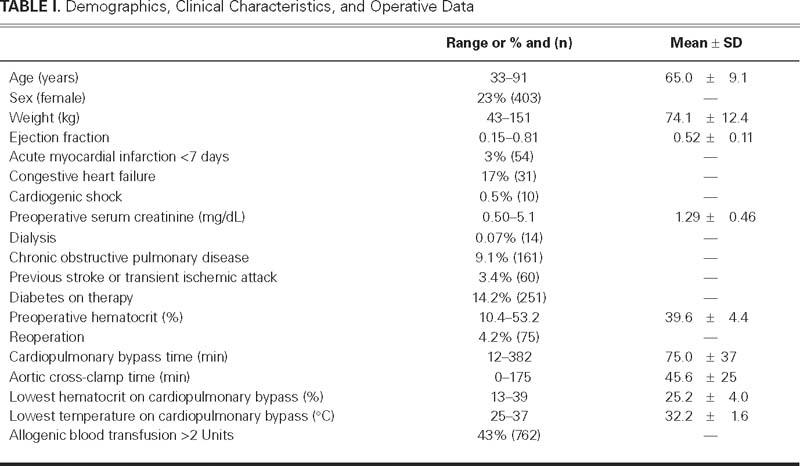
TABLE II. Univariate Morbidity and Mortality Risk Analysis for the Lowest Hematocrit Level on Bypass and the Other Significant Factors Identified
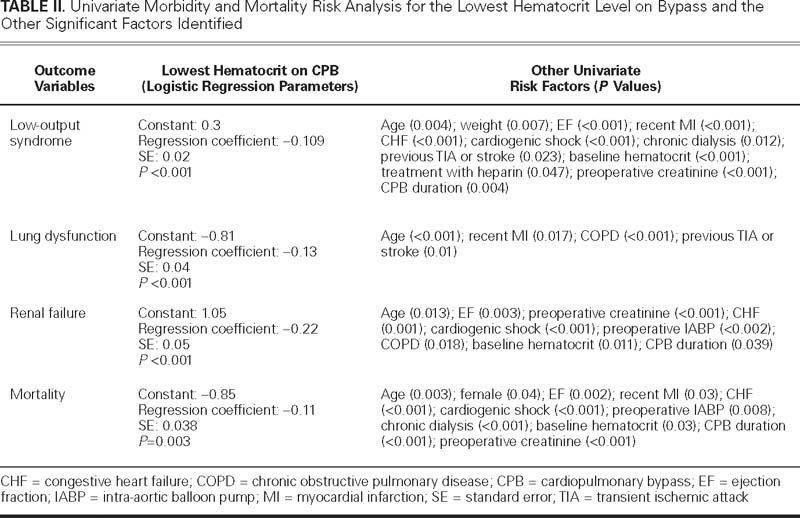
TABLE III. Multivariate Logistic Regression Analyses for Morbidity
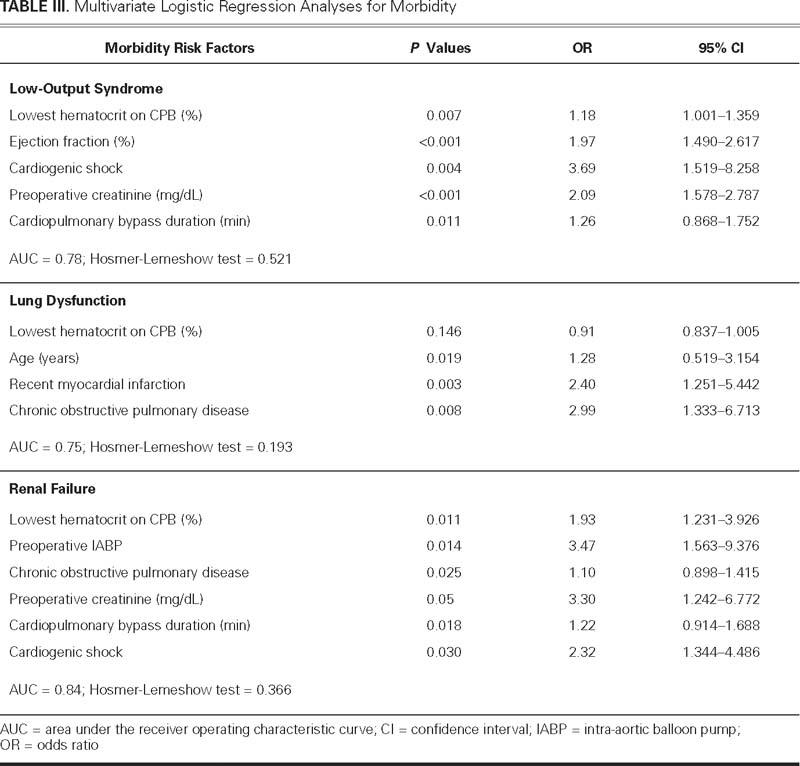
TABLE IV. Multivariate Logistic Regression Analysis for Mortality
The relationship between LOS, renal failure, and the cutoff values of hematocrit on CPB with respect to the need for blood transfusions is shown in Figure 1. Low-output syndrome was higher in patients being transfused (P <0.001), but no differences related to the lowest hematocrit within each group were found (Fig. 1). Renal failure was significantly higher in patients receiving transfusions (P <0.001), and within that group it doubled in patients with a hematocrit level on CPB less than 23% (2.1% vs 4.3%, P <0.001) (Fig. 2).
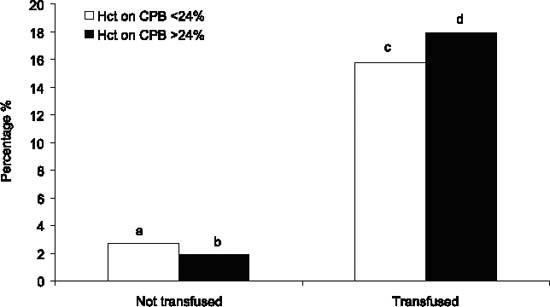
Fig. 1 Relative roles of the lowest hematocrit (Hct) on cardiopulmonary bypass (CPB) and of transfusion in determining low-output syndrome (see text for details).
• a versus b: P=NS
• c versus d: P=NS
• c and d versus a or b: P <0.001.
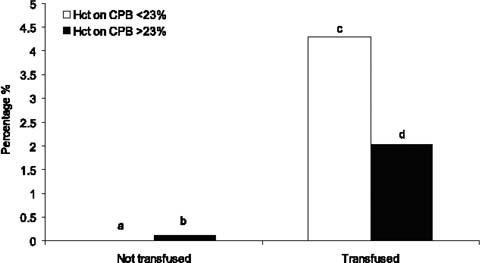
Fig. 2 Relative role of the lowest hematocrit (Hct) on cardiopulmonary bypass (CPB), and transfusions in determining renal failure (see text for details).
• a versus b: P=NS
• c versus d: P<0.01
• c and d versus a or b: P <0.001.
Discussion
Despite a number of studies that point out several drawbacks of extremely low values of hematocrit during CPB, the acceptable limit of hemodilutional anemia remains unclear.
The nadir hematocrit on CPB reflects demographic variables (sex and body size), comorbid conditions (renal failure and preoperative anemia), variations in CPB management (fluid administration, body temperature, and duration), and variations in local transfusion guidelines for management of CPB-related anemia. Each of those variables can cause hemodilutional anemia during CPB but can itself be a risk factor for poor outcome. For example, a longer CPB time means administration of more cardioplegic solution and larger fluid volume (that is, higher hemodilution). At the same time, a longer CPB time is itself a risk factor for mortality and morbidity.15 Due to the complexity of the model itself, drawing conclusions from a multivariate analysis without analyzing all the potential factors can induce misleading results even if a large population is analyzed.
A 2nd major point of concern is the role of transfusions. Allogenic blood transfusions, often required whenever major complications occur, correlate to pre- and intraoperative factors and are a risk factor for adverse events. Because they are both a cause and an effect of poor outcomes, blood transfusions should not be analyzed with conventional multivariate risk analysis. Yet excluding transfusions from a study that focuses on intraoperative anemia can produce uncertain results.
In our observational study of 1,766 consecutive CABG patients, we tried to overcome these problems by including several CPB-related variables in the analysis, and then by including blood transfusions and postoperative bleeding as interactive factors, in a subsequent step of the analysis.
Our findings showed that 1) the nadir hematocrit on CPB is an independent risk factor for LOS and renal failure; 2) after blood transfusions are included in the analysis, LOS is associated with transfusions, and the nadir hematocrit on CPB loses statistical significance; 3) after blood transfusions are included in the analysis, renal failure is significantly associated with transfusions, and within the group with hematocrit values of less than 23% on CPB, transfusions double the risk of renal failure; 4) ROC curve analyses show similar hematocrit cutoff values for LOS and renal failure; and 5) mortality does not correlate with the nadir hematocrit on CPB.
Three studies showed that nadir hematocrit on CPB is a risk factor for death. Fang and colleagues,9 in a series of 2,738 CABG patients, identified 2 cutoff values of hematocrit on CPB that were related to mortality: 14% for low-risk patients and 17% for high-risk patients. Fang's cutoff values are extremely low, and we were unable to compare them with our data, since a cutoff point lower than 17% would have included only 40 of our patients. DeFoe and associates10 used a statistical approach that analyzed 5 classes of lowest intraoperative hematocrit (<19%, 19%–20%, 21%–22%, 23%–24%, and ≥25%), and showed that the lowest hematocrit on bypass was significantly associated with in-hospital death. Finally, Habib and colleagues12 analyzed data from 5,000 consecutive cardiac operations and found a strong association between severity of hemodilution on CPB and death. Our results did not show the nadir hematocrit on CPB to be a mortality risk factor. However, our study included only 1,766 patients in comparison with the 2,738 patients studied by Fang, the 6,980 patients examined by DeFoe, and the 5,000 patients examined by Habib. Moreover, only 45 deaths (2.5%) were observed in the present study. These elements suggest that our study could be statistically underpowered to detect any significant relationship between anemia and mortality. Large observational studies might offer more certain evidence of a link between low on-pump hematocrit values and death.
A further uncertain issue is the association between hematocrit levels and myocardial dysfunction. Spiess and coworkers16 demonstrated that a hematocrit value of more than 34% after CPB is a risk factor for myocardial infarction. Hardy and colleagues17 showed that the anemia after surgery was associated with hemodynamic instability. DeFoe and colleagues10 found a significant association between low hematocrit values, failure of weaning from CPB, and the need for an intra-aortic balloon pump. In our series, ROC curve analysis showed that by choosing a hematocrit cutoff on CPB of less than 24%, the risk of LOS was unmodified. On the other hand, transfused patients showed an increased LOS rate not related to the hematocrit cutoff value. Transfusions are often given in the hope of improving cardiac output and oxygen delivery to the tissues. Although transfusions may appear to be a risk factor for myocardial infarction,16 our findings could be accounted for by our more liberal transfusional policy in regard to patients who have cardiac dysfunction after CPB.
Renal failure was higher in transfused patients, and the risk of renal impairment doubled when the transfusions were associated with a nadir hematocrit on CPB of less than 23%. These results confirm our previous findings6 and agree with those of Stafford-Smith, Hardy, and Karkouti.7,17–18 There is evidence that perfusing the kidney with severely anemic blood during CPB increases the risk of postoperative renal failure. The peculiar physiology of the kidney could play a role. Under normal conditions, the oxygen content of the renal tissues is low, and the medullar tissue is nearly hypoxic.19 Severe hemodilution leads to a further decrease in the blood arterial oxygen content and may cause renal injury.18,19
In conclusion, the nadir hematocrit on CPB plays a role in affecting the outcome after CABG surgery. Our findings show that severe anemia on CPB is a likely determinant of LOS and a definite risk factor for postoperative renal failure. In addition, the role of transfusions has been established for acute renal failure. Because the nadir hematocrit on CPB is one of the major determinants of allogenic blood transfusions, its role should be considered in triggering transfusion-related complications. Limiting the hemodilutional anemia (by reducing the size of the oxygenator and circuits, limiting the intraoperative fluid administration, or reducing the priming volume) might improve outcomes after coronary surgery. Proof that less hemodilution improves outcomes in this setting awaits a sound prospective goal-oriented trial that investigates one or more strategies to reduce anemia during CPB.
Footnotes
Address for reprints: Sabino Scolletta, MD, Department of Surgery and Bioengineering, Unit of Cardiothoracic Surgery, University of Siena, Viale Bracci 14, 53100 Siena, Italy. E-mail: scolletta@unisi.it
References
- 1.Kaplan JA, editor. Cardiac anesthesia. 3rd ed. Philadelphia: Saunders; 1993.
- 2.Kay PH, editor. Techniques in extracorporeal circulation. 3rd ed. Oxford: Butterworth-Heinemann; 1992.
- 3.Jonas RA, Elliott MJ, editors. Cardiopulmonary bypass in neonates, infants, and young children. Oxford: Butterworth-Heinemann; 1994.
- 4.Messmer K, Sunder-Plassmann L, Klovekorn WP, Holper K. Circulatory significance of hemodilution: rheological changes and limitations. Adv Microcirc 1972;4:1–77.
- 5.Kawaguchi A, Bergsland J, Subramanian S. Total bloodless open heart surgery in the pediatric age group. Circulation 1984;70(3 Pt 2):I30–7. [PubMed]
- 6.Ranucci M, Pavesi M, Mazza E, Bertucci C, Frigiola A, Menicanti L, et al. Risk factors for renal dysfunction after coronary surgery: the role of cardiopulmonary bypass technique. Perfusion 1994;9:319–26. [DOI] [PubMed]
- 7.Stafford-Smith M, Conlon PJ, White WD, Newman MF, King S, Grocott HP, et al. Low hematocrit but not perfusion pressure during CPB is predictive for renal failure following CABG surgery [abstract]. Anesth Analg 1998;86:SCA102.
- 8.Mossad E, Estafanous F. Con: a hematocrit of 20% is not adequate for separation from cardiopulmonary bypass. J Cardiothorac Vasc Anesth 1996;10:294–5. [DOI] [PubMed]
- 9.Fang WC, Helm RE, Krieger KH, Rosengart TK, DuBois WJ, Sason C, et al. Impact of minimum hematocrit during cardiopulmonary bypass on mortality in patients undergoing coronary artery surgery. Circulation 1997;96(9 Suppl):II-194–9. [PubMed]
- 10.DeFoe GR, Ross CS, Olmstead EM, Surgenor SD, Fillinger MP, Groom RC, et al. Lowest hematocrit on bypass and adverse outcomes associated with coronary artery bypass grafting. Northern New England Cardiovascular Disease Study Group. Ann Thorac Surg 2001;71:769–76. [DOI] [PubMed]
- 11.Swaminathan M, Phillips-Bute BG, Conlon PJ, Smith PK, Newman MF, Stafford-Smith M. The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg 2003;76:784–92. [DOI] [PubMed]
- 12.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg 2003;125:1438–50. [DOI] [PubMed]
- 13.Shore-Lesserson L. Myocardial injury in cardiac surgery: the role of transfusion. J Cardiothorac Vasc Anesth 2000;14(3 Suppl 1):11–4; discussion 37–8. [PubMed]
- 14.Fransen E, Maessen J, Dentener M, Senden N, Buurman W. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest 1999;116:1233–9. [DOI] [PubMed]
- 15.Higgins TL, Estafanous FG, Loop FD, Beck GJ, Lee JC, Starr NJ, et al. ICU admission score for predicting morbidity and mortality risk after coronary artery bypass grafting. Ann Thorac Surg 1997;64:1050–8. [DOI] [PubMed]
- 16.Spiess BD, Ley C, Body SC, Siegel LC, Stover EP, Maddi R, et al. Hematocrit value on intensive care unit entry influences the frequency of Q-wave myocardial infarction after coronary artery bypass grafting. The Institutions of the Multicenter Study of Perioperative Ischemia (McSPI) Research Group. J Thorac Cardiovasc Surg 1998;116:460–7. [DOI] [PubMed]
- 17.Hardy JF, Martineau R, Couturier A, Belisle S, Cartier R, Carrier M. Influence of haemoglobin concentration after extracorporeal circulation on mortality and morbidity in patients undergoing cardiac surgery. Br J Anaesth 1998;81 Suppl 1:38–45. [PubMed]
- 18.Karkouti K, Beattie WS, Wijeysundera DN, Rao V, Chan C, Dattilo KM, et al. Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg 2005;129:391–400. [DOI] [PubMed]
- 19.Brezis M, Rosen S, Silva P, Epstein FH. Renal ischemia: a new perspective. Kidney Int 1984;26:375–83. [DOI] [PubMed]


