Abstract
Cardiac echinococcosis is a rare but potentially very serious complication of hydatid disease. It is a diagnostic and therapeutic challenge due to the variability of signs and symptoms at presentation and to its numerous, often unpredictable, preoperative complications. Our clinical experiences with 7 cases of cardiac echinococcosis are reported, and the diagnostic and therapeutic considerations for the management of patients are discussed, together with a review of the literature.
Key words: Cerebrovascular accident, echinococcosis/diagnosis/surgery/ultrasonography, heart diseases/parasitology, cardiac tamponade, rupture
Hydatid cyst is a human parasitic disease caused by the larval stage of the cestode tapeworm Echinococcus granulosus, which infests the gut of dogs—its definitive hosts. Human beings may serve as incidental hosts by the ingestion of ova in vegetables or water contaminated with dog feces. Hydatid disease is endemic in cattle-raising areas of the world, notably in the Mediterranean countries, the Middle East, South America, Australia, and New Zealand.1,2 The incidence of hydatidosis in the Turkish population has been reported as 1:20,000.2
Hydatid cysts are commonly located in the liver (>65% of cases) and the lungs (25%). However, even in countries where hydatid disease is endemic, only isolated, sporadic cases of cardiac involvement (0.05% to 2% of all cases) have been reported.1–4 At our institution, the ratio of cardiac hydatid cysts to pulmonary hydatid cysts that underwent surgical ablation between 1978 and 1995 was 0.7%.2
Cardiac hydatic disease was first mentioned by Williams in 1836. In 1846, Griesinger reported 15 autopsy cases. The 1st successful surgical intervention was performed by Long in 1932. By 1964, only 42 successfully treated cases had been reported in the literature. In 1962, Arturico and colleagues reported the 1st successful operation for cardiac echinococcosis with cardiopulmonary bypass.1,2 Because there is no specific clinical picture, the diagnosis of cardiac hydatid disease usually arises from suspicion. When echinococcosis is diagnosed, the treatment of choice for even asymptomatic cases is surgical ablation, due to the risk of cystic rupture.1–4 We present herein a retrospective analysis of the diagnostic and therapeutic considerations in 7 cases of cardiac hydatid cysts, with a review of the literature.
Patients and Methods
From January 1993 through December 2003, 7 patients with cardiac hydatid disease underwent surgical treatment in our institution. The first 3 cases and the 5th have previously been reported.2,5 The clinical data on the patients are summarized in Table I. Four patients were male and 3 were female; their ages ranged from 6 to 38 years (mean, 19.8 years). Two of these patients had undergone previous operations for cardiac (Patient 1) and pericardial (Patient 4) hydatid cysts 10 and 2 years before, respectively; and a 3rd patient (Patient 2) had been operated on twice for hepatic and pulmonary cysts, 12 and 8 years before, respectively. The signs and symptoms at the time of presentation were nonspecific. The diagnosis of cardiac hydatid cyst was established during thoracotomy for a misdiagnosed paracardiac pulmonary cyst in Patient 2. In the other 6 cases, cardiac hydatid disease was diagnosed by echocardiography, which had been requested during investigations for other causes. In 1 patient with pericardial rupture of a hydatid cyst (Patient 3), the clinical picture at presentation closely mimicked the signs and symptoms of acute abdomen, which subsequently led to laparotomy. The patient was then referred to our department with symptoms of acute cardiac tamponade. Acute stroke was the presenting symptom in a 16-year-old patient (Patient 7).
TABLE I. Clinical Data in 7 Patients with Cardiac Hydatid Cysts
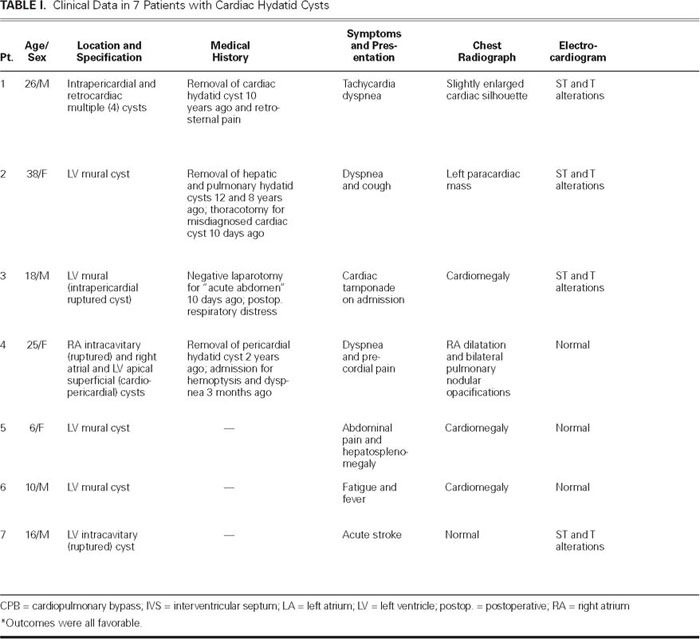
Table. Continued.
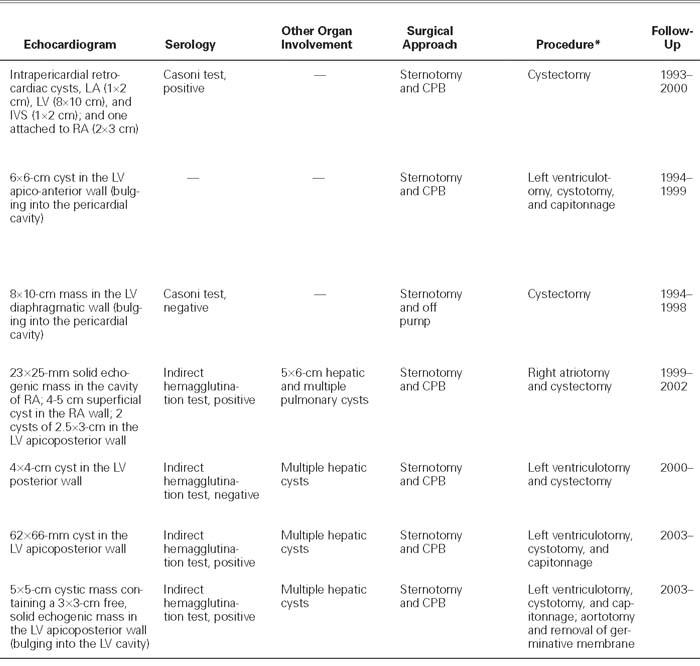
An abnormal cardiac silhouette was present in all patients except one (Patient 7, Fig. 1). The electrocardiogram showed ST-segment and T-wave alterations resembling ischemia in 4 patients (1, 2, 3, and 7). Two-dimensional echocardiography was diagnostic in all cases; hydatid cysts were recognized in the left ventricular free wall in 5 patients (2, 3, 5, 6, and 7), and in the right atrial wall in 1 case (Patient 4). (In patients 4 and 7, the cysts had ruptured into the right atrium and left ventricle, respectively.) In patients 1 and 4, there were multiple intrapericardial cysts. These findings were also confirmed by computed tomography and magnetic resonance imaging (Fig. 2). Results of serological examination by indirect hemagglutination test were positive in 3 of the 4 patients tested (patients 4, 6, and 7). Results of Casoni's intradermal test were positive in 1 of the 2 patients tested (Patient 1).
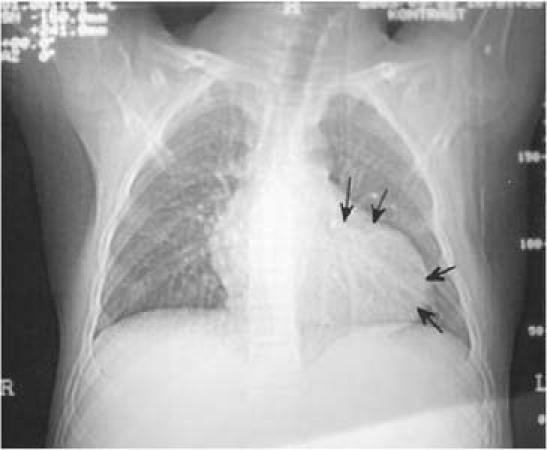
Fig. 1 Patient 2. Posteroanterior chest radiograph shows bulging of the left lower border of the heart (arrows).
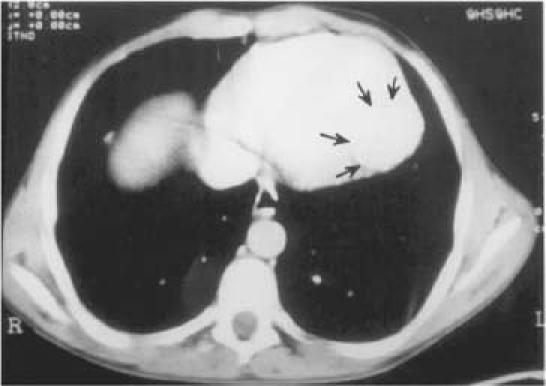
Fig. 2 Patient 6. Computed tomographic scan shows a 62- × 66-mm mass (arrows) in the apicoposterior wall of the left ventricle.
Other organ involvement was present in 4 cases. Three of these patients (5, 6, and 7) had multiple hepatic cysts, and the last (Patient 4) had multiple hepatic and bilateral pulmonary cysts. The hepatic cysts were diagnosed by ultrasonography.
The operation was performed through a median sternotomy in all 7 patients. One of these (Patient 3) was operated on with the beating-heart technique, while the remainder were operated on under cardiopulmonary bypass. In 5 patients (2, 3, 5, 6, and 7), the cysts were found in the left ventricular free wall (Fig. 3). In 2 patients (1 and 4) who had undergone previous operations in provincial hospitals for removal of cardiac and pericardial hydatid cysts, there were multiple superficial epicardial cysts growing into the pericardial cavity.
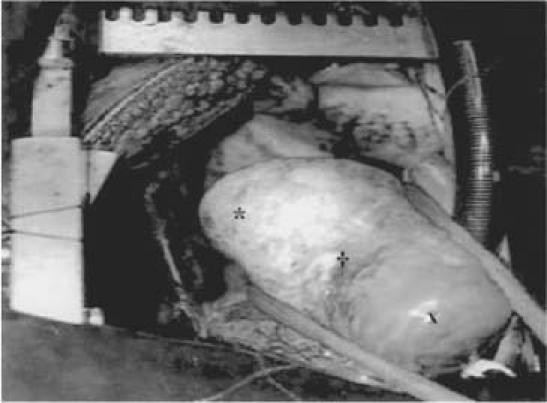
Fig. 3 Patient 5. Operative view shows a 4- × 4-cm cyst located in the posterior wall of the left ventricle.
* = posterior wall of the left ventricle; † = cyst; x = apex of the left ventricle
The operative field was wrapped with towels moistened with 1% povidone-iodine solution, which was also instilled into the cysts. In 5 patients (2, 3, 5, 6, and 7), longitudinal left ventriculotomy was performed on the prominent portion of the left ventricular wall. The right intra-atrial cyst in Patient 4 was removed by right atriotomy. After the cysts were opened, the germinative membrane was removed in 5 patients (1, 2, 3, 5, and 6). In Patient 4, the cyst had previously ruptured, and the mass within it was not characteristic. In Patient 7, the mass that had been observed within the cystic cavity on echocardiography the day before the operation could no longer be seen when the cyst was incised during the operation. There was a communication between the cystic cavity and the left ventricular chamber. After termination of cardiopulmonary bypass, the germinative membrane was found by abdominal Doppler ultrasonography to be occluding the terminus of the aorta and was removed (Fig. 4). In superficially located cysts (patients 1, 3, and 5), the cavity was left open at the end of cystectomy. In 3 patients (2, 6, and 7), capitonnage was performed to obliterate the cystic cavity. The operations for removal of concomitant lung and hepatic cysts in Patients 4, 5, 6, and 7 were deferred. Prophylactic medical therapy with albendazole was started in 3 cases (Patients 3, 4, and 7).
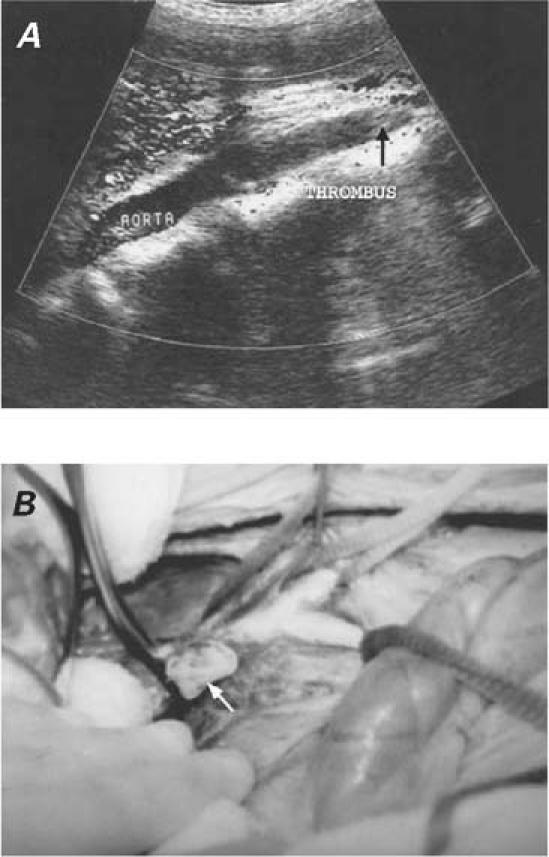
Fig. 4 Patient 7. A) Postoperative Doppler ultrasonogram of the abdominal aorta shows occlusion of the terminal aorta by the embolized germinative membrane (arrow). B) The germinative membrane was enucleated (arrow) from the abdominal aorta.
Results
The postoperative period was uneventful for all patients. The patients were discharged from the hospital between 6 and 14 days postoperatively (mean, 10 days). The patients' follow-up periods ranged from 6 months to 7 years (mean follow-up, 3.3 years). During the follow-up, no evidence of cardiac abnormalities or recurrences was detected during repeat echocardiography. In the patient who had presented with stroke (Patient 7), hemiparesis and aphasia improved substantially about a month after his discharge from the hospital, and no neurologic sequelae were detected during the 5-month follow-up evaluation. A secondary cyst formation in the area of cerebral infarct has not yet been detected.
Discussion
The hexacanth embryos of the parasite released from the ova into the human intestine enter the portal or lymphatic circulation. A few of these embryos may escape from the vascular beds of the liver and lungs and travel to the myocardium via the coronary circulation. When this occurs, the left ventricle is the most frequently involved site (60%), followed by the right ventricle (15%), the interventricular septum (9%), the left atrium (8%), the right atrium (4%), and the interatrial septum (2%).2,4,6,7 Primary pericardial cysts are extremely rare, and they generally develop secondary to intrapericardial rupture of a myocardial cyst or to spillage of cysts' contents during surgical removal.2,8,9 In 5 of our cases (Patients 2, 3, 5, 6, and 7), the cysts were localized in the left ventricular free wall. Of the 4 cysts present in Patient 4, 1 was within the right atrium, whereas the other 3 were located epicardially, in the apex and in the right atrial wall. In Patient 1, there were 4 pericardial cysts at various locations in the atrial and ventricular walls. Both patients who had multiple pericardial cysts (Patients 1 and 4) had undergone previous operations for the removal of cardiac and pericardial cysts at provincial hospitals.
Cysts in the ventricular walls grow toward either the epicardium or the endocardium. Subepicardial cysts grow more easily toward the pericardial cavity and can attain large diameters; cysts in a subendocardial position have a higher potential for intracavitary growth. Rupture is a serious complication of cardiac hydatid cysts. Intrapericardial rupture of a cardiac hydatid cyst occurs in about 10% of cases1,2,10 and can lead to acute pericarditis,11 which can eventually progress to constrictive pericarditis.12 On the other hand, a cyst bulging into a cardiac chamber can rupture spontaneously; discharge of its contents is encountered in about 40% of patients2-4,7 (as in Patients 4 and 7). The anatomicopathological consequences of intracardiac rupture are germinative membrane embolization to the pulmonary6,13,14 or systemic6,15–18 circulation (as in Patient 7), and eruption embolization of secondary cysts either into the lungs (as in Patient 4) or into various other organs supplied by the systemic circulation. The overall effect can give rise to symptoms such as those associated with the compression of a coronary artery, with a disturbance in the valvular mechanisms (clinically simulating mitral, pulmonary, aortic, or tricuspid valve stenosis or regurgitation), or with outflow tract obstruction or a variety of conduction defects (caused by the involvement of the interventricular septum).1–3,19
The clinical picture of a cardiac hydatid cyst depends on the age, size, location, and integrity of the cyst. Because of their slow growth (approximately 1 cm per year), cardiac hydatid cysts rarely present in early childhood. However, cardiac echinococcosis in children has recently been reported in a few studies.8,20,21 Actually, 2 of our patients (5 and 6) were 6 and 10 years old, respectively, and the mean age of our group was 19.8 years. There was no specific clinical picture that could lead to the correct preoperative diagnosis. On the other hand, in endemic geographic areas, the patient's history yields important clues about the diagnosis. In Table I, we summarize the clinical data on 7 patients with cardiac hydatid cysts. The presenting clinical picture may vary from no symptoms to congestive heart failure or even sudden death. It is estimated that 10% of patients remain asymptomatic for many years, although they are under the continuous threat of rupture. Further, it has been suggested that about 20% of fatal cases present with sudden death, having shown no previous signs or symptoms of cardiac echinococcosis.2,13,22
An anaphylactic reaction and profound circulatory collapse may follow intracavitary rupture.2,6,13,15 Although no history of acute allergic reaction was noted in Patients 4 and 5, in whom hydatid cysts had ruptured into the right atrial and left ventricular cavities, Patient 3 of our series presented with generalized rash, fever, nausea, vomiting, and acute respiratory distress, all of which were associated with intrapericardial rupture.
Angina secondary to the compression of a coronary artery may mimic coronary artery disease.1–3,19 Precordial or retrosternal chest pain may also arise secondary to intrapericardial rupture and acute pericarditis.1,11 In this situation, the pain may also radiate to the epigastrium and, as in Patient 3, it may closely mimic acute abdomen.2
Although infrequent, acute cardiac tamponade secondary to the rupture of intrapericardial cyst (as in Patient 3) has been reported.8–10 Acute stroke as a presenting symptom of cardiac hydatidosis, as in Patient 7 of our series, is exceptionally rare, and only a few cases have been reported in the literature.20,21,23,24 Because intracranial embolism of the germinative membrane is invariably fatal,23 nonfatal stroke (as in Patient 7) must result from smaller constituents of the ruptured cyst, rather than the germinative membrane. Limb ischemia secondary to germinative-membrane embolization may be the presenting symptom.15–18 The acute distal aortic embolism in Patient 7 was possibly secondary to migration of the germinative membrane from the ruptured cyst during the operation, before the aortic cross-clamp was applied.
In the diagnosis of cardiac hydatid cysts, especially in endemic geographic areas, suspicion is essential. Although the electrocardiogram and chest radiograph can be abnormal, they are usually not diagnostic.1,2 Casoni's intradermal test, the Weinberg reaction, and peripheral blood eosinophil count are not reliable, due to false negative results. On the other hand, the indirect hemagglutination test is highly sensitive and specific for hydatid disesase.3 As an informative, efficient, easy-to-perform, and highly sensitive noninvasive technique to detect and localize the cyst before surgery, echocardiography is the diagnostic method of choice for cardiac hydatid cysts.2,3,22 In all but 1 case (Patient 2), the preoperative diagnosis was established by echocardiography during investigations for other suspected causes. Computed tomography and magnetic resonance imaging are additional useful diagnostic methods.9
The treatment of choice even for asymptomatic cardiac hydatid cysts is surgical excision, which yields complete recovery and excellent prognosis.3 Although superficially located cysts can successfully be removed with a beating-heart (off-pump) technique,9,25 resection under cardiopulmonary bypass, since 1962, has been considered the safest method, with the least risk of spillage of cyst contents during the procedure.1–3 In our series, cardiopulmonary bypass was used in 6 patients. In Patient 3, the operation was performed on a beating heart. It is advisable to place an additional filter on the venous side of the circuit to prevent the passage of hydatid particles to the pump, especially in rupture cases.6,26 As a rule, the heart should not be manipulated before the application of the cross-clamp. In Patient 7, unless distal aortic embolization of the germinative membrane somehow occurred during the preoperative period, it occurred intraoperatively, despite great care to prevent such a complication. In operations for cysts located in the right side of the heart, the pulmonary artery can be clamped to avoid pulmonary embolism.6,26 After removal of a superficially located cyst, the cystic cavity can be left open as in Patients 1, 3, and 5; these pouches will close in time via secondary sealing, which occurs spontaneously, without surgical intervention.2,9 Supplemental medical therapy with mebendazole or albendazole is recommended to reduce the risk of recurrence, especially in the event of intracardiac rupture. In order to exclude the possibility of recurrence due to inadvertent spillage or a small cyst not noticed at the time of the operation, serologic and echocardiographic monitoring is recommended during the first 5 postoperative years.27
In conclusion, cardiac hydatid cysts are rare and may present with a variety of signs and symptoms. The possibility of hydatid disease should be kept in mind, especially in endemic zones. Due to the high risk of associated complications, cardiac hydatid cysts should be removed surgically, even in asymptomatic patients. During the operation, measures should be taken to prevent perioperative embolization of a germinative membrane. Surgical excision under cardiopulmonary bypass is the treatment of choice.
Footnotes
Address for reprints: Hafize Yaliniz, MD, Department of Cardiovascular Surgery, University of Cukurova, Balcali 01330, Adana, Turkey. E-mail: hafize101@yahoo.com
References
- 1.Ameli M, Mobarhan HA, Nouraii SS. Surgical treatment of hydatid cysts of the heart: report of six cases. J Thorac Cardiovasc Surg 1989;98(5 Pt 2):892–901. [PubMed]
- 2.Salih OK, Celik SK, Topcuoglu MS, Kisacikoglu B, Tokcan A. Surgical treatment of hydatid cysts of the heart: a report of 3 cases and a review of the literature. Can J Surg 1998;41: 321–7. [PMC free article] [PubMed]
- 3.Miralles A, Bracamonte L, Pavie A, Bors V, Rabago G, Gandjbakhch I, Cabrol C. Cardiac echinococcosis. Surgical treatment and results. J Thorac Cardiovasc Surg 1994;107: 184–90. [PubMed]
- 4.Abid A, Ben Omrane S, Kaouel K, Marghli A, Dhiab M, Abid N, et al. Intracavitary cardiac hydatid cyst. Cardiovasc Surg 2003;11:521–5. [DOI] [PubMed]
- 5.Yaliniz H, Tokcan A, Ulus T, Kisacikoglu B, Salih OK, Topcuoglu MS, et al. A rare presentation of cardiac hydatid cyst: stroke and acute aortic occlusion. Heart Surg Forum 2004;7:E364–6. [DOI] [PubMed]
- 6.Ozer N, Aytemir K, Kuru G, Atalar E, Ozer Y, Ovunc K, et al. Hydatid cyst of the heart as a rare cause of embolization: report of 5 cases and review of published reports. J Am Soc Echocardiogr 2001;14:299–302. [DOI] [PubMed]
- 7.Kaplan M, Demirtas M, Cimen S, Ozler A. Cardiac hydatid cysts with intracavitary expansion. Ann Thorac Surg 2001; 71:1587–90. [DOI] [PubMed]
- 8.Narin N, Mese T, Unal N, Pinarli S, Cangar S. Pericardial hydatid cyst with a fatal course. Acta Paediatr Jpn 1996;38:61–2. [DOI] [PubMed]
- 9.Birincioglu CL, Bardakci H, Kucuker SA, Ulus AT, Arda K, Yamak B, Tasdemir O. A clinical dilemma: cardiac and pericardiac echinococcosis. Ann Thorac Surg 1999;68:1290–4. [DOI] [PubMed]
- 10.Demirtas M, Usal A, San M, Birand A. Hydatid disease presenting as cardiac tamponade. A case history. Angiology 1996; 47:601–7. [DOI] [PubMed]
- 11.Di Bello R, Mantero ME, Dubra J, Sanjines A. Hydatid cyst of the left ventricle of the heart. Acute hydatid pericarditis. Am J Cardiol 1967;19:603–6. [DOI] [PubMed]
- 12.DiBello R, Abo JC, Borges UL. Hydatid constrictive pericarditis. A new case and review of the literature. J Thorac Cardiovasc Surg 1970;59:530–2. [PubMed]
- 13.Bayezid O, Ocal A, Isik O, Okay T, Yakut C. A case of cardiac hydatid cyst localized on the interventricular septum and causing pulmonary emboli. J Cardiovasc Surg (Torino) 1991;32:324–6. [PubMed]
- 14.Pasaoglu I, Dogan R, Hazan E, Oram A, Bozer AY. Right ventricular hydatid cyst causing recurrent pulmonary emboli. Eur J Cardiothorac Surg 1992;6:161–3. [DOI] [PubMed]
- 15.Madariaga I, de la Fuente A, Lezaun R, Imizcoz MA, Carmona JR, Urquia M, de los Arcos E. Cardiac echinococcosis and systemic embolism. Report of a case. Thorac Cardiovasc Surg 1984;32:57–9. [DOI] [PubMed]
- 16.Rosenberg T, Panayiotopoulos YP, Bastounis E, Papalambros E, Balas P. Acute abdominal aorta embolism caused by primary cardiac echinococcus cyst. Eur J Vasc Surg 1993;7:582–5. [DOI] [PubMed]
- 17.Unlu Y, Ceviz M, Karaoglanoglu N, Becit N, Kocak H. Arterial embolism caused by a ruptured hydatid cyst in the heart: report of a case. Surg Today 2002;32:989–91. [DOI] [PubMed]
- 18.Ceyran H, Tasdemir K, Tezcaner T, Asgun F, Karahan OI, Emirogullari ON, Andac H. A rare cause of peripheral arterial embolism: ruptured cardiac hydatid cyst. Vasa 2002;31:129–31. [DOI] [PubMed]
- 19.Rivera R, Delcan JL. Surgical treatment of coronary insufficiency produced by cardiac echinococcosis. Chest 1980;78: 849–52. [DOI] [PubMed]
- 20.Cakir O, Eren N, Kilinc N. Cardiac hydatic cyst causing cerebral emboli in a child. Pediatr Cardiol 2002;23:555–6. [DOI] [PubMed]
- 21.Byard RW, Bourne AJ. Cardiac echinococcosis with fatal intracerebral embolism. Arch Dis Child 1991;66:155–6. [DOI] [PMC free article] [PubMed]
- 22.Kanadasi M, Demirtas M, San M, Ozer C, Soyupak SK, Kisacikoglu B. Mobile right atrial hydatid cyst with multiorgan involvement. Catheter Cardiovasc Interv 2000;49:204–7. [PubMed]
- 23.Singh NP, Arora SK, Gupta A, Anuradha S, Sridhara G, Agarwal SK, Gulati P. Stroke: a rare presentation of cardiac hydatidosis. Neurol India 2003;51:120–1. [PubMed]
- 24.Benomar A, Yahyaoui M, Birouk N, Vidailhet M, Chkili T. Middle cerebral artery occlusion due to hydatid cysts of myocardial and intraventricular cavity cardiac origin. Two cases. Stroke 1994;25:886–8. [DOI] [PubMed]
- 25.Rossouw GJ, Knott-Craig CJ, Erasmus PE. Cardiac echinococcosis: cyst removal in a beating heart. Ann Thorac Surg 1992; 53:328–9. [DOI] [PubMed]
- 26.Thameur H, Abdelmoula S, Chenik S, Bey M, Ziadi M, Mestiri T, et al. Cardiopericardial hydatid cysts. World J Surg 2001; 25:58–67. [DOI] [PubMed]
- 27.Kutay V, Ekim H, Yakut C. Infected myocardial hydatid cyst imitating left ventricular aneurysm. Cardiovasc Surg 2003; 11:239–41. [DOI] [PubMed]


