Abstract
Introduction
Southeast Asian women have low levels of Papanicolaou (Pap) testing participation. We conducted a group-randomized controlled trial to evaluate a cervical cancer screening intervention program targeting Seattle’s Cambodian refugee community.
Methods
Women who completed a baseline, community-based survey were eligible for the trial. Neighborhoods were the unit of randomization. Three hundred and seventy survey participants living in 17 neighborhoods were randomized to intervention or control status. Intervention group women received home visits by outreach workers and were invited to group meetings in neighborhood settings. The primary outcome measure was self-reported Pap testing in the year prior to completing a follow-up survey.
Results
The proportion of women in the intervention group reporting recent cervical cancer screening increased from 44% at baseline to 61% at follow-up (+17%). The corresponding proportions among the control group were 51 and 62% (+11%). These temporal increases were statistically significant in both the intervention (P < 0.001) and control (P = 0.027) groups.
Discussion
This study was unable to document an increase in Pap testing use specifically in the neighborhood-based outreach intervention group; rather, we found an increase in both intervention and control groups. A general awareness of the project among women and their health care providers as well as other ongoing cervical cancer screening promotional efforts may all have contributed to increases in Pap testing rates.
Keywords: Cambodian Americans, Pap testing, Outreach
1. Introduction
The majority of Cambodian Americans were forced to leave their home country because of the political and personal persecution imposed by the Khmer Rouge during the mid-1970s, and were relocated from refugee camps in Thailand and the Philippines [1,2]. Compared to the general United States (US) population as well as other immigrant groups, Cambodian refugees are both economically disadvantaged and linguistically isolated; nearly one-half live below the poverty level and over 90% speak Khmer at home [3]. Cambodia is a largely agrarian society and before the revolutionary period the majority of Cambodians lived in rural or semi-rural settings [4]. Further, biomedicine was only available in urban centers and was usually crisis orientated [2,5]. Therefore, Cambodian immigrants are particularly unfamiliar with Western culture and biomedical concepts of prevention [6].
One of the six US national goals for reducing racial/ethnic health disparities specifically addresses cancer screening rates [7]. California data show that Southeast Asian (i.e. Cambodian, Hmong, Laotian, and Vietnamese) women have markedly elevated invasive cervical cancer incidence and mortality rates. Between 1988 and 1992, age-adjusted incidence rates were 35.2 per 100,000 women for Southeast Asians compared to 7.5 per 100,000 women for non-Latin Whites [8]. Additionally, studies conducted in Minnesota and Texas have suggested that Cambodian immigrants have very low rates of Papanicolaou (Pap) testing participation [9,10]. For example, Yi surveyed a convenience sample of Cambodian women served by community organizations in Houston. Although two-thirds (66%) of the respondents had a regular physician, only 13% had been screened in the previous year [10].
In any intervention program designed for racial/ethnic minority groups, the most acceptable interventionists are indigenous members of the target population [11,12]. In addition, door-to-door canvassing is believed to be an effective way of recruiting minority women to cancer control programs [13]. Finally, it has been demonstrated that in-home as well as small-group educational sessions can increase Pap testing participation by less acculturated Asian women [14,15]. We conducted a group-randomized controlled trial to evaluate a Pap testing intervention program targeting Seattle’s Cambodian refugee community. Our multi-faceted intervention was delivered by bilingual, bicultural Cambodian women; it included home visits, group meetings in neighborhood settings, and logistic assistance accessing screening services. In this paper, we report our findings with respect to the impact, acceptability, and feasibility of our intervention approach.
2. Materials and methods
2.1. Study design
2.1.1. Overview
The overall study design is summarized in Fig. 1. Seattle’s Cambodian population is concentrated in the southern and central regions of the city which include most of the low income census tracts [16]. Trial participants were women who participated in a community-based survey of Cambodian American women, aged 18 and older, conducted in these areas of Seattle. This study was approved by the Institutional Review Board at the Fred Hutchinson Cancer Research Center.
Fig. 1.
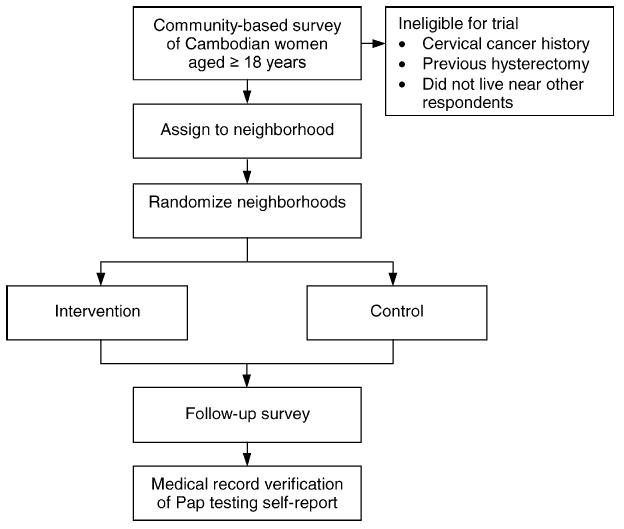
Overview of study design.
2.1.2. Baseline survey
Our baseline survey sampling, development, recruitment, and implementation methods have been described in detail elsewhere [17]. To identify Cambodian households in the target regions, we linked a list of Cambodian names to public-housing rosters, telephone book listings, and motor vehicle registration records. The questionnaire took about 45 min to complete; and included sociodemographic and acculturation items as well as questions related to Pap testing, mammography, and access to health care. All survey items were developed in English, translated into Khmer, back-translated to insure lexical equivalence, reconciled, and pre-tested [18]. The questionnaires were administered in respondents’ homes by bilingual, bicultural Cambodian women. When a household included two or more eligible women, we asked to speak with the oldest woman. The total estimated response rate (i.e. the response rate among all eligible households in the initial survey sample) was 72%, and the cooperation rate (i.e. the response rate among eligible and reachable households) was 89%.
2.1.3. Randomization
Four hundred and twenty-four women completed the baseline survey. We excluded 54 survey respondents because they reported a personal history of invasive cervical cancer and/or a previous hysterectomy, participated in an earlier qualitative study about cervical cancer, or did not live in close proximity to other respondents (and, therefore, could not reasonably be assigned to a neighborhood group). A geographical mapping program was then used to assign each of the remaining 370 survey participants to 17 neighborhoods. These neighborhoods were divided into four blocks: four “large” public-housing neighborhoods that included between 41 and 60 women; two “small” public-housing neighborhoods that included between 18 and 20 women; four “large” non-public-housing neighborhoods that included between 17 and 31 women; and seven “small” non-public-housing neighborhoods that included between 7 and 12 women. We randomized the 17 neighborhoods to an intervention or control arm within each of the four blocks. All participants in the same neighborhood were, therefore, randomized together to one of the two arms. We adopted this group-randomization scheme because the intervention included small group meetings in neighborhood settings. The block-randomization scheme was employed in order to approximately balance the total number of participants and the number of public-housing residents between the two arms.
2.1.4. Follow-up survey
We attempted to survey women in the intervention and control groups a second time approximately 1 year after their first survey. As at baseline, questionnaire items were developed in English, translated into Khmer, back-translated to insure lexical equivalence, reconciled, and pre-tested; and the interviewers were all bilingual, bicultural Cambodian women [18]. Eleven follow-up survey contact attempts (including at least one evening and one weekend attempt) were made as needed for each participant. Contact information for friends and relatives (provided at the time of the baseline survey), the National Change of Address System, and the most recent telephone book for Metropolitan Seattle were used in attempts to trace women who moved in the interval between the two surveys. Identical procedures were followed for the intervention and control groups (regardless of whether women in the experimental group had received the intervention program).
As in the baseline survey, women were asked whether they had ever had a Pap test and, if so, whether they had been screened in the last 12 months. Respondents were also asked knowledge and belief questions relevant to the educational content of the intervention program. Specifically, they were asked the following: Do you think a woman needs a Pap smear if she is not sleeping with a man? Do you think a woman needs a Pap smear after menopause? Do you think a woman needs a Pap smear if she has no health problems? Do you think Pap smears could help you live longer? Do you think Pap smears are embarrassing? Do you think Pap smears are painful or uncomfortable? Are you afraid of having Pap smears? and Do you think Cambodian or American women are more likely to get cervical cancer?
Finally, several follow-up survey questions aimed to assess a possible intervention effect from our baseline survey as well as diffusion of our intervention program to the control group. Women were asked: Have you been shown a video about Pap testing? Has anyone visited your home to talk with you about Pap testing? and Have you been to a meeting where Pap testing was discussed?
The follow-up survey response is summarized in Table 1. There was a statistically significant difference between the proportions of intervention and control women who completed the follow-up survey (P < 0.05). This differential loss to follow-up was largely the result of unexpected closures in one public-housing area that forced intervention group women to move.
Table 1.
Summary of follow-up survey response
| Intervention group (N = 196)
|
Control group (N = 174)
|
|||
|---|---|---|---|---|
| Final survey disposition | n | Percentage | n | Percentage |
| Completed | 144 | 73 | 145 | 83 |
| Refused | 20 | 10 | 16 | 9 |
| Unable to contact | 5 | 3 | 1 | 1 |
| Moved | 27 | 14 | 12 | 7 |
2.1.5. Medical record requests
Those women who responded “yes” to the follow-up survey question addressing cervical cancer screening in the last 12 months were asked to provide information about the month and year of their last screening, as well as information about the clinic or doctor’s office where testing was performed. Each woman was then asked to sign a medical release form giving project staff permission to request medical record verification of her self-reported Pap test.
2.2. Intervention program
2.2.1. Overview
Low levels of acculturation, limited English-language proficiency, and scant knowledge of Western medicine may preclude Southeast Asians from receiving and understanding publicly disseminated information [19,20]. Further, Southeast Asians often have feelings of alienation and social isolation from the general US society, tend to stay close to their own neighborhoods, and often need individualized assistance accessing preventive services [19]. Therefore, we chose to implement a neighborhood-based intervention program that was delivered by bilingual, bicultural outreach workers from Seattle’s Cambodian community. The components of our multi-faceted intervention program are shown in Fig. 2. Women in the intervention group received the program sequentially over a period of approximately 9 months.
Fig. 2.
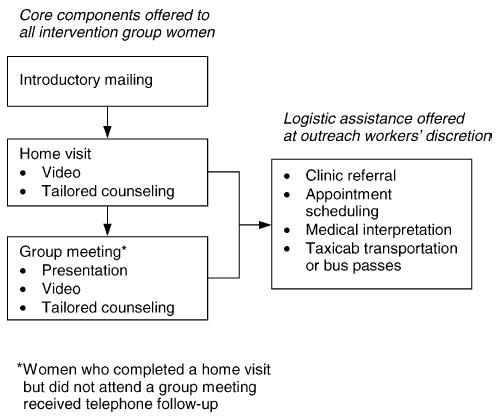
Summary of outreach worker intervention.
2.2.2. Home visits
Between 1 and 4 weeks after an introductory mailing, intervention women were visited at home by one of four bicultural, bilingual female outreach workers. If the woman was home and did not refuse intervention, the outreach worker either completed an in-home educational visit or arranged to return at a more convenient time. Ten attempts were made to contact each woman. Following a model developed by the Community House Calls program, the outreach workers were trained to act as role models, provide social support, and serve as cultural mediators between women and health care facilities [21]. They were also trained to use visual aids and provide tailored responses to each woman’s individual barriers to cervical cancer screening [22]. For example, if a woman indicated she did not believe Cambodian women are susceptible to cervical cancer, the outreach worker could show graphs documenting high cervical cancer rates and low Pap testing levels among Southeast Asian women. During each home visit, the outreach worker asked the woman if they would watch a Khmer-language video about Pap testing together. A portable VCR was available for use in the small proportion of Cambodian homes without VCR equipment. Finally, the outreach workers offered the following tailored logistic assistance, as necessary: clinic referral and assistance with appointment scheduling, medical interpretation during clinic visits for Pap testing, and transportation assistance (i.e. taxicab transportation to and from clinic appointments or two bus passes) [16,23,24].
2.2.3. Group meetings
Outreach workers routinely invited each intervention woman to one of several group meetings scheduled in their neighborhood after completing a home visit. These meetings were held at local community centers and light refreshments were provided. During the meetings, outreach workers made a short presentation about cervical cancer and Pap testing using visual aids (i.e. an anatomy model, a speculum and Pap testing kit, a photograph of an Asian American female physician performing a Pap test, and graphs showing high cervical cancer and low Pap testing rates among Southeast Asians). In addition, as during home visits, the video was shown, tailored responses were used to address barriers, and logistic assistance was offered, if appropriate [22,23]. Outreach workers attempted to provide telephone follow-up to intervention women who completed a home visit, but did not attend a group meeting.
2.3. Data analysis
Our primary outcome measure was self-reported Pap testing in the previous year. Secondary outcome measures included self-reports with respect to at least one previous Pap smear (i.e. “ever” versus “never” screened) as well as medical record verification of recent cervical cancer screening. We conducted an “intent-to-treat” analysis of our trial and included all randomized women with follow-up data, regardless of whether women in the experimental group had received the intervention program components [25]. In bivariate comparisons, we used χ2 tests, McNemar’s tests, and Fisher’s exact tests (when necessary) [26].
To evaluate our intervention effects on the primary outcome formally, we used a generalized linear mixed model for binary outcomes that took into account both the block- and group-randomized design of our intervention study as random effects [27,28]. The log odds of having a self-reported Pap test in the previous year were assessed between and within the intervention and control arms. In contrast to large individually-randomized trials, a group-randomized study of a small number of units (17 in this study) is subject to potential imbalance between arms with respect to baseline characteristics and follow-up completion. Indeed, we observed differential rates in the follow-up completion between the two arms (see above). To adjust for the imbalance, we used the predicted log odds of having a self-reported Pap test in the previous year (from a logistic regression model constructed previously with a set of baseline characteristics) as an “offset” term [17,29]. With the “offset” term calculated for each participant, we were able to estimate the change in odds of having a self-reported Pap test in the last 12 months at follow-up from that predicted at baseline in each arm. The difference between the two arms in the odds change was estimated and tested as our main evaluation of the intervention effect.
We used χ2 and Fisher’s exact tests to examine any differences with respect to cervical cancer screening knowledge and beliefs between intervention and control group women at follow-up [26].
3. Results
3.1. Study group characteristics
Nearly three-quarters (74%) of the study group completed their baseline survey in Khmer; the remaining participants completed the questionnaire in English. The baseline characteristics of the women who completed the follow-up survey are shown in Table 2. There were no statistically significant differences between the intervention and control groups with respect to age, marital status, education, housing type, years since immigration, and age at immigration. However, the difference between the proportion of intervention (56%) and control (66%) women who lived in public-housing was of borderline significance (P = 0.08). The two groups had similar Pap testing histories (i.e. there were no significant differences in the proportion of women who had ever and recently been screened for cervical cancer). The intraclass correlation within the designated neighborhoods was 0.001 for ever having had a Pap test and <0.001 for recent Pap testing.
Table 2.
Baseline characteristics of women with follow-up data
| Intervention group (N = 144)
|
Control group (N = 145)
|
|||
|---|---|---|---|---|
| Characteristic | n | Percentage | n | Percentage |
| Sociodemographics | ||||
| Age (years) | ||||
| 18–39 | 48 | 34 | 48 | 33 |
| 40–59 | 67 | 47 | 68 | 47 |
| 60+ | 28 | 20 | 28 | 19 |
| Marital status | ||||
| Never married | 23 | 16 | 24 | 17 |
| Currently married | 67 | 47 | 65 | 45 |
| Previously married | 52 | 37 | 55 | 38 |
| Any formal education | 78 | 58 | 77 | 54 |
| Public-housing resident | 80 | 56 | 95 | 66 |
| 10+ years since immigration | 121 | 84 | 123 | 86 |
| <40 years of age at immigration | 93 | 65 | 93 | 65 |
| Pap testing behavior | ||||
| Ever screened | 108 | 75 | 112 | 77 |
| Screened within the previous year | 64 | 44 | 74 | 51 |
3.2. Outcome evaluation
3.2.1. Bivariate results
As shown in Table 3, the proportion of women in the intervention group reporting recent cervical cancer screening increased from 44% at baseline to 61% at follow-up (+17%). The corresponding proportions among the control group were 51 and 62% (+11%). These temporal increases were statistically significant in both the intervention (P < 0.001) and control (P = 0.027) groups. Similarly, the proportions of intervention and control women reporting they had ever been screened for cervical cancer increased from 75 to 85% (+10%, P = 0.011) and from 77 to 84% (+7%, P = 0.059). Self-reported Pap testing levels for recently screened and ever screened were almost identical in the two groups at follow-up (P = 0.83 and 0.91, respectively).
Table 3.
Self-reported Pap testing behavior at baseline and follow-up
| Study group
|
|||
|---|---|---|---|
| Variable | Intervention (N = 144), n (%) | Control (N = 145), n (%) | Difference between intervention and control groups (%) |
| Pap test in the last 12 months | |||
| Baseline | 64 (44) | 74 (51) | −7 |
| Follow-up | 87 (61) | 90 (62) | −1 |
| Change | (+17) | (+11) | +6 |
| Ever had a Pap test | |||
| Baseline | 108 (75) | 112 (77) | −2 |
| Follow-up | 121 (85) | 122 (84) | +1 |
| Change | (+10) | (+7) | +3 |
3.2.2. Model-based results
Based on the generalized linear mixed model analysis, the odds of having a self-reported Pap test in the previous year increased at follow-up from that predicted at baseline by a factor of 2.28 (95% confidence interval 1.23–4.23, P = 0.012) in the intervention arm and 2.32 (95% confidence interval 1.22–4.42, P = 0.015) in the control arm. There was no difference in these odds increases between the two arms: the increase of the odds in the intervention group was 0.98 (95% confidence interval 0.40–2.40, P = 0.97) times the increase of the odds in the control group.
3.2.3. Medical record verification
We were able to request medical records for 82 (94%) of the 87 intervention group women and 84 (93%) of the 90 control group women who reported having received a Pap test in the previous 12 months. The remaining women either chose not to sign the medical release form or were unable to provide sufficient information about the health care facility where they had been screened. The outcomes of our medical record requests are given in Table 4. We were able to confirm womens’ self-reports of recent Pap testing for 58% of the intervention group and 54% of the control group. There were no statistically significant differences between the two groups with respect to the outcomes of our medical record verification (P = 0.50).
Table 4.
Medical record verification of recent Pap testing
| Outcome of medical record requests | Intervention group (N = 82), n (%) | Control group (N = 84), n (%) |
|---|---|---|
| Cytology report received confirming Pap testing in the previous 12 months | 48 (59) | 45 (54) |
| Cytology report received indicating last Pap test was performed more than 12 months prior to follow-up survey | 18 (22) | 17 (20) |
| Clinic reported no record of Pap testing | 11 (13) | 11 (13) |
| Clinic reported no record of patient | 5 (6) | 11 (13) |
3.2.4. Knowledge and beliefs
At follow-up, intervention group women were more likely to believe Pap testing is embarrassing than control women (P = 0.04). There were no other significant differences between the two groups for the knowledge and belief items.
3.3. Process evaluation
One hundred and fifty-four (79%) of the 196 women in the intervention arm completed a home visit. Twenty-seven (14%) had moved since the baseline survey, 6 (3%) could not be contacted after 10 attempts, and 9 (5%) refused a home visit. Sixty-one (40%) of the 154 women who completed a home visit also attended a group meeting and 65 (42%) completed follow-up telephone calls. The outreach workers showed the video to 88 (57%) of the women during completed home visits or group meetings. Finally, 47 (31%) of the women received one or more forms of logistic assistance (clinic referral—42, assistance with appointment scheduling—16, medical interpretation at a clinic visit—9, and transportation assistance—5). Among the control group, 8% reported they had been shown a video about Pap testing, 33% stated someone had visited them at home to talk about Pap testing, and 8% said they had been to a meeting where Pap testing was discussed at follow-up.
4. Discussion
The proportion of Cambodian American women reporting Pap testing in the previous 12 months increased significantly in both our intervention and control neighborhoods during the study period. Further, improvements in screening rates were similar in the intervention and control arms. There are several possible explanations for these findings. First, it is likely that our baseline survey had an intervention effect (at follow-up, one-third of the control group reported that someone had visited them at home to talk about Pap testing) [30]. Second, the Cambodian community in Seattle is relatively small and concentrated in certain areas of the city. Qualitative information from our Cambodian staff members suggests that the project was widely discussed (e.g. Cambodian women approached our outreach workers and asked them questions about Pap testing in community settings such as grocery stores). It is possible that general awareness about the project contributed to the observed increases in recent Pap testing among intervention and control women. Gotay et al. have pointed out that while such contamination is a problem for researchers, from the public health perspective, it represents a powerful tool. This Hawaii-based research group suggested that using social networks and family constellations for health education in close-knit ethnic minority communities is a promising strategy [31].
The majority of Cambodians in Seattle receive their health care at the city’s county hospital or one of several community clinics. Many of the physicians practicing at these health care facilities were aware of the study and efforts to promote cervical cancer screening in the Cambodian community. This awareness could also have contributed to the changes in Pap testing rates during the study period. Finally, the Seattle/King County Health Department participates in the national Breast and Cervical Cancer Control Program and specifically targets lower income residents of central and south Seattle. Seventeen Cambodian women enrolled in this program between the beginning of our baseline survey and the end of our follow-up survey (Lin Song PhD, Public Health—Seattle/King County, personal communication). However, we do not have information about where these women lived (i.e. whether they lived in our study neighborhoods).
Other investigators have found that home visits can be effective in increasing the uptake of cervical cancer screening among Asian immigrants to Western countries. McAvoy and Raza conducted a randomized controlled trial to evaluate the effects of health education on the uptake of cervical cancer screening among non-English speaking women, originally from the Indian sub-continent. Women with no record of Pap testing were randomized to one of three experimental groups or control status. Nearly one-half (47%) of women who were shown a video during a home visit by a bicultural, bilingual outreach worker adhered to screening recommendations compared to 37% who were visited and given a leaflet. In contrast only 5% of women who were not contacted and 11% of women who were sent a leaflet in the mail completed a Pap smear [15].
A study conducted by McPhee et al. is also relevant to this report. Specifically, this research group documented the effectiveness of small-group cervical cancer screening educational sessions given by indigenous Vietnamese lay health workers in California. Educational sessions were provided to Vietnamese women in the Tenderloin District of San Francisco while Vietnamese women in central Sacramento served as controls. The proportions of women reporting at least one previous Pap smear at baseline were similar in the two communities (46% in San Francisco and 40% in Sacramento). At follow-up, a significantly higher proportion of San Francisco respondents reported they had ever received Pap testing (66% versus 42% in Sacramento) [14].
Other research groups have examined why community-based efforts to promote cervical cancer screening have often had disappointing results [32,33]. Suarez et al. have suggested that program exposures might be too brief or lack sufficient intensity [33]. While we found that in-home educational sessions are feasible and acceptable to Cambodian women (<10% of reachable women refused a home visit), only a minority chose to watch the video with an outreach worker or attend a group meeting. Additionally, Sung et al. have pointed out that promoting disease prevention in extremely disadvantaged populations, such as Cambodian Americans, is particularly difficult because of the low priority given to preventive services. Understandably, members of such communities are often primarily concerned with more immediate medical and social service needs [30].
Nguyen et al. have recently reported that the accuracy of Pap testing self-report is higher in Whites (84%) than in Chinese (66%) or Filipino (67%) women [34]. We were only able to confirm the self-reported recent cervical cancer screening behavior of 56% of the Cambodian women who participated in our follow-up survey. In some instances where we were unable to verify Pap smear self-reports, women may actually have been screened. For example, Cambodian naming systems can lead to problems finding medical charts [35]. However, 21% of women who self-reported a Pap test in the last year were found to have received their last screening over 12 months before they completed their follow-up survey. Asian societies place a very high value on politeness. Therefore, the over-reporting of a behavior perceived as being desirable to others may be more of an issue in Asian American populations than other groups.
In conclusion, studies have previously shown that community-based cancer control interventions delivered by outreach workers and lay health workers have significant potential [14,15,36]. However, we were unable to document an increase in Pap testing participation specifically in the neighborhood-based outreach intervention group; rather, we found an increase in both intervention and control groups. Participation in our baseline survey, a general awareness of the project among Cambodian women as well as their health care providers, and other ongoing cervical cancer screening promotional efforts may all have contributed to increases in Pap testing rates. We did not ask women why they had their last Pap test on our follow-up survey. Our findings reinforce the importance of including survey items about “cues to action” when evaluating cancer control interventions. Finally, our results indicate self-reports may not be a valid measure of screening participation in Cambodian communities.
Acknowledgments
Supported by grant #70922 and cooperative agreement #86322 from the National Cancer Institute.
References
- 1.Kulig JC. Sexuality beliefs among Cambodians: implications for health care providers. Health Care Women Int. 1994;15:69–74. doi: 10.1080/07399339409516096. [DOI] [PubMed] [Google Scholar]
- 2.Ouch C, Park Y. The Cambodian community in the United States. Seattle: The Cross Cultural Health Care Program; 2000. [Google Scholar]
- 3.Department of Commerce. We the Asian Americans. Washington, DC: Department of Commerce; 1993. [Google Scholar]
- 4.Kiernan B. Genocide and democracy in Cambodia: the Khmer Rouge, the United States, and the international community. New Haven: Yale University Press; 1993. [Google Scholar]
- 5.Frye BA. Cultural themes in health care decision-making among Cambodian refugee women. J Community Health Nurs. 1991;8:33–44. doi: 10.1207/s15327655jchn0801_4. [DOI] [PubMed] [Google Scholar]
- 6.Uba L. Cultural barriers to health care for Southeast Asian refugees in the US. Am J Public Health. 1992;107:544–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Department of Health and Human Services. Racial and ethnic disparities in health. Washington, DC: Department of Health and Human Services; 1998. [Google Scholar]
- 8.Perkins CI, Morris CR, Wright WE, et al. Cancer incidence and mortality in California by detailed race/ethnicity. Sacramento: California Department of Health Services; 1995. [Google Scholar]
- 9.Kelly AW, Chacori MDF, Wollan PC, et al. A program to increase breast and cervical cancer screening for Cambodian women in a mid-Western community. Mayo Clin Proc. 1996;71:437–44. doi: 10.4065/71.5.437. [DOI] [PubMed] [Google Scholar]
- 10.Yi JK. Factors affecting cervical cancer screening among Cambodian women in Houston. Texas Fam Community Health. 1996;18:49–57. [Google Scholar]
- 11.Chen MS. The indigenous model and its application to heart health for Southeast Asians. Health Educ. 1989;20:48–51. [PubMed] [Google Scholar]
- 12.Giblin PT. Effective utilization and evaluation of indigenous health care workers. Public Health Rep. 1989;104:361–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Sung JFC, Cootes RJ, Williams JE, et al. Cancer screening education among Black women in inner-city Atlanta. Public Health Rep. 1992;107:381–8. [PMC free article] [PubMed] [Google Scholar]
- 14.Bird JA, McPhee SJ, Ha NT, et al. Opening pathways to cancer screening for Vietnamese American women: lay health workers hold the key. Prev Med. 1998;27:821–9. doi: 10.1006/pmed.1998.0365. [DOI] [PubMed] [Google Scholar]
- 15.McAvoy BR, Raza R. Can health education increase uptake of cervical smear testing among Asian women. Br Med J. 1991;302:833–6. doi: 10.1136/bmj.302.6780.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor VM, Jackson JC, Schwartz SM, et al. Cervical cancer control in a Cambodian American population. Asian Am Pac Isl J Health. 1998;6:241–7. [PubMed] [Google Scholar]
- 17.Taylor VM, Schwartz SM, Jackson JC, et al. Cervical cancer screening among Cambodian American women. Cancer Epidemiol Biomark Prev. 1999;8:541–6. [PubMed] [Google Scholar]
- 18.Eyton J, Neuwirth G. Cross-cultural validity: ethnocentrism in health studies with special reference to the Vietnamese. Soc Sci Med. 1984;18:447–53. doi: 10.1016/0277-9536(84)90061-3. [DOI] [PubMed] [Google Scholar]
- 19.McPhee SJ, Bird JA, Ha NT, et al. Pathways to early cancer detection for Vietnamese women: health is gold. Health Educ Q Suppl. 1996;23:60–75. [Google Scholar]
- 20.Nguyen MD. Culture shock—a review of Vietnamese culture and its concepts of health and disease. West J Med. 1985;142:409–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson-Carroll LM, Jackson JC, Graham E. Seattle: Harborview Medical Center; 1995. Beyond medical interpretation: the role of interpreter cultural mediators (ICMS)—selecting, training, and supporting the outreach staff. [Google Scholar]
- 22.King ES, Rimer BK, Seay J, et al. Promoting mammography use through progressive interventions: is it effective? Am J Public Health. 1994;84:104–6. doi: 10.2105/ajph.84.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson JC, Taylor VM, Chitnarong K, et al. Development of a cervical cancer control intervention program targeting Cambodian American women. J Community Health. 2000;25:359–77. doi: 10.1023/a:1005123700284. [DOI] [PubMed] [Google Scholar]
- 24.Mahloch J, Jackson JC, Chitnarong K, et al. Bridging cultures through the development of a Cambodian cervical cancer screening video. J Cancer Educ. 1999;14:109–14. doi: 10.1080/08858199909528591. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein AR. Intention-to-treat policy for analyzing randomized trials: statistical distortions and neglected clinical challenges. In: Cramer JA, Spilker B, editors. Patient compliance in medical practice and clinical trials. New York: Raven Press; 1991. [Google Scholar]
- 26.Rosner B. Fundamentals of biostatistics. Boston: Duxbury; 1995. [Google Scholar]
- 27.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J Am Stat Assoc. 1993;88:1065–73. [Google Scholar]
- 28.Murray DM. Design and analysis of group-randomized trials. New York: Oxford University Press; 1998. [Google Scholar]
- 29.McCullagh, Nelder JA. Generalized linear models. London: Chapman & Hall; 1989. [Google Scholar]
- 30.Sung JEC, Blumenthal DS, Cootes RJ, et al. Effect of a cancer screening intervention conducted by lay health workers among inner-city women. Am J Prev Med. 1997;13:51–7. [PubMed] [Google Scholar]
- 31.Gotay CC, Banner RO, Matsunaga DS, et al. Impact of a culturally appropriate intervention on breast and cervical cancer screening among native Hawaiian women. Prev Med. 2000;31:529–37. doi: 10.1006/pmed.2000.0732. [DOI] [PubMed] [Google Scholar]
- 32.Marcus AC, Crane LA. A review of cervical cancer screening intervention research: implications for public health programs and future research. Prev Med. 1998;27:13–31. doi: 10.1006/pmed.1997.0251. [DOI] [PubMed] [Google Scholar]
- 33.Suarez L, Roche RA, Pulley L, et al. Why a peer intervention program for Mexican American women failed to modify the secular trend. Am J Prev Med. 1997;13:411–7. [PubMed] [Google Scholar]
- 34.Nguyen TT, McPhee SJ, Somkin CP, et al. How valid are Pap smear and mammogram reports in a multi-ethnic population? J Gen Intern Med. 2001;16:160. [Google Scholar]
- 35.Schwartz SM, Taylor VM, Kuniyuki A, et al. Development and application of a database of Cambodian names for cancer prevention research; Presented at the American Public Health Association annual meeting; Chicago. 1999. [Google Scholar]
- 36.Navarro AM, Senn KL, McNicholas LJ, et al. Por La Vida model enhances use of cancer screening teats among Latins. Am J Prev Med. 1998;15:32–41. doi: 10.1016/s0749-3797(98)00023-3. [DOI] [PubMed] [Google Scholar]


