Abstract
OBJECTIVES
Depression is common in persons with HIV infection and with alcohol problems, and it has important prognostic implications. Neurocognitive dysfunction has been reported with chronic hepatitis C virus (HCV) infection. We hypothesized that HCV infection is associated with more depressive symptoms in HIV-infected persons with a history of alcohol problems.
METHODS
We performed a cross-sectional analysis of baseline data from a prospective cohort study of 391 HIV-infected subjects with a history of alcohol problems, of whom 59% were HCV antibody (Ab) positive and 49% were HCV RNA-positive. We assessed depressive symptoms (Center for Epidemiologic Studies Depression [CES-D]) and past month alcohol consumption. In the primary analysis, we evaluated whether there were more depressive symptoms in HCV Ab-positive and RNA-positive subjects in unadjusted analyses and adjusting for alcohol consumption, gender, age, race, CD4 count, homelessness, drug dependence, and medical comorbidity.
RESULTS
Mean CES-D scores were higher in subjects who were HCV Ab-positive compared with those who were HCV Ab-negative (24.3 vs 19.0; p < 0.001). In adjusted analyses, the difference in CES-D scores between HCV Ab-positive and Ab-negative subjects persisted (24.0 vs 19.0; p < 0.001). Unadjusted mean CES-D scores were also significantly higher in HCV RNA-positive subjects compared with those who were RNA-negative, and the difference remained significant (24.6 vs 19.3; p < 0.001) in adjusted analyses.
CONCLUSIONS
HCV/HIV coinfected persons with a history of alcohol problems have more depressive symptoms than those without HCV, and this association is unexplained by a variety of population characteristics. These data suggest that HCV may have a direct effect on neuropsychiatric function.
INTRODUCTION
Depression in HIV-infected patients is a common but under-diagnosed condition with important prognostic implications (1). Depressive symptoms have been associated with poor medication adherence, more rapid HIV disease progression, and increased mortality (2). Better understanding of the factors contributing to depression and its detrimental effect on the course of HIV infection may be gained by studying the effect of significant comorbidities.
Alcohol use and hepatitis C virus (HCV) infection are also common in HIV-infected patients, particularly those with a history of injection drug use. Alcohol use is clearly associated with depression and may exacerbate it (3, 4). Chronic HCV infection has been associated with neurocognitive symptoms, perhaps mediated by a direct effect on the central nervous system (5). Interferon, a component of the treatment regimen for HCV infection, can worsen depressive symptoms (6).
In order to better understand the relation between HCV infection and depressive symptoms in the context of HIV disease, we studied a cohort of HIV-infected patients with a history of alcohol problems. We tested the hypothesis that HCV infection is associated with more depressive symptoms in these HIV-infected subjects.
METHODS
Subject Recruitment
Study subjects were participants in the HIV-LIVE (HIV-Longitudinal Interrelationships of Viruses and Ethanol) study, a prospective, observational cohort study of HIV-infected patients with past or current alcohol problems. The present study is a cross-sectional analysis of data collected at entry into the HIV-LIVE cohort.
A total of 401 subjects were recruited from several different sources including: (1) a previous cohort study of people with HIV and alcohol problems (N = 154, 38%) (7); (2) the Diagnostic Evaluation Unit (DEU), an intake clinic for HIV-infected patients at Boston Medical Center (BMC) (N = 88, 22%) (8); (3) the HIV Primary Care and Specialty Clinics at Beth Israel Deaconess Medical Center (BIDMC) (N = 31, 8%); and (4) additional health care centers, homeless shelters, drug treatment programs, other studies, subject referrals, and flyers (N = 128, 32%). Enrollment began in August 2001, and ended in July 2003.
Eligibility criteria for the study included the following:
Documented HIV Ab test by ELISA and confirmed by Western blot (medical record or tested at enrollment).
Two or more affirmative responses to the CAGE alcohol screening questionnaire (9, 10) or physician-investigator diagnosis of alcoholism.
Ability to speak English or Spanish.
At least one contact person who was likely to know the subject’s whereabouts.
Exclusion criteria included: (1) scoring <21 on the 30-item Folstein Mini-Mental State Examination (MMSE) (11); and (2) a trained interviewer assessment that the patient was incapable of comprehending informed consent or of answering the interview questions.
If an eligible individual agreed to participate in this study, a research associate scheduled an appointment for the first interview at BMC’s General Clinical Research Center (GCRC) or BIDMC’s Clinical Research Center (CRC). All subjects who met the eligibility criteria and wished to participate in the study provided written informed consent prior to enrollment. The Institutional Review Boards of BMC and BIDMC approved this study. Additional privacy protection was secured by the issuance of a Certificate of Confidentiality by the Department of Health and Human Services to protect subjects from release of their research data even under a court order or subpoena.
Subject Assessment
After enrollment, subjects received an interviewer-administered assessment. The assessment included questions on the following: demographics; depressive symptoms (Center for Epidemiologic Studies Depression [CES-D] scale) (12); medical comorbidity by a validated interview measure (13); current and lifetime alcohol use and dependence (Composite International Diagnostic Interview [CIDI]) (14); current drug dependence (CIDI Short Form); and HIV risk behaviors (Risk Assessment Battery [RAB], modified version) (15). Past month alcohol consumption was assessed using a validated calendar method (16). Heavy alcohol consumption was defined as more than 14 drinks per week or more than 4 drinks on any one occasion for men aged 65 yr and younger; or more than 7 drinks per week or more than 3 drinks on any one occasion for women and anyone over the age of 65 yr. Moderate use was defined as 1 or more drinks in the past 30 days but less than the “heavy” category. Abstinent was defined as no drinks in the past 30 days. Homelessness was defined as having spent at least one night either on the street or in a shelter in the 6 months prior to the interview.
All subjects in this cohort were Ab tested for HCV infection. Those who were Ab-positive had HCV testing by RNA measurement using polymerase chain reaction testing to verify the presence of active infection.
Primary Outcome
The primary study outcome was depressive symptoms, which were assessed using the CES-D (12). The CES-D is a short self-report tool intended to assess depressive symptoms in the general population. It consists of 20 questions concerning mood and behavior over the past week with results reported as rarely or none of the time (<1 day), some or a little of the time (1–2 days), occasionally or a moderate amount of the time (3–4 days), or most or all of the time (5–7 days). CES-D scores can range from 0 to 60. Higher CES-D scores reflect the presence of more depressive symptoms.
Primary Independent Variable
The main independent variable was HCV status, which was defined in two ways: (1) HCV Ab-positive versus Ab-negative and (2) HCV RNA-positive versus RNA-negative. Examination of the independent variable in this manner was deemed important to identify a potential biologic effect of HCV infection on depressive symptoms. For the purpose of the analysis, HCV Ab-negative subjects were assumed to be HCV RNA-negative (17).
Statistical Analyses
χ2 and Wilcoxon rank sum tests were used to compare subject characteristics by HCV serologic status. Multiple linear regression models were used to assess the cross-sectional association between HCV infection and depressive symptoms. Separate analyses were performed for each method of defining HCV status. Covariates examined included alcohol consumption (abstinent vs moderate vs heavy) (18), gender, age, race (black vs white vs Hispanic vs other), CD4 cell count, homelessness (yes vs no), diagnosis of drug dependence (yes vs no), and medical comorbidity. Self-reported information was available on whether subjects ever used injection drugs. However, this variable was highly correlated with HCV infection status, whereas drug dependence diagnosis was not. Thus, drug dependence diagnosis was included as the covariate in regression analyses to avoid potential collinearity.
Secondary analyses were conducted modeling CES-D as a binary outcome (CES-D ≥ 23 vs CES-D < 23) and also modeling CES-D as a continuous outcome excluding those questions (1, 5, 7, 11, 20) that reflect somatic symptoms. Additional analyses were conducted to assess the following potential confounders: MMSE; ever received interferon therapy; educational level (high school vs not); employment status (yes vs no); income level (above vs below median); and current injection drug use (within 6 months). To assess the potential bias from including subjects who were previously on interferon therapy, the primary analysis was repeated excluding those subjects.
All analyses were conducted using two-sided significance tests defining p < 0.05 as statistically significant. Analyses were performed using SAS software (version 8.2; SAS Institute, Cary, NC).
RESULTS
Of the 401 HIV-infected subjects with current or past alcohol problems enrolled in the HIV-LIVE cohort, 391 had available HCV Ab test results. Of these 391 subjects, 231 (59%) were HCV Ab-positive (Fig. 1). Of the 213 HCV Ab-positive subjects who were tested for RNA, 183 (86%) had a detectable level. One additional subject did not have available HCV Ab results, but tested HCV RNA-negative and was included only in the HCV RNA analyses. Only one HCV-infected subject in the study was receiving interferon therapy at baseline, and only 17 of 231 (7.4%) HCV Ab-positive subjects had received interferon therapy ever. Of those 17 subjects, 10 had received it for more than 3 months, and 6 had received it for more than 6 months.
Figure 1.
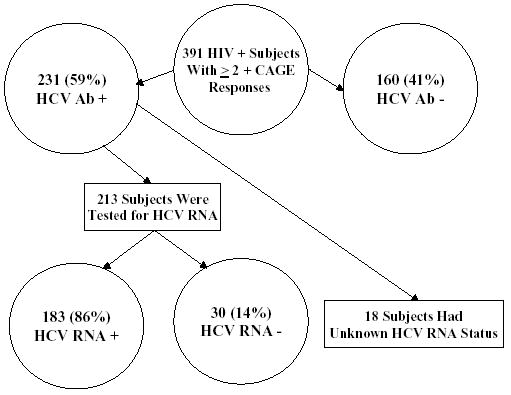
Hepatitis C serologic status of a cohort of HIV-infected subjects with current or past alcohol problems.
Characteristics of the cohort reflected the urban setting of this study: 75% were men with a median age of 42 yr; 41% were black, 33% white, and 19% Hispanic; 25% were homeless; and 43% met criteria for current drug dependence (past 12 months). Thirty-two percent reported heavy alcohol consumption, 11% had moderate alcohol consumption, and 58% were abstinent in the past 30 days. The median CD4 cell count was 402/mm3 (interquartile range 241–624/mm3), and the median HIV log RNA was 2.9 copies/mL (interquartile range 0.0–4.1 copies/mL). The median number of medical comorbidities was 1 (interquartile range 0–6).
Characteristics of subjects who were HCV Ab-positive versus those who were HCV Ab-negative are listed in Table 1. HCV Ab-positive subjects were more likely to be men, older, homeless, abstinent from alcohol, have injection drug use as their primary HIV-risk behavior, and have a lower MMSE score. HCV Ab-positive subjects also had a lower median CD4 cell count and more medical comorbidity.
Table 1.
Characteristics of HIV-Infected Subjects with Current or Past Alcohol Problems
| Characteristic | HCV Ab-Positive (N = 231) | HCV Ab-Negative (N = 160) |
|---|---|---|
| Male, N (%)* | 165 (71%) | 130 (81%) |
| Median (IQR) age* | 44.4 (39.7, 48.1) | 39.9 (35.6, 45.7) |
| Race, N (%) | ||
| Black | 87 (38%) | 75 (47%) |
| White | 77 (33%) | 52 (33%) |
| Hispanic | 53 (23%) | 21 (13%) |
| Other | 14 (6%) | 12 (7%) |
| Homelessness, N (%)* | 69 (30%) | 29 (18%) |
| Drug dependence, N (%) | 101 (44%) | 65 (41%) |
| Alcohol consumption, N (%)* | ||
| Abstinent | 145 (63%) | 78 (49%) |
| Moderate | 18 (8%) | 23 (14%) |
| Heavy | 68 (29%) | 58 (37%) |
| Primary HIV risk behavior, N (%)* | ||
| Injection drug use | 156 (75%) | 12 (8%) |
| Men sex with men | 9 (4%) | 75 (50%) |
| Other | 44 (21%) | 64 (42%) |
| Median (IQR) CD4 cell count* | 362 (232, 546) | 472 (291, 698) |
| Median (IQR) HIV log RNA | 3.0 (0, 4.1) | 2.9 (0, 4.1) |
| Median (IQR) medical comorbidity* | 2 (0, 6) | 1 (0, 6) |
| Receiving interferon therapy, N (%) | 1 (0.4%) | 0 (0.0%) |
| Mean (SD) MMSE score* | 26.94 (2.34) | 27.64 (2.14) |
p < 0.05.
IQR = interquartile range; SD = standard deviation.
Unadjusted mean CES-D scores were higher in the 231 subjects who were HCV Ab-positive compared with the 160 who were Ab-negative (24.3 vs 19.0; p = 0.001) (Table 2). In adjusted analyses, the difference in CES-D scores between HCV Ab-positive and Ab-negative subjects remained significant (24.0 vs 19.0; p = 0.001). Unadjusted mean CES-D scores were significantly higher in the 183 HCV RNA-positive subjects compared with the 191 who were RNA-negative (24.8 vs 19.2; p < 0.001) (Table 3). The difference in CES-D scores remained significant (24.6 vs 19.3; p < 0.001) in adjusted analyses.
Table 2.
Bivariate and Multivariable Analysis of the Impact of HCV Antibody Status on Depressive Symptoms
| Mean Depressive Symptoms (SE)Unadjusted | Mean Depressive Symptoms (SE)Adjusted* | |
|---|---|---|
| HCV Ab-positive (N = 231) | 24.3 (0.88) | 24.0 (0.86) |
| HCV Ab-negative (N = 160) | 19.0 (0.90) | 19.0 (1.04) |
| P-value | <0.001 | <0.001 |
Bivariate and multivariable analysis of the impact of HCV antibody status on depressive symptoms is measured by CES-D score.
Adjusted for alcohol consumption, gender, age, race, CD4 count, homelessness, drug dependence, and medical comorbidity.
Table 3.
Bivariate and Mutivariable Analysis of the Impact of HCV RNA Status on Depressive Symptoms
| Mean Depressive Symptoms (SE) Unadjusted | Mean Depressive Symptoms (SE) Adjusted* | |
|---|---|---|
| HCV RNA-positive (N = 183) | 24.8 (1.00) | 24.6 (0.95) |
| HCV RNA-negative (N = 191) | 19.2 (.85) | 19.3 (0.92) |
| P-value | <0.001 | <0.001 |
Bivariate and mutivariable analysis of the impact of HCV RNA status on depressive symptoms is measured by CES-D score.
Adjusted for alcohol consumption, gender, age, race, CD4 count, homelessness, drug dependence, and medical comorbidity.
In order to assess the effect of using a clinically relevant CES-D threshold, we repeated our primary analysis with CES-D < 23 vs CES-D ≥ 23 as the dependent variable and still found a significant association between HCV status and depressive symptoms (HCV RNA, adjusted analysis: OR 2.78 [1.73, 4.49]). In order to determine whether somatically focused CES-D questions may have influenced the results, we repeated our primary analysis excluding those five questions and still found a comparable significant association between HCV status and depressive symptoms (Table 4).
Table 4.
Adjusted Mean Difference in CES-D Score in Secondary Analyses
| Secondary Analysis | HCV Ab+ vs Ab− | HCV RNA + vs RNA− |
|---|---|---|
| CES-D without somatic questions as the dependent variable | 3.4 | 3.8 |
| Primary model adjusting for MMSE | 4.7 | 4.9 |
| Primary model excluding interferon users | 5.2 | 5.2 |
| Primary model adjusting for educational level | 4.0 | 4.5 |
| Primary model adjusting for employment status | 4.3 | 4.6 |
| Primary model adjusting for income level | 4.2 | 4.6 |
| Primary model adjusting for current drug use | 5.1 | 5.3 |
Primary model examines CES-D as continuous variable and is adjusted for alcohol consumption, gender, age, race, CD4 count, homelessness, drug dependence, and medical comorbidity.
All adjusted mean differences p < 0.05.
In order to assess whether MMSE was a confounder, we repeated our primary analysis adjusting for MMSE score and still found a significant association between HCV status and depressive symptoms (Table 4). In order to determine whether prior interferon therapy may have affected the results, we repeated our primary analysis excluding subjects who had ever received interferon therapy and still found a significant association between HCV status and depressive symptoms (Table 4).
We also examined whether sociodemographic factors may have influenced the results. Our primary analysis was repeated adjusting for educational level, employment status, and income level and still showed a significant difference in CES-D scores by HCV status (Table 4). In addition, we repeated our primary analysis adjusting for self-reported current injection drug use and still found a significant difference in CES-D scores by HCV status (Table 4).
DISCUSSION
In this cohort of HIV-infected subjects with current or past alcohol problems, depressive symptoms were significantly more frequent in those coinfected with HCV. Other population characteristics, including alcohol consumption, gender, age, race, CD4 count, homelessness, drug dependence, and medical comorbidity, did not account for this observed difference. Significant differences in depressive symptoms between HCV-infected and uninfected subjects were still noted when using a clinically relevant CES-D threshold, excluding CES-D questions with somatic content, adjusting for MMSE score, excluding subjects who had ever received interferon therapy, and adjusting for additional sociodemographic factors and current injection drug use.
Depression is common in patients with chronic HCV infection, and most authors have attributed it to a psychological response to a chronic progressive medical condition or drug use itself (19–26). In one blinded study of 309 injection drug users, 57.2% of subjects with HCV infection had significant depressive symptomatology based on CES-D test results compared with 48.2% of HCV-negative controls (27). None of the HCV-infected subjects were receiving interferon therapy. However, another study comparing 295 injection drug users who were HIV+/HCV− (N = 81), HIV−/HCV+ (N = 62), and HIV−/HCV− (N = 152) found no differences in psychological morbidity on several affective scales among these groups (28).
Several studies have described an association between chronic HCV infection and neurocognitive dysfunction that appears independent of liver disease severity. Forton et al. used a computer-based cognitive battery to demonstrate selective impairments of attention, concentration, and psychomotor speed in patients without significant disease on liver biopsy (5). Fatigue, depression, or a history of drug abuse did not account for these findings. Hilsabeck et al. described neuropsychological impairment in 49% of HCV-infected patients without cirrhosis (29). McAndrews et al. evaluated a cohort of HCV-infected subjects, screened to exclude relevant comorbidities, with neuropsychological tests (30). Compared to controls, subjects with HCV infection were observed to have somewhat poorer learning ability. Ryan et al. compared coinfected subjects with advanced HIV disease to subjects without HCV infection using neurocognitive testing and psychiatric interviews (31). Forty-two percent of each group met criteria for major depression, but coinfected subjects exhibited diminished neurocognitive capabilities.
Neuroradiologic and neurophysiologic studies have indicated the possibility of an underlying biological mechanism for neurocognitive dysfunction in HCV-infected patients (32, 33). Forton et al. showed altered brain metabolism using proton magnetic resonance spectroscopy in patients with chronic HCV infection (32). Kramer et al. demonstrated mild abnormalities on neuroelectrophysiologic testing in this patient population not attributable to drug or alcohol use or cirrhosis (33). HCV genomic sequences have also been detected in postmortem brain tissues along with evidence of viral replication (34, 35). Thus, in addition to epidemiological evidence for an association between HCV infection and depressive symptoms, neuropsychological testing and physiological data from neuroimaging provide support for a potential biologic basis for this observation.
Depression affects half of the HIV-infected population at some time in the course of their disease, occurring twice as frequently as in seronegative persons (1, 36–39). However, because of the clinical focus on other complications, it may not always be diagnosed (39, 40). Depression in this patient population has been associated with decreased adherence to medical therapy (41) and increased mortality (2, 42). Recognition that HIV-infected patients who also have hepatitis C may be prone to more depressive symptoms has important management implications.
This study has several limitations. While the CES-D is a well-validated scale for depressive symptoms, use of other instruments, such as the Beck Depression Inventory, might have yielded different results. Interpretations of the importance of differences in CES-D scores vary. However, the observed differences in this study have generally been considered clinically important (2). Whether these study findings are applicable to other populations with chronic HCV infection would need to be confirmed. The cross-sectional, observational nature of this study limits our ability to establish a causal link between HCV infection and depressive symptoms. In addition, we cannot distinguish between the effects of injection drug use and HCV serostatus on depressive symptoms. An alternative, but less compelling, explanation for these findings would be that depressed persons are more likely to inject drugs, which leads to HCV infection.
In summary, HCV infection appears to be associated with more depressive symptoms in patients with HIV infection who have a history of alcohol problems. Recent literature suggests that HCV infection has a direct effect on the central nervous system, which may be responsible for this observation.
Clinicians should be alert for depressive symptoms in HIV/HCV coinfected patients and initiate treatment for depression when appropriate. Institution of antidepressant therapy may enhance medical adherence, which is key to successful antiretroviral management, and the patient’s ability to tolerate treatment for HCV infection. Researchers should focus future efforts on understanding the potential biologic reasons for the association observed in this study. Further research may better delineate the contributions of HCV and other factors in the development of depressive symptoms in HIV-infected patients.
STUDY HIGHLIGHTS
What is Current Knowledge
Depression is common in HIV-infected persons and persons with alcohol problems and has important prognostic implications.
Neurocognitive dysfunction has been reported with chronic hepatitis C virus (HCV) infection.
What is New Here
HCV/HIV co-infected persons with a history of alcohol problems have more depressive symptoms than those without HCV.
This association is unexplained by a variety of population characteristics.
HCV may have a direct effect on neuropsychiatric function.
Acknowledgments
The authors appreciate the contributions of the staff researchers on the project (Rachel Levison, Elizabeth Curley, Eileen O’Connor) and data management assistance (Vincent Faber). Support for this study came from the following grants from the National Institute of Alcohol Abuse and Alcoholism (NIAAA) of the NIH: RO1-AA13766 (Clinical Impact of HCV and Alcohol in HIV-Infected Persons), RO1-AA11785 (Medication Adherence in Alcohol Abusing HIV Patients); RO1-AA10870 (Enhanced Linkage of Alcohol Abusers to Primary Care). This research was conducted in part in the General Clinical Research Center at Boston University School of Medicine, USPHS Grant MO1 RR00533, and the Clinical Research Center at Beth Israel Deaconess Medical Center, USPHS Grant MO1 RR01032.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Howard Libman, M.D.
The authors declared no conflicts of interest.
References
- 1.Fairfield KM, Libman H, David RB, et al. Detecting depression: Providing high quality primary care for HIV-infected patients. Am J Med Qual. 2001;16:71–4. doi: 10.1177/106286060101600205. [DOI] [PubMed] [Google Scholar]
- 2.Ickovics JR, Hamburger ME, Vlahov D, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis for the HIV Epidemiology Research Study. JAMA. 2001;285:1466–74. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan LE, Fiellin DA, O’Connor PG. The prevalence and impact of alcohol problems in major depression: A systematic review. Am J Med. 2005;118:330–41. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Saitz R. Unhealthy alcohol use. N Engl J Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- 5.Forton DM, Taylor-Robinson DS, Thomas HC. Cerebral dysfunction in chronic hepatitis C infection. J Viral Hepat. 2003;10:81–6. doi: 10.1046/j.1365-2893.2003.00416.x. [DOI] [PubMed] [Google Scholar]
- 6.Scalori A, Pozzi M, Bellia V, et al. Interferon-induced depression: Prevalence and management. Dig Liver Dis. 2005;37:102–7. doi: 10.1016/j.dld.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 7.Samet JH, Horton NJ, Traphagen ET, et al. Alcohol consumption and HIV disease progression: Are they related? Alcohol Clin Exp Res. 2003;27:862–7. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- 8.Samet JH, Libman H, LaBelle C, et al. A model clinic for the initial evaluation and establishment of primary care for persons infected with human immunodeficiency virus. Arch Intern Med. 1995;155:1629–33. [PubMed] [Google Scholar]
- 9.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: Validation of a new alcoholism screening instrument. Am J Psychiatry. 1974;131:1121–3. doi: 10.1176/ajp.131.10.1121. [DOI] [PubMed] [Google Scholar]
- 10.Buchsbaum DG, Buchanan RG, Centor RM, et al. Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med. 1991;115:774–7. doi: 10.7326/0003-4819-115-10-774. [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 12.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 13.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Robins LN, Wing J, Wittchen HU, et al. The composite international diagnostic interview. An epidemiologic instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Arch Gen Psychiatry. 1988;45:1069–77. doi: 10.1001/archpsyc.1988.01800360017003. [DOI] [PubMed] [Google Scholar]
- 15.Navaline HA, Snider EC, Petro CJ, et al. An automated version of the Risk Assessment Battery (RAB): Enhancing the assessment of risk behaviors. AIDS Res and Hum Retro-viruses. 1994;10:S281–3. [PubMed] [Google Scholar]
- 16.Sobell LC, Sobell MB Handbook of psychiatric measures. American Psychiatric Association. Washington, DC: American Psychiatric Association; 1996. Alcohol timeline followback (TLFB) pp. 477–9. [Google Scholar]
- 17.Thio CL, Nolt KR, Astemborski J, et al. Screening for hepatitis C virus in human immunodeficiency virus-infected individuals. J Clin Microbiol. 2000;38:575–7. doi: 10.1128/jcm.38.2.575-577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Helping patients who drink too much: A clinician’s guide. www.niaaa.nih.gov/publications/practitioner/guide.pdf.
- 19.Lee DH, Jamal H, Regenstein FG, et al. Morbidity of chronic hepatitis C as seen in a tertiary care medical center. Dig Dis Sci. 1997;42:186–91. doi: 10.1023/a:1018818012378. [DOI] [PubMed] [Google Scholar]
- 20.Dwight MM, Kowdly KV, Russo JE, et al. Depression, fatigue, and functional disability in patients with chronic hepatitis C. J Psychosom Res. 2000;49:311–7. doi: 10.1016/s0022-3999(00)00155-0. [DOI] [PubMed] [Google Scholar]
- 21.Fontana RJ, Hussain KB, Schwartz SM, et al. Emotional distress in chronic hepatitis C patients not receiving antiviral therapy. J Hepatol. 2002;36:401–7. doi: 10.1016/s0168-8278(01)00280-x. [DOI] [PubMed] [Google Scholar]
- 22.Goulding C, O’Connell P, Murray FE. Prevalence of fibromyalgia, anxiety and depression in chronic hepatitis C virus infection: Relationship to RT-PCT status and mode of acquisition. Eur J Gastroenterol Hepatol. 2001;13:507–11. doi: 10.1097/00042737-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Kraus MR, Schafer A, Csef H, et al. Emotional state, coping styles, and somatic variables in patients with chronic hepatitis C. Psychosomatics. 2000;41:377–84. doi: 10.1176/appi.psy.41.5.377. [DOI] [PubMed] [Google Scholar]
- 24.Forton DM, Thomas HC, Murphy CA, et al. Hepatitis C and cognitive impairment in a cohort of patients with mild liver disease. Hepatology. 2002;35:433–9. doi: 10.1053/jhep.2002.30688. [DOI] [PubMed] [Google Scholar]
- 25.Lehman CL, Cheung RC. Depression, anxiety, post-traumatic stress, and alcohol-related problems among veterans with chronic hepatitis C. Am J Gastroenterol. 2002;97:2640–6. doi: 10.1111/j.1572-0241.2002.06042.x. [DOI] [PubMed] [Google Scholar]
- 26.McDonald J, Jayasuriya J, Bindley P, et al. Fatigue and psychological disorders in chronic hepatitis C. J Gastroenterol Hepatol. 2002;17:171–6. doi: 10.1046/j.1440-1746.2002.02669.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson ME, Fisher DG, Fenaughty A, et al. Hepatitis C virus and depression in drug users. Am J Gastroenterol. 1998;93:785–9. doi: 10.1111/j.1572-0241.1998.225_a.x. [DOI] [PubMed] [Google Scholar]
- 28.Grassi L, Mondardini D, Pavanati M, et al. Suicide probability and psychological morbidity secondary to HIV infection: A control study of HIV-seropositive, hepatitis C virus (HCV)-seropositive and HIV(HCV-seronegative injecting drug users. J Affect Disord. 2001;64:195–202. doi: 10.1016/s0165-0327(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 29.Hilsabeck RC, Perry W, Hassanein TI. Neuropsychological impairment in patients with chronic hepatitis C. Hepatology. 2002;35:440–6. doi: 10.1053/jhep.2002.31257. [DOI] [PubMed] [Google Scholar]
- 30.McAndrews MP, Farcnik K, Carlen P, et al. Prevalence and significance of neurocognitive dysfunction in hepatitis C in the absence of correlated risk factors. Hepatology. 2005;41:801–8. doi: 10.1002/hep.20635. [DOI] [PubMed] [Google Scholar]
- 31.Ryan EL, Morgello S, Isaacs K, et al. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62:957–62. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Forton DM, Allsop JM, Main J, et al. Evidence for a cerebral effect of the hepatitis C virus. Lancet. 2001;358:38–9. doi: 10.1016/S0140-6736(00)05270-3. [DOI] [PubMed] [Google Scholar]
- 33.Kramer L, Bauer E, Funk G, et al. Subclinical impairment of brain function in chronic hepatitis C infection. J Hepatol. 2002;37:349–54. doi: 10.1016/s0168-8278(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 34.Forton DM, Karayiannis P, Mahmud N, et al. Identification of unique hepatitis C virus quasispecies in the central nervous system and comparative analysis of internal translational efficiency of brain, liver, and serum variants. J Virol. 2004;78:5170–83. doi: 10.1128/JVI.78.10.5170-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radkowski M, Wilkinson J, Nowicki M, et al. Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: Evidence of replication. J Virol. 2002;76:600–8. doi: 10.1128/JVI.76.2.600-608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58:721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 37.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 38.Kilbourne AM, Justice AC, Rabeneck L, et al. General medical and psychiatric comorbidity among HIV-infected veterans in the post-HAART era. J Clin Epidemiol. 2001;54(suppl 1):22–8. doi: 10.1016/s0895-4356(01)00443-7. [DOI] [PubMed] [Google Scholar]
- 39.Penzak SR, Reddy YS, Grimsley SR. Depression in patients with HIV infection. Am J Health Syst Pharm. 2000;57:376–86. doi: 10.1093/ajhp/57.4.376. [DOI] [PubMed] [Google Scholar]
- 40.Asch SM, Kilbourne AM, Gifford AL, et al. Underdiagnosis of depression in HIV: Who are we missing? J Gen Intern Med. 2003;18:450–60. doi: 10.1046/j.1525-1497.2003.20938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Starace F, Ammassari A, Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(suppl 3):S136–9. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 42.Leserman J, Jackson ED, Petitto HM, et al. Progression to AIDS: The effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]


