Abstract
The immunocytokine scFvMEL/TNF, a fusion protein composed of human tumor necrosis factor (TNF) and a single-chain Fv antibody (scFv) scFvMEL targeting the melanoma gp240 antigen, demonstrates impressive cytotoxic effects against human melanoma cell lines in vitro. Pharmacokinetic studies of 125I-scFvMEL/TNF in BALB/c mice showed that the construct clears from the circulation with a terminal-phase half-life of 17.6 hours after intravenous administration. The maximum tolerated dose of scFvMEL/TNF in nude mice was 4 mg/kg, i.v., on a daily x5 schedule. There were no changes in gross pathology, clinical chemistry, or hematologic parameters in mice treated at doses of up to 3 mg/kg. Therapeutic studies at a dose of 2.5 mg/kg on athymic mice bearing established (∼ 50 mm3) human melanoma A375GFP xenograft tumors transfected with green fluorescent protein demonstrated potent tumor suppression and complete tumor regression of all lesions. There was no subsequent outgrowth of tumors from mice rendered tumor-free. These data show that scFvMEL/TNF can target melanoma cells in vivo and can result in pronounced antimelanoma effects after systemic administration. Toxicology studies indicate the relative safety of this agent at doses that are therapeutically effective and provide guidance to projected phase I starting doses on patients at this schedule.
Keywords: Tumor necrosis factor, melanoma, immunocytokine, single-chain Fv antibody, tumor xenograft
Introduction
Through molecular engineering, proteins can be modified to significantly enhance their biologic activities. There have been numerous fusion proteins designed to combine the specific cell-binding characteristics of antibodies or growth factors with the cytotoxic or growth-mod ulatory effects of toxins, cytokines, or proapoptotic proteins [1–4]. Studies by Pastan and Kreitman [5], Pastan [6], Chan and Murphy [7], Murphy et al. [8], Reisfeld et al. [9,10], Xiang et al. [11], Rosenblum [1], Rosenblum et al. [12,13], and Dadachova et al. [14] have all demonstrated the preclinical and clinical potentials of these hybrid molecules.
The high-molecular-weight melanoma-associated glycoprotein gp240 has previously been demonstrated in a majority (80%) of melanoma cell lines and fresh tumor samples [15]. Recently, the gp240 antigen was found in 44 of 66 lobular breast cancer biopsy specimens [16], suggesting a possible common origin for some types of breast cancer and melanoma. More importantly, the gp240 antigen is either not expressed or expressed in low levels in normal cells [17,18], thus making this an interesting target for therapeutic intervention. Monoclonal antibodies (mAbs) targeting the gp240 antigen, such as ZME-018 and 9.2.27, have been extensively studied in melanoma patients and have demonstrated in numerous clinical trials an impressive ability to localize in metastatic tumors after systemic administration [19,20].
The antibody ZME-018 possesses high specificity for melanoma and is minimally reactive with a variety of normal tissues, making it a promising candidate as a targeting carrier [12,19,21,22]. Full-length mAbs are capable of selectively targeting tumor cells while avoiding normal tissues and are able to kill tumor cells through a variety of intrinsic (immunologic) and extrinsic (by the use of carried cytotoxic agents) modes. However, these molecules are large, poorly diffusible into areas of bulky disease, and highly immunogenic, rendering repeated infusions problematic. These advantages and disadvantages must be carefully considered in the application of mAb in cancer treatment.
Single-chain Fv antibodies (scFvs) consist of the antibody VL and VH domains linked by a designed flexible peptide tether [23]. Compared to intact IgG, scFvs have the following advantages: 1) smaller size; 2) structural simplicity with comparable antigen-binding affinities; 3) stability greater than that of analogous two-chain Fab fragments [24,25]. Several studies have shown that the smaller size of scFvs provides better penetration into tumor tissues, improved pharmacokinetics, and reduction in the immunogenicity observed with intravenous administration of Fabs, compared to that of intact murine antibodies [24–27].
The single-chain antibody scFvMEL retains the same binding affinity and specificity of the parental ZME-018 antibody, which recognizes the surface domain of the gp240 antigen [15,19]. It has been used extensively in our laboratory to target gp240-bearing cells in vitro and using xenograft models [3,12,20,28–31]. This antibody binds to target cells and is efficiently internalized, making this an excellent direct carrier of toxins or other cytotoxic payloads to gp240 antigen-positive cells.
Tumor necrosis factor (TNF) is a cytotoxic polypeptide that is secreted primarily by activated macrophages, and it shares some sequence homology (30%) with another peptide hormone, lymphotoxin (LTor TNF-β), which is secreted by activated lymphocytes [32]. Purified recombinant human (rhu) TNF-α is a single-chain nonglycosylated polypeptide with a molecular mass of 17 kDa, although it polymerizes into a compact non-disulfide-linked trimer in solution. TNF mediates a wide spectrum of systemic and cellular responses, including fever, shock, tissue injury, tumor necrosis, induction of other cytokines and immunoregulatory molecules, cell proliferation, differentiation, and apoptosis [33]. In vitro, TNF is cytostatic or cytotoxic to a number of human tumor cells, including SKBR-3 breast carcinoma and A375-M human melanoma [34,35]. However, the use of TNF in cancer therapy is restricted by severe dose-limiting toxicity and limited clinical efficacy against many tumor types [32]. A variety of strategies have been suggested for the use of the antitumor properties of this agent and for the simultaneous reduction of its systemic side effects, including the transfer of the TNF gene into tumor cells [36], tumor-infiltrating lymphocytes [37], and endothelial cells lining the artery, leading to local generation of TNF [38], stimulation of the local synthesis of TNF in situ [39], and antibody-mediated delivery [4,28,40,41].
Previous studies in our laboratory initially demonstrated that immunocytokines consisting of chemical conjugates of human TNF and mAbs displayed impressive targeted cytotoxic properties against tumor cells in culture, appeared to be cytotoxic agents that are far superior to native TNF, and were active against TNF-resistant tumor cells [4,12,28]. In addition, studies in xenograft models suggested that antibody-TNF conjugates readily accumulate specifically in tumor tissues and demonstrate in vivo antitumor activity that is superior to that of native TNF [12]. Based on these original observations, we designed and constructed a second-generation recombinant fusion construct composed of the recombinant single-chain anti-gp240 antibody scFvMEL that targets human melanoma cells and contains human TNF as a cytotoxic effector molecule, designated scFvMEL/TNF [4]. This fusion protein, compared to native TNF, was shown to enhance the in vitro killing of both TNF-sensitive and TNF-resistant human melanoma cells. In addition, radiolabeled scFvMEL/TNF was shown to localize effectively in human melanoma xenografts after intravenous administration.
The purpose of the current study was to evaluate the in vivo pharmacokinetics, toxicity, and therapeutic effects of scFvMEL/TNF in murine models to identify parameters that are critical for the clinical development of this agent.
Materials and Methods
Cell Lines
A375-M (human melanoma, gp240 antigen-positive, TNF-sensitive), AAB-527 (human melanoma, gp240 antigenpositive, TNF-resistant), SKBR3-HP (human breast cancer, gp240-negative, TNF-sensitive), and H4 (human neuroglioma, gp240-negative, TNF-resistant) were maintained in culture using Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) containing antibiotics (0.05 mg/ml), added glutamine (2 mM), sodium pyruvate (1 mM), nonessential amino acids (0.1 mM), and MEM vitamins specific for A375-M and AAB-527. All cells were routinely grown at a density of 7 x 106 cells per T-75 flask, subcultured twice weekly, routinely tested, and found to be free of Mycoplasma contamination using Mycoplasma Plus PCR Primer Sets (Stratagene, Cedar Creek, TX). Tissue culture media and supplements were purchased from Life Technologies, Inc. (Rockville, MD).
Stable Transfection of A375 Cell Lines
The plasmid pcDNA3-EGFP was produced by inserting a HindIII/XhoI fragment containing the enhanced green fluorescent protein (EGFP) coding sequence from pCMV-EGFP into the same sites of pcDNA3.1. Lipofectamine 2000 reagent (Invitrogen Life Technologies, Carlsbad, CA) was used for transfection. Briefly, cells were cultured for 24 hours in six-well plates with 1 ml/well DMEM with 10% FBS until 60% to 70% confluence had been reached. Liposomal DNA (Lipofectamine-pcDNA3-GFP complex) or nonliposomal DNA (pcDNA3) was directly added into culture plates at a proportion of 2 µg of DNA per 106 cells. To produce A375 cells that stably express EGFP, G418 (400 µg/ml) selection was started 24 hours after transfection. After 10 days of selection with G418, surviving cells were examined by fluorescence microscopy. Fluorescent colonies were picked and expanded. Cellular expression of GFP was evaluated using a fluorescence/visible light microscope setup to directly assess the percentage of total cells fluorescing. The stable transfection clone A375GFP-B1 was further characterized, including its response to TNF and scFvMEL/TNF, and the expression of the gp240 antigen was compared to that of the parental cell line. This clone was then used for in vivo studies.
Production of scFvMEL/TNF, scFvMEL, and rhuTNF
The scFvMEL/TNF fusion gene was constructed using polymerase chain reaction-based construction methods. The fusion gene was finally cloned into a bacterial expression vector pET32a(+) (Novagen, Madison, MI), and soluble fusion protein was expressed and purified as previously described [4].
The rhuTNF gene from plasmid pET32scFvMEL/TNF was subcloned into the pET32a(+) vector, and the protein was expressed in Escherichia coli Origami (DE3) cells. The expression and purification of TNF were the same as those of the fusion protein scFvMEL/TNF. The biologic activity of TNF was assessed using a standard biologic assay, which depends on cytotoxicity to a standard L-929 murine fibroblast cell line, as previously described [28]. rhuTNFα (Roche Molecular Biochemicals, Indianapolis, IN) was used as standard. The biologic activity of the final purified huTNF was 2 x10 7 U/mg protein.
The scFvMEL gene was amplified by polymerase chain reaction from plasmid pET32scFvMEL/TNF, and the gene was cloned into the pET21 b vector, creating plasmid pET21scFvMEL. The scFvMEL protein was expressed in E. coli AD494 (DE3) plysS. Biologically active scFvMEL protein was obtained by refolding an insoluble inclusion body based on a procedure described by Steinle et al. [42]. The final purified scFvMEL protein was shown to have target antigen-binding activity, as assessed by enzyme-linked immunosorbent assay [4], which was identical to that of the scFvMEL/TNF construct.
In Vitro Cytotoxicity of scFvMEL/TNF and TNF
To examine the cytotoxicity of scFvMEL/TNF and TNF, cells were plated into 96-well plates at a density of 3000 cells/well and allowed to adhere for 24 hours at 37°C in 5% CO2. After 24 hours, the medium was replaced with one containing different concentrations of either scFvMEL/TNF or TNF. The effect of scFvMEL/TNF and TNF on the growth of tumor cells in culture was determined by crystal violet (0.5% in 20% methanol) staining and was solubilized with Sorenson's buffer (0.1 M sodium citrate, pH 4.5, in 50% ethanol), as previously described [28]. Cell plates were read at 630 nm (Bio-Tek Instruments, Winooski, VT). Percent control refers to the percentage of cells in drug-treated wells compared to that in control (untreated) wells.
Protein Labeling Using p-Iodobenzoate
Proteins were labeled with 125I (Dupont, Wilmington, DE) by the p-iodobenzoate method, as previously described [12].
Animal Model Studies
Pharmacokinetic study Female BALB/c mice (4–6 weeks old) were injected with 5 µg of total protein in 200 µl of normal saline (2 µCi of 125I-scFvMEL/TNF per mouse). At 1, 2, 4, 8, 24, 48, and 72 hours after injection, three mice were sacrificed by cervical dislocation at each assay time. Blood samples were removed from the chest cavity, weighed, and counted to determine the total radioactivity in a γ-counter (model 5360; Packard, Meriden, CT). The blood samples were also centrifuged, and plasma was decanted and counted to determine radioactivity. The results from plasma determinations of radio-activity were analyzed by a least squares nonlinear regression (PK Analyst; MicroMath, Inc., St. Louis, MO) program.
In vivo toxicity study To determine the maximum tolerated dose (MTD) and the lethal dose (LD) for scFvMEL/TNF, female BALB/c mice (4–6 weeks old) were assigned to six groups (three to five mice per group). scFvMEL/TNF, at six total doses per group, was administered through the tail vein daily for 5 days, and mice were observed everyday to establish the MTD and the LD. The MTD was defined as the highest total dose that could be administered without lethality. Then, five groups (five mice per group) of female BALB/c mice (4–6 weeks old) were treated (intravenously through the tail vein) with either saline or four doses of the drug, using the same schedule. The total doses administered to each group were 1, 2, 3, and 4 mg/kg, and these doses corresponded to 25%, 50%, 75%, and 100% of the previously established MTD. Seven days after the last injection (day 12), the animals were sacrificed with carbon dioxide; terminally bled for hematologic parameters [including complete blood count (CBC)] and clinical chemistry analysis [including sodium, potassium, calcium, phosphorus, chloride, total bilirubin (TBIL), total protein (TP), albumin (Alb), globulin (Glob), alkaline phosphatase (Alk), creatinine (Cr), blood urea nitrogen (BUN), aspartate aminotransferase (AST), and alanine aminotransferease (ALT)]; and subjected to complete necropsy. Sections of the heart, lungs, spleen, kidneys, liver, gallbladder, skeletal muscles, aorta, brain, pituitary gland, eyes, lacrimal glands, sciatic nerve, spinal cord, pancreas, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, lymph nodes (mesenteric and mandibular), thymus, adrenal gland, larynx/pharynx, tongue, thyroid gland, parathyroid glands, trachea, esophagus, injection site, skin, mammary glands, urinary bladder, ovary, uterus, cervix, femur with knee joint, sternum, and bone marrow were fixed by immersion in a neutral-buffered 10% formalin solution. The tissues were embedded in paraffin blocks, sectioned at a nominal 5-µm section, stained with hematoxylin and eosin, and examined by a certified veterinary pathologist at the Department of Veterinary Medicine and Surgery of the University of Texas M. D. Anderson Cancer Center (Houston, TX).
In vivo efficacy study BABL/c (nu/nu) mice (4–6 weeks old) were injected subcutaneously with 3 x 106 A375GFP log-phase melanoma cells in the right flank. The tumors were allowed to establish for 2 weeks before the start of therapy, and the mice were divided into four groups. Each group had five mice with 30- to 50-mm3 established tumors. The mice were injected (intravenously through the tail vein) daily for 5 days with saline, scFvMEL (2.5 mg/kg), scFvMEL (0.2 mg/kg) plus TNF (0.2 mg/kg), or scFvMEL/TNF (2.5 mg/kg, 60% MTD). At the end of the therapy, the tumors were monitored weekly using Xenogen IVIS 200 Imaging System (Xenogen Co., Alameda, CA) and every 2 or 3 days with a caliper. Tumor volume was calculated with the formula: tumor volume = (width)2 x length/2 [43].
Statistical Analysis
Data were analyzed using paired t-test (Prism 3.0; Micro-Math, Inc., St. Louis, MO). Data are presented as mean ± SEM. A difference was regarded as significant if P < .05.
Results
Cytotoxicity of scFvMEL/TNF In Vitro
The cytotoxicity of scFvMEL/TNF was assessed against log-phase antigen-positive, TNF-sensitive human melanoma A375-M cells and antigen-positive, TNF-resistant human melanoma AAB-527 cells, respectively. We also compared these effects with antigen-negative, TNF-sensitive human breast cancer SKBR3-HP and antigen-negative, TNF-resistant human neuroglioma H4 cells. The results showed that, against antigen-positive A375-M cells, scFvMEL/TNF (IC50 = 0.1 nM) appeared to be approximately 10-fold more active than native TNF (IC50 = 1.4 nM) (P < .0001). Against TNF-sensitive, antigen-negative SKBR3-HP cells, the cytotoxicity of scFvMEL/TNF (IC50 = 2.5 nM) showed a dose-response curve similar to that of authentic TNF (IC50 = 2.7 nM) (P > .05). Against antigen-negative, TNF-resistant H4 cells, scFvMEL/TNF demonstrated no cytotoxic effects at doses of up to 1000 nM. However, against antigen-positive, TNF-resistant human melanoma AAB-527 cells, scFvMEL/TNF showed significant dose-related cytotoxic effects (IC50 = 20 nM). In contrast, these AAB-527 cells were resistant to the cytotoxic effects of TNF at concentrations of up to 5000 nM (Table 1). These results demonstrate that scFvMEL/TNF appeared to be significantly more active than native TNF against gp240 antigen-positive cells. More importantly, the fusion construct appeared to be able to overcome TNF resistance on gp240 antigen-positive, TNF-resistant AAB-527 cells.
Table 1.
Cytotoxicity* of scFvMEL/TNF Versus TNF on Various Human Cell Lines.
| Cell Lines | Cell Features | IC50 of scFvMEL/TNF (nM) | IC50 of TNF (nM) | Targeting Index† | P |
| A375-M | gp240+, TNF-sensitive | 0.1 ± 0.013 | 1.37 ± 0.020 | 15 | < .0001 |
| AAB-527 | gp240+, TNF-resistant | 20.62 ± 1.125 | > 5000‡ | > 250 | - |
| SKBR3-HP | gp240-, TNF-sensitive | 2.55 ± 0.085 | 2.71 ± 0.014 | 1 | > .05 |
| H4 | gp240-, TNF-resistant | > 1000‡ | > 5000‡ | - | - |
Seventy-two-hour treatment.
Targeting index = (IC50 of TNF)/(IC50 of scFvMEL/TNF).
No cytotoxic effects at indicated concentrations.
In Vivo Pharmacokinetics of scFvMEL/TNF
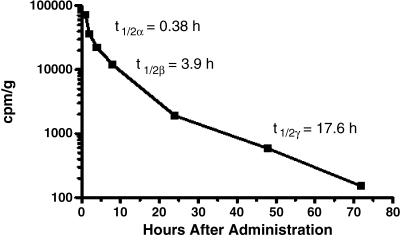
As described in the Materials and Methods section, scFvMEL/TNF was radiolabeled using the p-iodobenzoate method. The blood samples from BALB/c mice were centrifuged, and plasma was decanted and counted to determine radioactivity at different time points after injection. The results from plasma determinations of radioactivity were analyzed by a least squares nonlinear regression program. Pharmacology studies of scFvMEL/TNF in mice show a triphasic clearance curve with calculated plasma half-lives of 0.38, 3.9, and 17.6 hours for the α, β, and γ phases, respectively (Figure 1). The immediate apparent volume of distribution (Vd) of scFvMEL/TNF was 19.5 ml. However, the plasma clearance of radiolabeled TNF, chemical conjugate ZME-TNF, and intact antibody ZME was biphasic and closely fit (γ2 > 0.94) an open two-compartment mathematical model. Comparatively, as shown in Table 2, the half-lives of full-length antibody ZME-018 and chemical conjugate ZME-TNF were similar in this model, with α-phase half-lives of 1.39 and 1.2 hours, respectively. In addition, the half-lives of the terminal phases were also similar at 41.3 and 36.1 hours, respectively. In contrast, the clearance of free TNF in this model was relatively rapid, with α-phase and α-phase half-lives of 27.1 minutes and 2.7 hours, respectively. The immediate apparent volume of distribution (Vd) for ZME-018 alone approximated the blood volume (1.9 ml), whereas TNF alone had a somewhat larger Vd (3.9 ml). The chemical conjugate ZME-TNF displayed a Vd (11.6 ml) that was higher that that of either ZME-018 or TNF, suggesting a greater distribution outside the vasculature. The fusion construct scFvMEL/TNF demonstrated the highest Vd (19.5ml), suggesting the most extensive extravascular disposition in all of its component agents.
Figure 1.
Pharmacokinetics of 125I-labeled scFvMEL/TNF in mice. The radiolabeled fusion construct was administered intravenously to BALB/c mice. Groups (five per group) were sacrificed at various times after administration. The radioactivity in plasma was assessed, and the results were analyzed by a least squares nonlinear regression. The data demonstrated a triphasic curve fit with calculated half-lives of 0.38, 3.9, and 17.6 hours for the α, β, and γ phases, respectively.
Table 2.
Pharmacokinetics of 125I-ZME, ZME-TNF, TNF, and scFvMEL/TNF.
| Parameters | Full-Length IgG ZME | Chemical Conjugate ZME-TNF | Cytokine TNF | Immunocytokine scFvMEL/TNF |
| Molecular | 160 | 190 | 51 | 135 |
| mass (kDa) | ||||
| T1/2α (hr) | 1.39 | 1.20 | 0.45 | 0.38 |
| T1/2 (hr) | - | - | - | 3.93 |
| T1/2γ (hr) | 41.30 | 36.05 | 2.68 | 17.59 |
| Vd (ml) | 1.91 | 11.68 | 3.95 | 19.50 |
T1/2α = plasma half-life, α phase; T1/2β = plasma half-life, β phase; T1/2γ = plasma half-life, terminal phase; Vd = immediate apparent volume of distribution.
Toxicity Studies of scFvMEL/TNF in BALB/c Mice
MTD and LD for scFvMEL/TNF The total doses of scFvMEL/TNF per group of mice administered through the tail vein daily for 5 days are 16.7, 8.3, 4.8, 4.0, 2.5, and 0.5 mg/kg, respectively. The percentages of deaths and survivors are summarized in Table 3. The LD25 of scFvMEL/TNF in mice was approximately 4.8 mg/kg. The LD100 of scFvMEL/TNF in mice was approximately 8.3 mg/kg. The MTD of scFvMEL/TNF in mice was the highest dose in which no drug-related deaths were noted and was approximately 4.0 mg/kg.
Table 3.
Survival of Mice after Five Consecutive Administrations of scFvMEL/TNF.
| Total Dose/Mouse (mg/kg) | Mice per Group (n) | Deaths (%) | Survivors (%) |
| 16.7 | 4 | 100 | 0 |
| 8.3* | 4 | 100 | 0 |
| 4.8† | 4 | 25 | 75 |
| 4.0‡ | 5 | 0 | 100 |
| 2.5 | 5 | 0 | 100 |
| 0.5 | 3 | 0 | 100 |
The LD100 of scFvMEL/TNF in mice is approximately 8.3 mg/kg.
The LD25 of scFvMEL/TNF in mice is approximately 4.8 mg/kg.
The MTD of scFvMEL/TNF in mice is approximately 4.0 mg/kg.
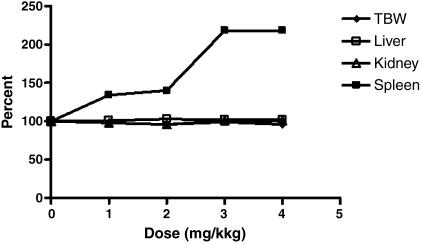
Mortality, gross pathology, and organ weight For mice treated at doses of up to and including the MTD, no deaths occurred in this study. In addition, there were no dose-related gross pathology findings observed even at the MTD level. A few gross lesions that were considered incidental and unrelated to the administration of scFvMEL/TNF were observed. scFvMEL/TNF caused a dose-related increase in relative spleen weights (relative to body weight) at doses of 1, 2, 3, and 4 mg/kg (Figure 2). The magnitude of the increase plateaus at 3 mg/kg. The increase in spleen weight correlated with increased extramedullar hematopoiesis in the red pulp and with increased follicular hyperplasia in the white pulp.
Figure 2.
Average terminal body weight (TBW) and relative organ weights in toxicology studies of scFvMEL/TNF. Five BALB/c mice per group were injected intravenously daily for 5 days with 1-, 2-, 3-, and 4-mg/kg total doses of scFvMEL/TNF, corresponding to 25%, 50%, 75%, and 100% of an established MTD. The vehicle control group consisted of saline. Seven days after the last injection (day 12), mice were sacrificed by exposure to CO2. The TBW of each mouse was measured before the performance of a complete necropsy, including the liver, kidneys, and spleen. The organ weight of individual animals was measured before the organs were fixed by immersion in a neutral-buffered 10% formalin solution. The relative organ weights (relative to body weight) were calculated as percentage of control (organ weight/TBW x 100). scFvMEL/TNF causes a dose-related increase in relative spleen weights (relative to body weight).
Clinical pathology No test substance-related alterations were noted in hematologic parameters [including hemoglobin (HGB), hematocrit (HCT), and CBC] (Table 4) and clinical chemistry parameters (including sodium, potassium, calcium, phosphorus, TBIL, TP, Alb, Glob, Alk, Cr, and BUN) (Table 5). There was a very slight increase in the mean levels of AST and ALT in the 2-, 3-, and 4-mg/kg groups when compared to those of the control saline group, but it was still within the reference range for this study. These minimal mean elevations in the treated groups were due to single animals in each group. No histopathologic correlations were observed in any of these animals, suggesting that elevations in these three animals may be spurious.
Table 4.
Group Means for Hematologic Parameters.
| Groups | HGB (g/dl) | HCT % | White Blood Cells (103 I-1) | Red Blood Cells (106 I-1) | Platelets (103 I-1) | Segmenters % | Lymphocytes % | Monocytes % | Eosinophils % | Basophils % |
| Saline | 14.7 | 41.0 | 10.8 | 9.4 | 768 | 12.2 | 81.3 | 1.7 | 3.6 | 1.2 |
| 1 mg/kg | 13.1 | 35.9 | 7.8 | 8.3 | 914 | 15.7 | 74.7 | 4.7 | 1.8 | 3.3 |
| 2 mg/kg | 13.8 | 38.0 | 7.9 | 8.8 | 944 | 20.4 | 75.6 | 1.6 | 1.4 | 0.9 |
| 3 mg/kg | 13.7 | 39.0 | 7.1 | 9.0 | 406 | 24.6 | 69.8 | 2.7 | 1.5 | 1.5 |
| 4 mg/kg | 12.7 | 36.0 | 4.8 | 8.2 | 584 | 30.6 | 57.2 | 7.3 | 1.8 | 3.1 |
| Reference range | 12.8–16.4 | 35.9–48.2 | 1.4–8.9 | 7.2–9.8 | 615–1802 | 1–35 | 54.8–92.4 | 1.5–9.7 | 1–6.6 | 0 |
Table 5.
Group Means for Clinical Chemistry Parameters.
| Groups | Na (mEq/l) | K (mEq/l) | Ca+ (mg/dl) | PO4 (mg/dl) | TBIL (mg/dl) | TP (g/dl) | Alb (g/dl) | Glob (g/dl) | Alk (IU/l) | Cr (mg/dl) | BUN (mg/dl) | AST (IU/l) | ALT (IU/l) |
| Saline | 153.1 | 7.1 | 10.3 | 8.8 | 0.4 | 5.5 | 3.8 | 1.7 | 140 | 0.2 | 21.7 | 133 | 58 |
| 1 mg/kg | 152.5 | 7.7 | 10.5 | 8.8 | 0.2 | 5.5 | 3.7 | 1.8 | 117 | 0.2 | 18.6 | 115 | 76 |
| 2 mg/kg | 151.6 | 7.9 | 10.3 | 8.8 | 0.3 | 5.6 | 3.8 | 1.9 | 123 | 0.2 | 17.6 | 216 | 179 |
| 3 mg/kg | 151.8 | 7.8 | 10.8 | 9.5 | 0.3 | 5.9 | 3.7 | 2.2 | 111 | 0.2 | 13.4 | 258 | 112 |
| 4 mg/kg | 150.2 | 8.8 | 10.9 | 10.7 | 0.3 | 5.9 | 3.7 | 2.2 | 134 | 0.2 | 18.7 | 376 | 199 |
| Reference range | 146–152 | 7–11 | 8–12 | 8.9–13 | 0–0.5 | 5.5–6 | 3–3.4 | 2.5–2.8 | 111–224 | 0–0.4 | 18–33 | <410 | <344 |
Histopathology Systemic administration of scFvMEL/TNF resulted in apparent dose-dependent lesions in the liver, lung, and spleen of female BALB/c mice (Table 6). The incidence and severity of these lesions appear to be dose-dependent and include increased extramedullary hematopoiesis of the liver and spleen at doses greater than or equal to 2, 3, and 4 mg/kg scFvMEL/TNF; pulmonary fibrin thrombi of the lung at doses greater than or equal to 4 mg/kg scFvMEL/TNF; and follicular hyperplasia of the splenic white pulp at doses greater than or equal to 1, 2, 3, and 4 mg/kg scFvMEL/TNF. The most sensitive indicators of scFvMEL/TNF-related effects appeared to be extramedullary hematopoiesis and follicular lymphoid hyperplasia of the spleen. The no-observed-adverse-effect level (NOAEL) for scFvMEL/TNF under the conditions of this study is 3 mg/kg, based on fibrin thrombi observed in the lungs of three of five animals in the 4-mg/kg group.
Table 6.
Summary Incidence: scFvMEL/TNF-Related Organ Morphologic Lesions and Lesion Average Grades*.
| Groups | Liver, Extramedullary Hematopoiesis, Increased | Lung, Thrombus, Fibrosed/Recanalized, Singular | Spleen, Extramedullary Hematopoiesis, Increased | Spleen, Follicular Cell Hyperplasia | ||||
| #P/T† | Grade | #P/T | Grade | #P/T | Grade | #P/T | Grade | |
| Saline | 2/5 | 1 | 0/5 | 2/5 | 1 | 2/5 | 1 | |
| 1 mg/kg | 1/5 | 1 | 0/5 | 5/5 | 1.5 | 5/5 | 2.8 | |
| 2 mg/kg | 4/5 | 1 | 0/5 | 5/5 | 2 | 5/5 | 3.2 | |
| 3 mg/kg | 5/5 | 1 | 0/5 | 5/5 | 4 | 5/5 | 3.4 | |
| 4 mg/kg | 5/5 | 1 | 3/5 | 1 | 5/5 | 4 | 5/5 | 4 |
The incidence of the lesion in the group: Grade 1 = modest, rare 5 to 10%; Grade 2 = mild, infrequent 10 to 20%; Grade 3 = moderate, frequent 20 to 50 %; Grade 4 = severe, extensive > 50 %.
#P/T, Numbers of positive mice /total mice
The dose-dependent extramedullary hematopoiesis observed was considered compound-related, but was not considered adverse. The exact mechanism for the increased extramedullary hematopoiesis is not known, but this can be caused by factors that stimulate hematopoiesis, such as proinflammatory proteins. Follicular lymphoid hyperplasia is considered an immunogenic response to a foreign protein in mice. This study did not attempt to measure the immunogenicity of the protein, which will be addressed in a more relevant species.
No significant lesions were observed in the kidneys, gallbladder, heart, skeletal muscles, aorta, brain, pituitary gland, eyes, lacrimal glands, sciatic nerve, spinal cord, pancreas, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, lymph nodes (mesenteric and mandibular), thymus, adrenal gland, larynx/pharynx, tongue, thyroid gland, parathyroid glands, trachea, esophagus, injection site, skin, mammary glands, urinary bladder, ovary, uterus, cervix, femur with knee joint, sternum, and bone marrow.
In Vivo Antitumor Effects of scFvMEL/TNF
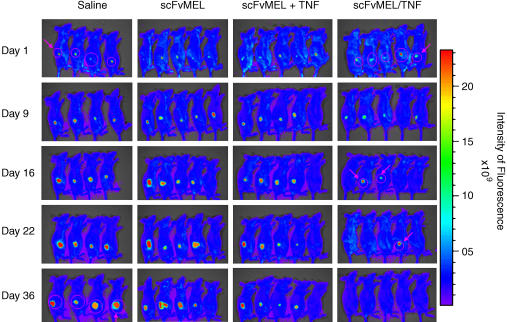
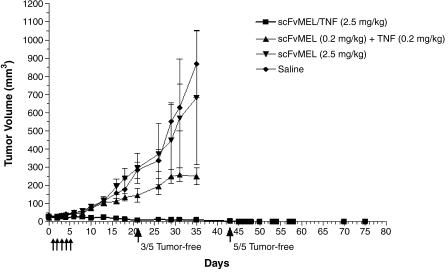
Groups of mice bearing established (30–50 mm3) A375GFP xenografts were treated (intravenously through the tail vein) daily (days 1–5) for 5 days with saline, scFvMEL (2.5 mg/kg), scFvMEL (0.2 mg/kg) plus TNF (0.2 mg/kg), or scFvMEL/TNF (2.5 mg/kg). The mice were observed, and their tumors were imaged by the Xenogen IVIS 200 Imaging System and measured using a caliper every 2 or 3 days. Three of five mice on day 16, four of five mice on day 22, and five of five mice on day 36 had no detectable tumors, as assessed (arrows) by an external imaging system. In contrast, tumor GFP signals in all mice in the saline and scFvMEL control groups dramatically increased with time (Figure 3). It was noted that, although tumor GFP signals were not detected in some mice, small tumors were still measurable using a caliper. When monitored by caliper measurement, all mice treated with either saline or scFvMEL alone had rapid tumor growth. Treatment with scFvMEL plus rhuTNF demonstrated moderate tumor growth inhibition probably due to TNF effects. However, scFvMEL/TNF treatment demonstrated potent antitumor activity, with three of five mice being tumor-free on day 21 and with complete tumor regression (five of five mice being tumor-free) on day 43. There was no subsequent outgrowth of tumors from mice rendered tumor-free up to the last day of measurement on day 75 (Figure 4). These data show that scFvMEL/TNF, compared to TNF alone or scFvMEL or scFvMEL plus TNF, can target melanoma cells in vivo and can result in pronounced and prolonged antimelanoma effects.
Figure 3.
Antitumor activity of scFvMEL/TNF in A375GFP tumor xenografts monitored by the Xenogen IVIS 200 Imaging System. Nude mice bearing established (50 mm3) human melanoma (A375GFP) tumors stably transfected with GFP growing in the right flank were treated intravenously with either saline (control), scFvMEL (2.5 mg/kg), scFvMEL (0.2 mg/kg) plus TNF (0.2 mg/kg), or scFvMEL/TNF (2.5 mg/kg; total dose) for five consecutive days. The tumors were monitored using the Xenogen IVIS 200 Imaging System after mice had been anesthetized once with Nembutal (50 mg/kg, i.p.) a week after treatment. On treatment with scFvMEL/TNF, tumor GFP signals (arrows) were not detected in three of five mice on day 16, in four of five mice on day 22, and in five of five mice on day 36, respectively, whereas tumor GFP signals dramatically increased with time in all mice in the saline and scFvMEL control groups, indicating tumor growth.
Figure 4.
Antitumor activity of scFvMEL/TNF in A375GFP tumor xenografts as monitored by caliper measurement. Nude mice bearing human melanoma (A375GFP) tumors were treated intravenously with either saline (control), scFvMEL (2.5 mg/kg), scFvMEL (0.2 mg/kg) plus rhuTNF (0.2 mg/kg), or scFvMEL/TNF (2.5 mg/kg; total dose) for five consecutive days (arrows). The treatment of mice bearing established (30–50 mm3) tumors with scFvMEL/TNF at a dose of 2.5 mg/kg resulted in potent tumor suppression and complete tumor regression of all lesions (with three of five mice being tumor-free on day 21, and with five of five mice being tumor-free on day 43). In contrast, all mice treated with either saline, scFvMEL alone, or scFvMEL plus TNF showed rapid tumor growth.
Discussion
Malignant melanoma is a primary example of a cancer that is highly metastatic and responds poorly to various treatments, including chemotherapy and γ-irradiation [44]. Novel therapeutic strategies targeting melanoma are currently under development in several laboratories [45]. We previously reported [4] a fusion construct, designated scFvMEL/TNF, that is composed of the antibody scFvMEL, which recognizes the surface domain of the gp240 antigen present in 80% of human melanoma cells. The antibody-specific delivery of TNF, compared to TNF alone, to the cell surface of melanoma cells resulted in augmented cytotoxicity. In addition, we demonstrated that antibody-TNF chemical conjugates and fusion constructs were capable of delivering TNF to tumors in vivo [4,12]. In this study, we further demonstrated that the fusion construct scFvMEL/TNF has potent antitumor activity after in vivo administration. In addition, the estimated MTD for the scFvMEL/TNF fusion construct (4 mg/kg) was found to be almost 10-fold higher than the MTD reported for TNF itself (0.3 mg/kg) [46], suggesting that the targeted construct is capable of directing the active TNF cytokine to tumor cells and away from tissue sites that cause toxicity.
TNF is known to not only possess direct cytotoxicity against tumor cells, but also induce tumor vessel disruption [47]. However, systemic administration of TNF protein has been shown to result in significant host toxicity without significant antitumor effects [48,49]. A variety of strategies have been suggested for the use of the antitumor properties of this agent and for the simultaneous reduction of its systemic side effects, including antibody-mediated delivery [4,40] or transfer of the TNF gene into tumor cells [36].
TNF as a cytotoxic payload for targeted therapeutics has undergone extensive preclinical evaluation by our group and others [4,12,28,50–52]. More recently, the novel immunocytokine scFv23/TNF targeting Her-2/neu-overexpressing malignancies has been shown to sensitize TNF-resistant Her-2/neu-overexpressing breast cancer cells to TNF-induced apoptosis [53]. These data suggest that TNF targeted to tumor cells, compared to that of TNF itself, may have fundamental differences in the cellular effects exerted by the fusion construct. Studies in our laboratory examining the mechanistic effects of scFvMEL/TNF versus TNF alone suggested that the fusion construct specifically inhibits SAPK/JNK pathways in melanoma cells resistant to TNF, whereas TNF itself does not [54]. Further studies detailing other mechanistic differences between these two molecules are still ongoing.
Antibodies for the targeted delivery of cytokines not only provide for enhanced localization to tumor tissues after in vivo administration but also possess the potential to increase the plasma half-life of therapeutic agents [55]. As shown in this study, scFvMEL/TNF has a relatively short serum half-life (17.6 hours) compared to mAb (ZME, 41.3 hours). The chemical conjugate ZME-TNF, consisting of the full-length IgG antibody ZM E-018 chemically coupled to TNF, demonstrated a much longer serum half-life (36 hours), thereby increasing the circulating time of biologically active TNF. The scFvMEL/TNF fusion construct, compared to TNF itself, therefore provides for a prolonged plasma half-life (2.7 hours), and previous studies [4] examining the tissue distribution of the construct in tumor xenograft models also demonstrate impressive localization of the agent in gp240-expressing tumors.
Because effective clinical management of solid tumors such as melanoma generally requires prolonged treatment regimens, the immunogenicity of this molecule is of concern. The constant domain (murine framework) regions of the scFvMEL molecule account for ∼37% of the total size of the construct. By comparison, mouse/human chimeric antibodies also contain 35% murine sequences. Therefore, based on this analysis, we expect the immunogenicity of the construct to be similar to that of chimeric antibodies. The immunogenicity of an antibody-cytokine containing full-length antibodies is due, at least in part, to the large size of the antibody itself and the long circulation time of the constructs. The serum half-life of the scFvMEL/TNF fusion construct was much shorter than that of the chemical conjugate, suggesting that the single-chain immunocytokine, by comparison, should be significantly less immunogenic due to its small size and relatively rapid clearance kinetics from the circulation. In addition to these properties, scFvMEL/TNF demonstrated a Vd (19.5 ml) that is higher than those of both TNF and chemical conjugate ZME-TNF, suggesting its relatively greater distribution outside the vasculature. Tissue distribution studies [4] of the scFvMEL/TNF construct indicated that tumor localization of the construct occurs efficiently by 72 hours. Concentrations of the fusion construct, compared to those of free TNF, were shown to be highest in tumor tissues. The tumor-targeting capability of the construct, compared to that of TNF, may also account for the increase in the apparent volume of distribution (Vd) of the construct. In addition, the smaller size of the scFvMEL/TNF construct, compared to that of the chemical conjugate ZME-TNF, may be responsible for its comparatively facile extravascular disposition. The short half-life observed with this construct suggests that dosing intervals of 24 or 48 hours appear to be optimal to achieving the maximal concentration of the agent in tumor tissues.
Of critical importance to the eventual clinical development of scFvMEL/TNF is an examination of MTD toxicity profile and efficacy studies of this fusion construct in well-characterized human melanoma xenograft models. The MTD of the fusion construct scFvMEL/TNF was 0.8 mg/kg daily for five consecutive days, corresponding to an MTD of 4 mg/kg (total dose). It is important to note that the impressive antitumor effects observed in vivo with the fusion construct were obtained at a dose of 2.5 mg/kg, which is approximately 60% of the MTD and is also below the NOAEL dose (3 mg/kg) determined from our toxicology studies.
Our results indicate that neither significant adverse effects nor organ toxicity was associated with intravenous injections of scFvMEL/TNF at doses of up to 100% of the MTD (4 mg/kg). The scFvMEL/TNF construct could be safely administered at this schedule at doses of up to 75% of the MTD (3.0 mg /kg). Although dose-related extramedullary hematopoiesis was noted in the liver and the spleen, and follicular cell (lymphoid) hyperplasia was noted in the spleen, none of these lesions was considered an adverse effect. Extramedullary hematopoiesis is the proliferation of erythrocytes, myelocytes, and megakaryocytes in the spleen and the liver, primarily. It occurs spontaneously in mice and has a higher incidence in female mice. The spleen is a major hematopoietic organ in mice. The increased incidence and severity of extramedullary hematopoiesis in mice can be caused by anything that will stimulate hematopoiesis. Most proinflammatory cytokines stimulate hematopoiesis through different growth factors, as do conditions that produce anoxia such as hemolysis or bone marrow toxicity. In this toxicity study, the specific cause of extramedullary hematopoiesis is unknown, but it may be related to the proinflammatory properties of TNF. Follicular lymphoid hyperplasia is an indication of the immunogenicity of this protein in mice. Immunogenicity for humans needs to be addressed in a more relevant species. In addition, we found no significant changes in hematologic parameters or changes in clinical chemistry parameters in mice even when they were treated at 100% of the MTD. A single recanalized thrombus in lung tissues was observed in three of five mice at MTD level. These thrombi occurred in only one vessel and did not compromise pulmonary function; however, they are considered biologically adverse and, for this reason, NOAEL was assessed at the 3-mg/kg (75% MTD) dose level. Therefore, the safe level for a phase I trial is well below MTD level. The cause of the thrombosis is speculative and may be related to the proinflammatory properties of TNF.
The MTD of scFvMEL/TNF (4 mg/kg) in mice was found to be significantly higher than that of rhuTNF alone (0.3 mg/kg) [46]. The causes of death in animals treated with 4.8 (LD25), 8.3 (LD100), and 16.7 mg/kg scFvMEL/TNF may have been related to the proinflammatory properties of huTNF. Studies recently demonstrated that rhuTNF induced severe toxicity at the MTD because intravenously administered TNF leads to the destruction of the normal tissue microvasculature [46,56], resulting in vascular congestion and hemorrhagic injury in normal organs such as the gastrointestinal tract and the thyroid gland. In addition, ulcers induced by rhuTNF were sufficient to cause lethal toxicity [46]. However, in the present study, scFvMEL/TNF showed no significant normal organ injury in mice despite its higher antitumor potency at 60% MTD. Thus, scFvMEL/TNF appears to have more selective activity toward tumors, compared with a rather nonspecific toxicity profile for rhuTNF.
Thus, careful assessment of the pharmacokinetics of the scFvMEL/TNF construct has provided a rationale for designing an optimal administration schedule. Assessment of the therapeutic efficacy of this schedule demonstrates impressive antitumor effects against well-established melanoma xenografts. Efficacy studies, combined with histopathology, clinical chemistry, and effects on clinical chemistry parameters, provide a rationale for designing phase I trials in patients with gp240-positive tumors such as melanoma and lobular breast cancer.
Acknowledgements
The authors thank Darnay G. Bryant for plasmid pcDNA3. We also thank Michelle McCall for her excellent assistance in the preparation of this manuscript.
Abbreviations
- TNF
tumor necrosis factor
- scFv
single-chain Fv antibody
- MTD
maximum tolerated dose
- LD
lethal dose
- GFP
green fluorescent protein
Footnotes
This research was conducted, in part, by the Clayton Foundation for Research.
References
- 1.Rosenblum M. Immunotoxins and toxin constructs in the treatment of leukemia and lymphoma. Adv Pharmacol. 2004;51:209–228. doi: 10.1016/S1054-3589(04)51009-8. [DOI] [PubMed] [Google Scholar]
- 2.Veenendaal LM, Jin H, Ran S, Cheung L, Navone N, Marks JW, Waltenberger J, Thorpe P, Rosenblum MG. In vitro and in vivo studies of a VEGF121/rGelonin chimeric fusion toxin targeting the neovasculature of solid tumors. Proc Natl Acad Sci USA. 2002;99:7866–7871. doi: 10.1073/pnas.122157899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Cheung LH, Hittelman WN, Rosenblum MG. Targeted delivery of human pro-apoptotic enzymes to tumor cells: in vitro studies describing a novel class of recombinant highly cytotoxic agents. Mol Cancer Ther. 2003;2:1341–1350. [PubMed] [Google Scholar]
- 4.Liu Y, Cheung LH, Marks JW, Rosenblum MG. Recombinant single-chain antibody fusion construct targeting human melanoma cells and containing tumor necrosis factor. Int J Cancer. 2004;108:549–557. doi: 10.1002/ijc.11524. [DOI] [PubMed] [Google Scholar]
- 5.Pastan I, Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Invest Drugs. 2002;3:1089–1091. [PubMed] [Google Scholar]
- 6.Pastan I. Immunotoxins containing Pseudomonas exotoxin A: a short history. Cancer Immunol Immunother. 2003;52:338–341. doi: 10.1007/s00262-002-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan MC, Murphy RM. Kinetics of cellular trafficking and cytotoxicity of 9.2.27-gelonin immunotoxins targeted against the highmolecular-weight melanoma-associated antigen. Cancer Immunol Immunother. 1999;47:321–329. doi: 10.1007/s002620050537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy JR, Lakkis FG, vanderSpek JC, Anderson P, Strom TB. Protein engineering of diphtheria toxin. Development of receptor-specific cytotoxic agents for the treatment of human disease. Target Diagn Ther. 1992;7:365–382. [PubMed] [Google Scholar]
- 9.Reisfeld RA, Gillies SD, Mendelsohn J, Varki NM, Becker JC. Involvement of B lymphocytes in the growth inhibition of human pulmonary melanoma metastases in athymic nu/nu mice by an anti-body-lymphotoxin fusion protein. Cancer Res. 1996;56:1707–1712. [PubMed] [Google Scholar]
- 10.Reisfeld RA, Becker JC, Gillies SD. Immunocytokines: a new approach to immunotherapy of melanoma. Melanoma Res. 1997;7(Suppl 2):S99–S106. [PubMed] [Google Scholar]
- 11.Xiang R, Lode HN, Dolman CS, Dreier T, Varki NM, Qian X, Lo KM, Lan Y, Super M, Gillies SD, et al. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 12.Rosenblum MG, Cheung L, Mujoo K, Murray JL. An antimelanoma immunotoxin containing recombinant human tumor necrosis factor: tissue disposition, pharmacokinetic, and therapeutic studies in xenograft models. Cancer Immunol Immunother. 1995;40:322–328. doi: 10.1007/BF01519633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenblum MG, Verschraegen CF, Murray JL, Kudelka AP, Gano J, Cheung L, Kavanagh JJ. Phase I study of 90Y-labeled B72.3 intraperitoneal administration in patients with ovarian cancer: effect of dose and EDTA coadministration on pharmacokinetics and toxicity. Clin Cancer Res. 1999;5:953–961. [PubMed] [Google Scholar]
- 14.Dadachova E, Nosanchuk JD, Shi L, Schweitzer AD, Frenkel A, Nosanchuk JS, Casadevall A. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proc Natl Acad Sci USA. 2004;101:14865–14870. doi: 10.1073/pnas.0406180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kantor RR, Ng AK, Giacomini P, Ferrone S. Analysis of the NIH workshop monoclonal antibodies to human melanoma antigens. Hybridoma. 1982;1:473–482. doi: 10.1089/hyb.1.1982.1.473. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Erba L, Calo-Gabrieli G, Caruso ML, Thomas R, Cortino G, Valentini AM, Muto P, Albrizio M, Pastena MI, Lastoria S. Immuno-histochemical reactivity of anti-melanoma monoclonal antibody 225.28S in human breast cancer biopsies. Anticancer Res. 2001;21:925–930. [PubMed] [Google Scholar]
- 17.Graf LH, Jr, Ferrone S. Human melanoma-associated antigens. Immunol Ser. 1989;43:643–679. [PubMed] [Google Scholar]
- 18.Kantor RR, Albino AP, Ng AK, Ferrone S. Biosynthesis and intracellular processing of four human melanoma associated antigens. Cancer Res. 1986;46:5223–5228. [PubMed] [Google Scholar]
- 19.Macey DJ, Denardo SJ, Denardo GL, Goodnight JK, Unger MW. Uptake of indium-111-labeled monoclonal antibody ZME-018 as a function of tumor size in a patient with melanoma. Am J Physiol Imaging. 1988;3:1–6. [PubMed] [Google Scholar]
- 20.Rosenblum MG, Levin B, Roh M, Hohn D, McCabe R, Thompson L, Cheung L, Murray JL. Clinical pharmacology and tissue disposition studies of 131I-labeled anticolorectal carcinoma human monoclonal antibody LiCO 16.88. Cancer Immunol Immunother. 1994;39:397–400. doi: 10.1007/BF01534427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mujoo K, Cheung L, Murray JL, Rosenblum MG. Pharmacokinetics, tissue distribution, and in vivo antitumor effects of the anti-melanoma immunotoxin ZME-gelonin. Cancer Immunol Immunother. 1995;40:339–345. doi: 10.1007/BF01519635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenblum MG, Murray JL, Cheung L, Rifkin R, Salmon S, Bartholomew R. A specific and potent immunotoxin composed of antibody ZME-018 and the plant toxin gelonin. Mol Biother. 1991;3:6–13. [PubMed] [Google Scholar]
- 23.Atwell JL, Breheney KA, Lawrence LJ, McCoy AJ, Kortt AA, Hudson PJ. scFv multimers of the anti-neuraminidase antibody NC10: length of the linker between VH and VL domains dictates precisely the transition between diabodies and triabodies. Protein Eng. 1999;12:597–604. doi: 10.1093/protein/12.7.597. [DOI] [PubMed] [Google Scholar]
- 24.Adams GP, Schier R. Generating improved single-chain Fv molecules for tumor targeting. J Immunol Methods. 1999;231:249–260. doi: 10.1016/s0022-1759(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 25.Colcher D, Pavlinkova G, Beresford G, Booth BJ, Choudhury A, Batra SK. Pharmacokinetics and biodistribution of genetically-engineered antibodies. Q J Nucl Med. 1998;42:225–241. [PubMed] [Google Scholar]
- 26.Bird RE, Hardman KD, Jacobson JW, Johnson S, Kaufman BM, Lee SM, Lee T, Pope SH, Riordan GS, Whitlow M. Single-chain antigen-binding proteins. Science. 1988;242:423–426. doi: 10.1126/science.3140379. [DOI] [PubMed] [Google Scholar]
- 27.Colcher D, Bird R, Roselli M, Hardman KD, Johnson S, Pope S, Dodd SW, Pantoliano MW, Milenic DE, Schlom J. In vivo tumor targeting of a recombinant single-chain antigen-binding protein. J Natl Cancer Inst. 1990;82:1191–1197. doi: 10.1093/jnci/82.14.1191. [DOI] [PubMed] [Google Scholar]
- 28.Rosenblum MG, Cheung L, Murray JL, Bartholomew R. Antibody-mediated delivery of tumor necrosis factor (TNF-alpha): improvement of cytotoxicity and reduction of cellular resistance. Cancer Commun. 1991;3:21–27. [PubMed] [Google Scholar]
- 29.Rosenblum MG, Cheung L, Kim SK, Mujoo K, Donato NJ, Murray JL. Cellular resistance to the antimelanoma immunotoxin ZME-gelonin and strategies to target resistant cells. Cancer Immunol Immunother. 1996;42:115–121. doi: 10.1007/s002620050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblum MG, Marks JW, Cheung LH. Comparative cytotoxicity and pharmacokinetics of antimelanoma immunotoxins containing either natural or recombinant gelonin. Cancer Chemother Pharmacol. 1999;44:343–348. doi: 10.1007/s002800050987. [DOI] [PubMed] [Google Scholar]
- 31.Rosenblum MG, Cheung LH, Liu Y, Marks JW., III Design, expression, purification, and characterization, in vitro and in vivo, of an antimelanoma single-chain Fv antibody fused to the toxin gelonin. Cancer Res. 2003;63:3995–4002. [PubMed] [Google Scholar]
- 32.Aggarwal BB, Natarajan K. Tumor necrosis factors: developments during the last decade. Eur Cytokine Netw. 1996;7:93–124. [PubMed] [Google Scholar]
- 33.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 34.Shiohara M, Gombart AF, Berman JD, Koike K, Komiyama A, Koeffler HP. Cytostatic effect of TNFalpha on cancer cells is independent of p21WAF1. Oncogene. 1997;15:1605–1609. doi: 10.1038/sj.onc.1201315. [DOI] [PubMed] [Google Scholar]
- 35.Zouboulis CC, Schroder K, Garbe C, Krasagakis K, Kruger S, Orfanos CE. Cytostatic and cytotoxic effects of recombinant tumor necrosis factor-alpha on sensitive human melanoma cells in vitro may result in selection of cells with enhanced markers of malignancy. J Invest Dermatol. 1990;95:223S–230S. doi: 10.1111/1523-1747.ep12875823. [DOI] [PubMed] [Google Scholar]
- 36.Koshita Y, Lu Y, Fujii S, Neda H, Matsuyama T, Satoh Y, Itoh Y, Takahashi M, Kato J, Sakamaki S. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with tumor necrosis factor-alpha cDNA for the gene therapy of cancer in humans. Efficacy of TNF-alpha gene-transduced tumor cells in treatment of established in vivo tumor. Int J Cancer. 1995;63:130–135. doi: 10.1002/ijc.2910630123. [DOI] [PubMed] [Google Scholar]
- 37.Hwu P, Yannelli J, Kriegler M, Anderson WF, Perez C, Chiang Y, Schwarz S, Cowherd R, Delgado C, Mule J. J Immunol. 1993;150:4104–4115. [PubMed] [Google Scholar]
- 38.Mizuguchi H, Nakagawa T, Toyosawa S, Nakanishi M, Imazu S, Nakanishi T, Tsutsumi Y, Nakagawa S, Hayakawa T, Ijuhin N, et al. Tumor necrosis factor alpha-mediated tumor regression by the in vivo transfer of genes into the artery that leads to tumors. Cancer Res. 1998;58:5725–5730. [PubMed] [Google Scholar]
- 39.Joseph WR, Cao Z, Mountjoy KG, Marshall ES, Baguley BC, Ching LM. Stimulation of tumors to synthesize tumor necrosis factor-alpha in situ using 5,6-dimethylxanthenone-4-acetic acid: a novel approach to cancer therapy. Cancer Res. 1999;59:633–638. [PubMed] [Google Scholar]
- 40.Scherf U, Benhar I, Webber KO, Pastan I, Brinkmann U. Cytotoxic and antitumor activity of a recombinant tumor necrosis factor-B1(Fv) fusion protein on LeY antigen-expressing human cancer cells. Clin Cancer Res. 1996;2:1523–1531. [PubMed] [Google Scholar]
- 41.Yang J, Moyana T, Xiang J. A genetically engineered singlechain FV/TNF molecule possesses the anti-tumor immunoreactivity of FV as well as the cytotoxic activity of tumor necrosis factor. Mol Immunol. 1995;32:873–881. doi: 10.1016/0161-5890(95)00051-f. [DOI] [PubMed] [Google Scholar]
- 42.Steinle A, Li P, Morris DL, Groh V, Lanier LL, Strong RK, Spies T. Interactions of human NKG2Dwith its ligands MICA, MICB, and homologs of the mouse RAE-1 protein family. Immunogenetics. 2001;53:279–287. doi: 10.1007/s002510100325. [DOI] [PubMed] [Google Scholar]
- 43.Osborne CK, Hobbs K, Clark GM. Effect of estrogens and antiestrogens on growth of human breast cancer cells in athymic nude mice. Cancer Res. 1985;45:584–590. [PubMed] [Google Scholar]
- 44.Helmbach H, Rossmann E, Kern MA, Schadendorf D. Drug-resistance in human melanoma. Int J Cancer. 2001;93:617–622. doi: 10.1002/ijc.1378. [DOI] [PubMed] [Google Scholar]
- 45.Leong SP. Future perspectives on malignant melanoma. Surg Clin North Am. 2003;83:453–456. doi: 10.1016/S0039-6109(02)00204-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuroda K, Miyata K, Fujita F, Koike M, Fujita M, Nomura M, Nakagawa S, Tsutsumi Y, Kawagoe T, Mitsuishi Y, et al. Human tumor necrosis factor-alpha mutant RGD-V29 (F4614) shows potent antitumor activity and reduced toxicity against human tumor xenografted nude mice. Cancer Lett. 2000;159:33–41. doi: 10.1016/s0304-3835(00)00529-2. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe N, Niitsu Y, Umeno H, Kuriyama H, Neda H, Yamauchi N, Maeda M, Urushizaki I. Toxic effect of tumor necrosis factor on tumor vasculature in mice. Cancer Res. 1988;48:2179–2183. [PubMed] [Google Scholar]
- 48.Moritz T, Niederle N, Baumann J, May D, Kurschel E, Osieka R, Kempeni J, Schlick E, Schmidt CG. Phase I study of recombinant human tumor necrosis factor alpha in advanced malignant disease. Cancer Immunol Immunother. 1989;29:144–150. doi: 10.1007/BF00199290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blick M, Sherwin SA, Rosenblum M, Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987;47:2986–2989. [PubMed] [Google Scholar]
- 50.Rosenblum MG, Horn SA, Cheung LH. A novel recombinant fusion toxin targeting HER-2/NEU-over-expressing cells and containing human tumor necrosis factor. Int J Cancer. 2000;88:267–273. doi: 10.1002/1097-0215(20001015)88:2<267::aid-ijc19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 51.Curnis F, Gasparri A, Sacchi A, Longhi R, Corti A. Coupling tumor necrosis factor-alpha with alpha V integrin ligands improves its antineoplastic activity. Cancer Res. 2004;64:565–571. doi: 10.1158/0008-5472.can-03-1753. [DOI] [PubMed] [Google Scholar]
- 52.Hoogenboom HR, Raus JC, Volckaert G. Targeting of tumor necrosis factor to tumor cells: secretion by myeloma cells of a genetically engineered antibody-tumor necrosis factor hybrid molecule. Biochim Biophys Acta. 1991;1096:345–354. doi: 10.1016/0925-4439(91)90071-g. [DOI] [PubMed] [Google Scholar]
- 53.Lyu MA, Rosenblum MG. The immunocytokine scFv23/TNF sensitizes HER-2/neu-overexpressing SKBR-3 cells to tumor necrosis factor (TNF) via up-regulation of TNF receptor-1. Mol Cancer Ther. 2005;4:1205–1213. doi: 10.1158/1535-7163.MCT-05-0014. [DOI] [PubMed] [Google Scholar]
- 54.Liu Y, Cheung LH, Rosenblum MG. A recombinant fusion protein scFvMEL/TNF decreases SAPK/JNK activation and induces IkB-alpha degradation in human melanoma cells. Proc AACR. 2001;42:503–2710. [Google Scholar]
- 55.Mihara M, Koishihara Y, Fukui H, Yasukawa K, Ohsugi Y. Murine anti-human IL-6 monoclonal antibody prolongs the half-life in circulating blood and thus prolongs the bioactivity of human IL-6 in mice. Immunology. 1991;74:55–59. [PMC free article] [PubMed] [Google Scholar]
- 56.Kuroda K, Miyata K, Shikama H, Kawagoe T, Nishimura K, Takeda K, Sakae N, Kato M. Novel muteins of human tumor necrosis factor with potent antitumor activity and less lethal toxicity in mice. Int J Cancer. 1995;63:152–157. doi: 10.1002/ijc.2910630127. [DOI] [PubMed] [Google Scholar]