Abstract
Angiopoietins (Ang) are involved in the remodeling, maturation, and stabilization of the vascular network. Ang-4 was discovered more recently; thus, its effect on angiogenesis and its interplay with other angiogenic factors have not been equivocally established. The role of Ang-4 in angiogenesis was tested in Matrigel chambers implanted into the subcutaneous space of nude mice. Ang-4 inhibited the angiogenic response of basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), and GLC19 tumor cells. In Matrigel chambers with Ang-4-transfected cells, the mean response was significantly lower than that of mock cells. Subcutaneous tumor interstitial fluid pressure (IFP) was significantly lower in Ang-4-transfected GLC19 tumors than in mock-transfected tumors. IFP reduction in Ang-4-transfected tumors was comparable to the reduction seen after bevacizumab treatment. In vitro, we examined the effect of recombinant Ang-4 on endothelial cell migration in Boyden chambers. Human umbilical vein endothelial cell (HUVEC) migration induced by bFGF and VEGF was inhibited by Ang-4 to control levels. In conclusion, we show that rhAng-4, as well as transfection with Ang-4, inhibits angiogenesis induced by GLC19 tumor cells and that Ang-4 expression reduces elevated tumor IFP. In addition, we demonstrate that rhAng-4 inhibits HUVEC migration and growth factor-induced angiogenesis.
Keywords: Ang-4, IFP, antiangiogenic, SCLC, migration
Introduction
Angiogenesis is considered essential to the growth and metastasis of solid tumors. It is a complex and dynamic process regulated by a delicate balance between angiogenic stimulators and angiogenic inhibitors that are present in the microenvironment. The best characterized proangiogenic factors are vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF). Numerous other proteins are known to play a role in angiogenesis, but less is known about the factors involved in remodeling and stabilizing the structure of primitive vessels. In particular, the first two members of the angiopoietin (Ang) family, Ang-1 and Ang-2, are considered important regulators of vascular remodeling and maturation. Ang are ligands for the endothelial cell (EC)-specific receptor tyrosine kinase, Tie-2. Ang-1 binding to Tie-2 induces receptor tyrosine phosphorylation, which recruits pericytes and smooth muscle cells into developing vessels and stabilizes mature vessels [1]. Moreover, Ang-1 confers resistance to leakage induced by VEGF [2]. Ang-1 is mitogenic for cultured ECs and induces proliferation in a dose-dependent manner [3]. Ang-2 does not induce Tie-2 phosphorylation on binding, but Ang-2 competitively inhibits Ang-1 binding [4,5]. Ang-2 is involved in the destabilization of the endothelium as part of permanent vascular remodeling in tumors [4,5]. The plastic state triggered by Ang-2 can lead to new vessel growth or vessel regression, depending on the presence of other proangiogenic factors. One theory is that the Ang-1/Ang-2 ratio in tumors is shifted in favor of Ang-2, which causes increased vascular plasticity [6]. Ang-1 promotes the in vivo growth of intracerebral gliomas in rats, which is associated with a well-differentiated and organized vascular structure, whereas overexpression of Ang-2 results in the opposite phenomenon [7]. In addition, Ang-2 has been shown to antagonize angiogenesis induced by bFGF and VEGF [8]. Recently, inhibition of Ang-2 was found to reduce tumor growth [9]. This is interesting, as previous reports have shown the antiangiogenic effects of Ang-2. The biologic roles of Ang-3 and Ang-4 and their effects on the interplay between multiple angiogenic factors within a tumor mass need clarification. High cytoplasmic expression of Ang-4 has been reported in human colorectal and gastric adenocarcinomas [10,11]; moreover, Ang-4 induced human umbilical vein endothelial cell (HUVEC) migration in an in vitro assay [12]. In mouse corneal assay, Ang-3 and Ang-4 are angiogenic in vivo [12]. However, overexpression of Ang-3 inhibited angiogenesis in the pulmonary metastasis of Lewis lung carcinoma and TA3 mammary carcinoma [13]; thus, only sparse and partly contradictory information exists regarding the activities of Ang-3 and Ang-4, and much remains to be learned about the biologic activity of the more recently discovered members of the Ang family [14]. The biologic action of Ang on tumor growth still remains uncertain and might depend on the unique mixture of angiogenic factors and their receptors in the microenvironment.
High permeability and leakage from tumor vessels are important contributing factors to increased interstitial fluid pressure (IFP) in solid tumors [15–18]. The overall aim of the present work was to investigate the effects of Ang-4 on in vivo angiogenesis. As Ang-1 and Ang-2 are regulators of vessel permeability and maturation, our aim was also to elucidate the effect of Ang-4 on tumor IFP, as IFP is regulated by vessel permeability.
Materials and Methods
Cell Cultures
HUVECs were obtained from Clonetics (Brøndby, Denmark) and cultured in an endothelial basal medium (EBM) 2 Bulletkit (Cambrex, Vallensbaek, Denmark). Experiments were performed on cells in passages 4 to 6.
The human small cell lung cancer (SCLC) cell line GLC19 was maintained with RPMI medium (GibcoBRL, Taastrup, Denmark) supplemented with 10% heat-inactivated fetal calf serum.
The cells were cultured in a humidified atmosphere with 5% CO2 at 37°C, without the addition of antibiotics.
Growth Factors
The following growth factors were used: recombinant human bFGF (Sigma-Aldrich, Vallensbaek, Denmark), recombinant human VEGF-A (R&D Systems, Abingdon, UK), and recombinant human Ang-4 (R&D Systems).
Migration Assays
In vitro EC migration was assessed with Boyden chamber assay. Polycarbonate membranes (Neuro Probe, Inc., Gaithersburg, MD) of 8 µm pore size were coated overnight in a 0.1-mg/ml solution of human collagen IV (Sigma-Aldrich). Serum-free EBM-2 growth medium, with 0.2% bovine serum albumin and growth factors as chemotactic stimuli, was applied to the bottom wells of a Neuro Probe AP48 chemotaxis chamber; a complete Bulletkit medium with serum and growth factors was used as positive control. In accordance with standard usage guidelines, coated membranes were applied, and a cell suspension containing 40,000 cells in serum-free EBM-2 medium was added to each upper well. After 4 hours of migration in a humidified chamber at 37°C and 5% CO2, the chambers were disassembled. The membranes were fixed in methanol and stained with Giemsa stain (Invitrogen, Taastrup, Denmark). The stained membranes were attached to a microscope slide, and cells were wiped off the non-migrated side of the filter. Migrated cells were counted under a microscope with a counting grid. All groups were studied in hexaduplicate in each experiment, and the experiment was performed thrice. Applied growth factor concentrations were chosen based on previous studies of EC migration [8].
Real-Time Monitoring of HUVEC Proliferation and Viability
The Real-Time Cell Electronic Sensing (RT-CES) system (ACEA Biosciences, Inc., San Diego, CA) uses an electronic impedance readout to noninvasively quantify adherent cell proliferation and viability in real time.
A x16 device was coated with gelatin (1 mg/ml) for 15 to 30 minutes at 37°C and 5% CO2 in a humidified atmosphere. Wells were washed twice with sterile water. At t = 0 hours, 50 µl of EBM-2 media with 2% fetal bovine serum (FBS) was added per well, and background impedance was measured. Then 5000 cells in 100 µl of culture media were applied per well. bFGF (10 ng/ml) and VEGF (3 ng/ml) were added immediately. At t = 5 hours, HUVECs attached, and Ang-4 was added in different concentrations (10, 40, or 200 ng/ml). Attachment, spread, and proliferation were continuously monitored every 10 minutes for a period of 24 hours.
The electronic readout of cell sensor impedance was displayed as an arbitrary cell index unit. The cell index at each time point was defined as Rn - Rb/Rb, where Rn is the cell electrode impedance of the well with cells and Rb is the background impedance of the well with media alone [19].
In addition, HUVEC proliferation was tested in a 96-well device. HUVECs were seeded, and growth factors were applied as described above. Cells were fixed and stained before the photography of the wells. Proliferation was calculated by computer-assisted cell counting.
Animals
Male 7-week-old athymic nude mice (NMRI-nu/nu) obtained from Taconic M&B (Lille Skensved, Denmark) were used. The mice were kept in groups of five in individually ventilated SealSafe cages (Scanbur BK A/S, Karlslunde, Denmark) and fed sterile food pellets and water ad libitum. Lighting was controlled in a 12-hour light/dark cycle. Institutional guidelines for animal welfare and experimental conduct were followed.
Matrigel Chambers
In vivo, angiogenic response was tested using a modified Z-chamber angiogenesis assay, as previously described [8,20].
All groups consisted of 16 to 20 chambers, corresponding to 8 to 10 animals (two chambers per animal). The animals were anesthetized using subcutaneous injections of 1 mg/kg xylazine (Rompun; Bayer, Kgs. Lyngby, Denmark) and 10 mg/kg ketamine (Ketalar; Pfizer, Sollentuna, Sweden) in an isotonic 0.9% NaCl solution. Immediately before implantation, appropriate portions of BD Matrigel (BD Biosciences, San Diego, CA) containing the growth factors and/or the tumor cells to be examined were prepared and kept on ice. For experiments with tumor cells, 5 x 106 GLC19 cells per chamber were resuspended in Matrigel; experiments were performed on cells in passages 102 to 107. Growth factor-reduced Matrigel was used for studies involving growth factors only, whereas standard Matrigel was used for studies with tumor cells. Growth factor concentrations were chosen based on previous studies of bFGF, VEGF, and Ang with Matrigel chamber assay [8].
Z-chambers (Lab Vision Corporation, Fremont, CA) were then filled with appropriate Matrigel mixture and implanted subcutaneously in male nude NMRI mice. Using blunt dissection through a 1.5-cm transversal incision in the neck region of each mouse, a subcutaneous pocket on either flank of the animal was formed. A chamber was inserted into each pouch, and the incision was closed with nonresorbable suture (Ethicon 6-0).
The chambers used were of the type TZ-014, with an outer diameter of 14 mm, a membrane pore size of 180 µm, and a chamber volume of approximately 0.2 ml.
Excision
The chambers were well tolerated, and only a few adhesive tissue reactions were observed at the time of removal. The location of the incision at a distance from the actual implantation site prevented the rigid chambers from inflicting mechanical stress on the wounds. No macroscopic signs of inflammation were observed.
Mice were sacrificed on day 12 (growth factor experiment) or on day 16 (tumor cell experiment), and the chambers were excised. The time points were chosen based on pilot studies showing an appropriate measurable angiogenic response on days 12 and 16, respectively. Care was taken to remove any tissue adhering to the outside of the chambers, which were then placed on a white surface. Using a setup consisting of an Olympus SC-40 microscope (Olympus, Melville, NY) fitted with a ring lighting system and a Leica DC-150 digital camera (Leica, Bannockburn, IL), the chambers were photographed from both sides. Images were saved as high-quality compressed images in JPEG format.
Quantification of Matrigel Chamber Angiogenesis
The quantification procedure was performed as described previously [14]. Briefly, the image processing software Paint Shop Pro 8.1 (Jasc Software, Eden Prairie, MN) was used to select the area of interest (AOI) for each chamber. Subsequently, the images were opened with the program Sigma Scan Pro 5.0 (SPSS Science, Chicago, IL) to detect the total number of red pixels for each image and then to calculate the corresponding area. These data were transferred to a spreadsheet, where the red area of each image was divided by the total number of pixels in the AOI, yielding the percentage of red coloration.
For each group, the mean values of the percentages of red pixels were calculated.
Histochemistry
After photography, chamber content was excised by cutting nylon membranes along the inner rim of the Plexiglas ring.
Chambers for paraffin sections were then fixed in 4% paraformaldehyde and embedded in paraffin blocks. Chambers for CD31 immunohistochemistry were frozen in pre-cooled isopentane. Sections were cut, air-dried, fixed in 4% paraformaldehyde, washed in phosphate-buffered saline nd Tris-buffered saline (pH 7.6), and incubated with 5% rabbit serum (X902; DAKO, Albertslund, Denmark) for 30 minutes. Incubation with rat anti-CD31 antibodies was carried out overnight at 4°C. An antibody solution was used in a 0.5-µg/ml dilution (PharMingen 553370; BD Biosciences). Then, sections were incubated with biotin-conjugated rabbit antirat immunoglobulin (E468; DAKO) in a 1:600 dilution for 30 minutes and incubated with alkaline phosphatase-conjugated streptavidin (D396; DAKO) for 30 minutes. The Fast Red Substrate System (K699; DAKO) was used as substrate for the alkaline phosphatase reaction. Sections were counterstained with hematoxylin.
Paraffin sections were used for Ki67 immunohistochemistry. NCL-Ki67p (Novocastra Laboratories, Ltd., Newcastle, UK) was used as a primary antibody in 1:4000 dilution.
Generation of Ang-4 Expression Plasmid and Transfection
A pDrive vector containing the full coding region of Ang-4 (Incyte Easy-to-Spot Human cDNA Clone; Open Biosystems, Huntsville, AL) was verified by sequencing (MWG biotech AG, Ebersberg, Germany). The Ang-4 coding region was cut out by BamHI and XbaI and ligated into the expression vector pcDNA3.1(-) (Invitrogen, Taastrup, Denmark). To verify the construct, HEK293 cells were transfected with the Ang-4 pcDNA3.1(-) construct or the empty pcDNA3.1(-) vector (mock) using Lipofectamine2000 (Invitrogen), according to the manufacturer's instructions. After 72 hours, media and cells were collected, and the expression of Ang-4 in cells and media was verified by Western blot analysis. Subsequently, GLC19 cells were transfected with Ang-4 pcDNA3.1 (-) or with mock vector, and were verified as described above. After 48 hours, 2 mg/ml G418 was added for the selection of transfected cells.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted with Trizol (Invitrogen) or with RNeasy minikit (Qiagen, Hilden, Germany), as described by the manufacturer. cDNA was generated by ThermoScript cDNA kit (Invitrogen) following the manufacturer's instructions. All RT-PCR fragments were amplified using the AmpliTaqGold System (Applied Biosystems, Naerum, Denmark) under the following conditions: 95°C for 5 minutes, 35 cycles at 95°C for 30 seconds, annealing at 60°C for 1 minute, and elongation at 72°C for 1 minute. All PCRs were finalized by a 10-minute elongation at 72°C. A control reaction with GAPDH primers (sense 5′-AACGGATTTGGTCGTATTGGGC-3′ and antisense 5′-TAAGCAGTTGGTGGTGCAGG-3′) was performed to verify the quality of the cDNA before performing PCR with primers for Ang-4 (368-bp product; sense 5′-TTCTGTCCACCAACAAGCTG-3′ and antisense 5′-CTCTGCACAGTCCTGGAACA-3′).
Western Blot Analysis
Protein was extracted from cell pellets or tissue homogenates with a lysis buffer [50 mM Tris (pH 7.5), 150 mM NaCl, 1% NP-4, 0.1% sodium dodecyl sulfate, 1% (wt/vol) natrium deoxycholate, 1 mM Na3VO4, 1 mM dithiothreitol, complete protease inhibitors (Roche Diagnostics, Hvidovre, Denmark)] and homogenized by sonication. Protein concentrations were determined using BCA protein assay (Pierce, Rockford, IL). Twenty micrograms of protein was separated on NuPage Bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes by semidry blotting. Protein detection was performed after blocking in 5% milk powder in Tris-buffered saline with 0.1% Tween-20 (TBST). The membrane was incubated overnight at 4°C with a primary antibody in TBST (0.1 µg/ml Ang-4, AF-964, R&D Systems; 1:80,000 α-tubulin, T-9026, Sigma, Vallensbaek, Denmark) and with horseradish peroxidase-coupled secondary antibody (DAKO) for 1 hour before detection with ECL+ (Amersham). Protein expression was visualized and quantified by chemifluorescence scanning on a STORM 840 (Molecular Dynamics, Inc., Sunnyvale, CA). α-Tubulin expression was used as loading control.
Tumor Xenografts
Xenografts were established by subcutaneous injection of tumor cells in the flank of nude mice. Cells (5 x 106) were mixed with 200 µl of Matrigel before injection. Tumors were measured thrice a week during tumor growth as two orthogonal diameters d1 and d2. Tumor volume was calculated according to the equation:
where k is an empirical constant equal to 0.67 [21].
Bevacizumab
Bevacizumab (Avastin; Roche Pharmaceuticals) is an anti-VEGF humanized monoclonal antibody that binds VEGF and prevents the interaction of VEGF with its receptors (Flt-1 and KDR) on the surface of ECs [22,23]. Bevacizumab was given as 200-µg intraperitoneal injections thrice a week, starting on the day before the implantation of Matrigel chambers or tumor cells.
IFP
Tumor IFP measurements were performed by wick-in-needle technique [24], with modifications [25]. Briefly, a 23-gauge needle with a 2- to 3-mm side hole 4 to 5 mm from the tip was filled with five surgical sutures (Ethicon 6-0). A sensing needle was coupled to a pressure sensor by a water column in polyethylene tubing (inner diameter, 0.58 mm) filled with heparinized saline (70 U/ml). The pressure sensor was connected to a MacLab/4e (ADInstruments, Ltd., Colorado Springs, CO) through an ML112 bridge amplifier (AD-Instruments, Ltd.). Pressure data from the sensor were collected from a MacLab/4e digitizer by SCSI interface using a personal computer with a PowerLab Chart software v. 4.2 (ADInstruments, Ltd.). The pressure-sensing system was calibrated against a water column of predefined height before each experiment, and calibration was checked at the end of each experiment. Calibration remained stable over time.
Because tumor IFP can vary with tumor size, IFP was measured only for tumors with a volume of 600 to 1000 mm3 to ensure comparability between groups.
Statistical Analysis
Statistical evaluations of differences between treated and untreated groups were performed using a two-tailed Mann-Whitney U test. The software package SPSS 12.0 was used for statistical analyses, and differences were accepted as statistically significant at P < .05.
Results
Ang-4 Inhibits EC Migration in Serum-Free and Growth Factor-Stimulated Conditions
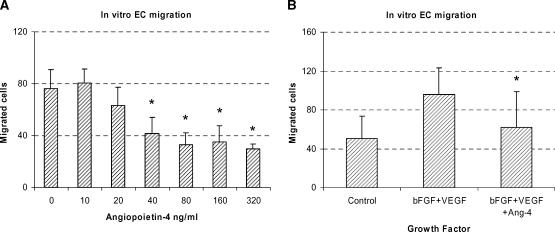
When Ang-4 was added to a serum-free medium, EC migration in the Boyden chamber assay was reduced in a dose-dependent manner (Figure 1A); the reduction from 40 to 320 ng/ml was significant (P = .002).
Figure 1.
In vitro EC migration in Boyden chambers. (A) Addition of 40 ng/ml Ang-4 to the serum-free medium significantly reduced the number of migrated cells from 76.2 to 41.5. Further addition of Ang-4 did not result in further inhibition of EC migration. (B) In a separate experiment, the combination of 50 ng/ml bFGF and 16 ng/ml VEGF caused EC migration significantly above the control level (mean, 96.1 vs 50.2 cells/field). This response was significantly inhibited by the addition of Ang-4 (40 ng/ml). Data are expressed as mean ± SD. Each column represents a mean value of six wells in each group. Migrated cells were counted at x250 magnification.
A combination of 50 ng/ml bFGF and 16 ng/ml VEGF significantly stimulated EC migration (P = .014) (Figure 1B). Further addition of 40 ng/ml Ang-4 to the medium containing bFGF and VEGF significantly inhibited migration (P =.038) (Figure 1B).
Effect of Ang-4 on EC Proliferation
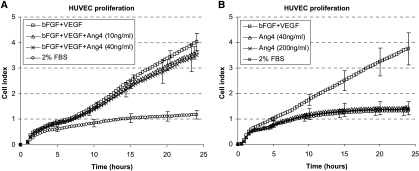
By means of the RT-CES system, we tested the effect of Ang-4 on HUVEC proliferation in a low-serum medium and in a medium with bFGF and VEGF. The combination of bFGF (10 ng/ml) and VEGF (3 ng/ml) significantly increased HUVEC proliferation (P = .029) (Figure 2A). However, HUVEC proliferation was not affected by Ang-4 in this system; neither the proliferation level under low serum conditions (Figure 2B) nor the proliferation induced by bFGF and VEGF was affected (Figure 2A). Similar results were obtained by the computer-assisted cell counting of cells cultured with the same growth factors in 96-well trays (data not shown).
Figure 2.
RT-CES measurements of HUVEC proliferation are shown. The combination of bFGF (10 ng/ml) and VEGF (3 ng/ml) significantly induced HUVEC proliferation (A and B). There was no significant effect of Ang-4 addition, neither when Ang-4 was applied in combination with bFGF and VEGF (A) nor when Ang-4 was applied as a single agent (B). After 24 hours, proliferation curves level off, and cell growth is no longer exponential. Beyond 24 hours, the bottom of the wells is completely covered by ECs, and there may be lack of media and growth factors. As expected, the lowest proliferation rate was found in the wells containing FBS alone, with no growth factors added.
Growth Factor-Induced In Vivo Angiogenesis in Matrigel Chambers Is Inhibited by Ang-4
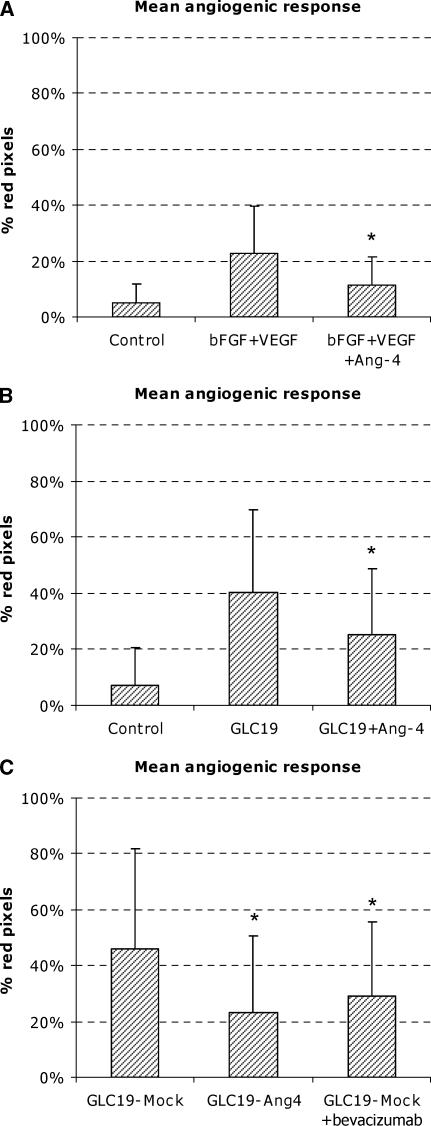
The addition of 750 ng/ml bFGF and 250 ng/ml VEGF to the Matrigel chambers on implantation induced a mean angiogenic response of 23% red pixels. This was significantly above the control level of 5% (P < .0001) (Figure 3A). By further addition of Ang-4 (500 ng/ml), the response was reduced to 11% red coloration, which was significantly less than the response to bFGF and VEGF alone (P = .014).
Figure 3.
In vivo angiogenic response in Matrigel chambers. (A) Growth factor-induced angiogenesis in Matrigel chambers. The combination of 750 ng/ml bFGF and 250 ng/ml VEGF induced angiogenesis on day 12 significantly above the control level. Addition of Ang-4 (500 ng/ml) significantly inhibited the response. (B) Tumor angiogenesis in Matrigel chambers induced by GLC19 SCLC cells. Tumor cells induced a highly significant angiogenic response by 16 days of implantation in Matrigel chambers. The addition of Ang-4 (1500 ng/ml) to tumor cells significantly inhibited angiogenic response. (C) Angiogenesis in Matrigel chambers induced by transfected tumor cells. Ang-4-transfected GLC19 cells induced an angiogenic response that was significantly lower than that of mock-transfected cells. Bevacizumab treatment of the mock-transfected cells also reduced angiogenic response. Each column represents a mean value of 18 to 20 chambers in each group. Data are expressed as mean ± SD.
Tumor Angiogenesis in Matrigel Chambers Is Inhibited by Ang-4
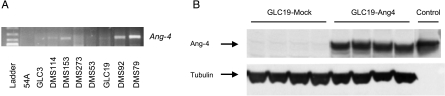
Ang-4 expression was evaluated by RT-PCR in a series of SCLC lines. In four of nine cell lines, a transcript was detected (Figure 6A). The SCLC cell line GLC19 was chosen for tumor cell studies because of its lack of endogenous Ang-4 production and its relatively high angiogenic activity in the in vivo Matrigel chambers in pilot studies. When grown as subcutaneous xenograft tumors, GLC19 has a relatively high vessel density and VEGF expression, compared with other SCLC lines [26].
Figure 6.
(A) RT-PCR ofcDNA from nine SCLC cell lines with primers specific for Ang-4. PCR products were separated on a 1.2% agarose gel and visualized by ethidium bromide staining. A ladder was included as size control. (B) A protein isolated from subcutaneous tumor xenografts was used for Western blot analysis with Ang-4 antibody. Ang-4 expression is retained in Ang-4-transfected GLC19 cells following growth in nude mice. Mock-transfected cells had no Ang-4 protein expression. Equal loading was confirmed by α-tubulin detection. Control, recombinant Ang-4.
Chambers with GLC19 tumor cells showed a mean angiogenic response of 40%, which is significantly above the cell-free control level of 7% (P < .0001) (Figure 3B). When 1500 ng/ml recombinant Ang-4 was added to tumor cells on implantation, the mean angiogenic response was significantly lower at 25% (P = .015) (Figure 3B). We found no further inhibition of tumor angiogenesis in the Matrigel chambers with the addition of a larger dose of Ang-4 (3000 ng/ml).
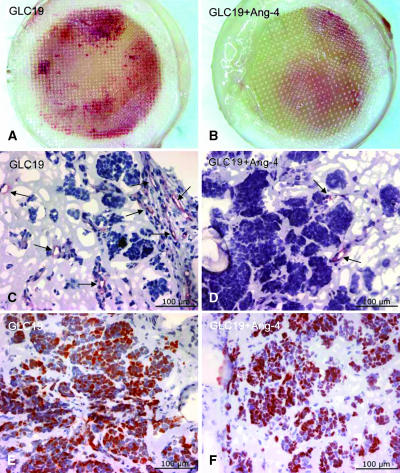
CD31 immunohistochemistry confirmed the widespread presence of ECs in the chambers with GLC19 tumor cells, indicating that the observed degree of red coloration does indeed reflect angiogenesis (Figure 4, A and C). The addition of Ang-4 to the GLC19 chambers resulted in less red coloration, which also was reflected by a sparser distribution of CD31+ structures in these chambers (Figure 4, B and D).
Figure 4.
Matrigel chambers and histology. (A and B) Digital photographs of Matrigel chambers with GLC19 SCLC cells (A) or the combination of GLC19 SCLC cells and rhAng-4 (B). We found only limited red coloration in Ang-4-treated chambers. (C and D) Anti-CD31 immunohistochemical staining of ECs in Matrigel chambers. Numerous structures inside GLC19 chambers were positively stained (C), whereas only sparse staining was seen in Ang-4-treated chambers (D). (E and F) Anti-Ki67 staining of Matrigel chambers showed proliferating cells in both groups. (C and D) Arrows show vascular structures.
To ensure the viability of tumor cells in the chambers, anti-Ki67 staining was applied to assess proliferation (Figure 4, E and F). Ki67 nuclear staining of Matrigel chambers showed proliferating cells in both untreated GLC19 chambers and chambers with GLC19 and Ang-4, indicating that reduced vascularity is caused by a direct effect on angiogenesis, rather than on cytotoxicity. Proliferation was pronounced in the periphery of the chambers, close to the membranes, whereas the center of the chambers showed very little proliferation. This was the case in chambers with and without Ang-4.
Transfection of GLC19 with Ang-4 Inhibits Tumor Angiogenesis in Matrigel Chambers
To further evaluate the role of Ang-4, we transfected GLC19 cells with Ang-4 or with an empty control vector (mock). The mean angiogenic response in Matrigel chambers with mock-transfected GLC19 tumor cells was 46% (Figure 3C). In Matrigel chambers with Ang-4-transfected cells, the mean response was 23%, which was significantly lower than the response of mock cells (P = .003) (Figure 3C). To demonstrate the effect of a known antiangiogenic substance in this model system, mice bearing chambers with mock-transfected cells were treated with bevacizumab. Similar to transfection with Ang-4, bevacizumab significantly lowered angiogenic response to 29% (Figure 3C).
Ang-4 Transfection Does Not Affect the Growth of Tumor Xenografts
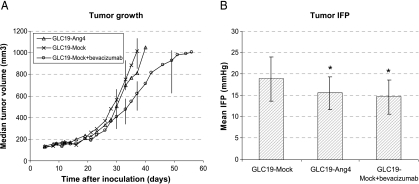
When Ang-4-transfected and mock-transfected GLC19 cells were grown as subcutaneous tumor xenografts, no difference in growth rate or growth delay was found (Figure 5A). To verify if Ang-4 transfection was intact following growth on mice, tumors were excised for protein isolation. Ang-4 expression was high in tumors from Ang-4-transfected GLC19 cells (Figure 6B). As a positive control for tumor growth inhibition, we used bevacizumab treatment, which significantly slowed the growth of subcutaneous tumors. We used the Kaplan-Meier log rank test of time until the tumor volume was ≥ 1000 mm3 (P = .005) (Figure 5A).
Figure 5.
GLC19-transfected cells grown as subcutaneous tumors on mice. (A) Each curve represents a median value of 10 tumors in each group. Transfection of GLC19 cells with Ang-4 did not affect tumor growth. We found no difference in growth between GLC19-Ang-4 and GLC19-mock tumors. Bevacizumab treatment significantly slowed tumor growth. Bars represent interquartile ranges. (B) IFP of subcutaneous tumors. All IFP recordings were carried out at tumor sizes of 600 to 1000 mm3. Each column represents a mean IFP of 12 to 17 tumors in each group. Data are expressed as mean ± SD.
Tumor IFP Is Decreased by Ang-4
Tumor IFP was significantly lower in Ang-4-transfected GLC19 xenografts than in mock-transfected xenografts (P = .027) (Figure 5B). The mean IFP in the two groups was 15.6 ± 3.8 and 18.9 ± 5.1 mm Hg, respectively. Furthermore, IFP was significantly lower in bevacizumab-treated tumors than in mock-transfected controls (P = .006) (Figure 5B). The mean IFP in bevacizumab-treated tumors was 14.6 ±4.0 mm Hg.
Discussion
The aim of the present study was to clarify the role of Ang-4 in angiogenesis. The effect of different Ang on the angiogenic process is complex, as exemplified by Ang-2. Ang-2 stimulates angiogenesis in the corneal model in the presence of VEGF [27], but it also competes with Ang-1 for the Tie-2 receptor. VEGF plays an important role in the effects of Ang-2. Ang-2 is thought to stimulate pericyte detachment and basal membrane degradation; in the presence of VEGF, this will allow EC sprouting and migration, facilitating the early phases of angiogenesis. If VEGF levels are low, the destabilization of the vessel wall will instead lead to an antiangiogenic effect of Ang-2.
The Matrigel chamber assay has the advantage of allowing the study of well-characterized combinations of angiogenic factors. We have previously shown that Ang-2 inhibits the angiogenesis induced by a bFGF/VEGF growth factor combination in the Matrigel chamber assay [8]. In the present study, we found a similar inhibitory effect of Ang-4. Furthermore, this study shows that Ang-4 inhibits EC migration in vitro and tumor cell-induced angiogenesis in vivo. The doses of growth factors used in the experiments were based on pilot studies with several doses and were in the same order as those used by other groups [12,13].
In several repeated experiments, we found that Ang-4 inhibited EC migration. Both migration in the serum-free medium and growth factor-stimulated migration were inhibited by Ang-4. In a different experimental setup, Lee et al. [12] found increased HUVEC migration on stimulation with Ang-4. This dissimilarity may be due to differences in culture conditions or in coating materials because both parameters can be crucial to the outcome of EC migration in the Boyden chamber assay.
To evaluate the effect of Ang-4 on EC proliferation, we used two different assays: RT-CES and computer-assisted cell counting. We found no increase in HUVEC proliferation after the addition of Ang-4. This is in accordance with previous findings [28].
The in vivo effect of Ang-4 may be site-specific or assay-specific and may be influenced by local differences in microenvironmental factors (e.g., growth factor levels). In our setup, Ang-4 was tested in the presence of GLC19 tumor cells, or of bFGF and VEGF in combination. Vessels in different regions of a tumor or in an experimental area of an in vivo system might be exposed to entirely different levels of VEGF and Ang. Ang-4 inhibited angiogenesis in the models used in the present study.
The angiogenic response induced by tumor cells appears to be more robust than the response induced by growth factors alone. The continuous secretion of VEGF and bFGF from tumor cells may cause higher local concentrations of growth factors compared to the gradual release of recombinant factors from the Matrigel chambers. Furthermore, secretion from tumor cells in the chamber might increase as cells proliferate, whereas the release of recombinant growth factors is likely to decrease as the deposit in the chamber is depleted. Moreover, tumor cells may produce other growth factors in addition to VEGF and bFGF, and their presence may constitute a proangiogenic stimulus as well.
The inhibitory effect of Ang-4 on angiogenesis driven by recombinant growth factors, together with the observation of a similar tumor cell proliferation in the treated and untreated groups, indicates that angiogenesis is affected directly by Ang-4 and not by toxicity in tumor cells. This is also supported by the similarity between the effects of Ang-4 and bevacizumab in the Matrigel chambers.
There was no difference in growth rate between Ang-4-transfected and mock-transfected tumor cells. Apparently, Ang-4-induced inhibition of angiogenesis was not enough to inhibit tumor growth. Angiogenesis was not completely blocked by Ang-4 in the Matrigel chambers, and the remaining angiogenesis was sufficient for the tumor to grow. Treatment with bevacizumab significantly slowed tumor growth, as expected [29,30]. VEGF receptors are now known to be present on some tumor cells [31–33]; accordingly, there may be a direct effect of anti-VEGF therapy (e.g., bevacizumab) on tumor cells themselves, which adds to the antiangiogenic effect. This might partly explain the observed difference in the tumor growth effects of bevacizumab and Ang-4.
Increased vascular permeability contributes to elevated IFP, which is characteristic of solid tumors [16,17]. VEGF is a proleakage factor, and anti-VEGF treatment by bevacizumab has been shown to reduce IFP in human tumor xenografts [34] and in rectal cancer patients [35], most likely by antileakage mechanisms as part of the vascular normalization process. In accordance with what has previously been shown, we observed a lower mean IFP in bevacizumab-treated tumor xenografts. A lower tumor IFP is attractive as it might increase the delivery of drugs to tumor cells [18]. In this study, we show that Ang-4 decreases IFP in tumors to a similar extent as treatment with bevacizumab. It has previously been shown that Ang-4 reduces thrombin-induced increase in the permeability of EC monolayers [28]. Furthermore, in a murine model of acute lung injury, Ang-4 has been shown to have a stabilizing effect on vessels, diminishing capillary leakage [36]. In conjunction with our results, these observations strongly indicate a reducing effect of Ang-4 on vascular permeability. Vascular integrity may well be regulated by a balance between proleakage factors VEGF and Ang-2, and antileakage factors Ang-1 and Ang-4. Thus, changes in any given factor may be less important than the overall balance between the proleakage and antileakage factors.
In conclusion, our study shows that, in a human SCLC tumor line, Ang-4 can contribute to the regulation of vascular maturation and remodeling as an angiogenesis inhibitor and as an antileakage factor.
Acknowledgements
We thank Pia G. Knudsen, Tina Larsen, Bente Møller, and Jette Christiansen for valuable technical assistance.
Footnotes
This research was supported by grants from the Danish Cancer Society, the Danish Medical Research Council and The A. P. Moeller Foundation for the advancement of medical science.
References
- 1.Suri C, Jones PF, Patan S, Bartunkova S, Maisonpierre PC, Davis S, Sato TN, Yancopoulos GD. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 2.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 3.Kanda S, Miyata Y, Mochizuki Y, Matsuyama T, Kanetake H. Angiopoietin 1 is mitogenic for cultured endothelial cells. Cancer Res. 2005;65:6820–6827. doi: 10.1158/0008-5472.CAN-05-0522. [DOI] [PubMed] [Google Scholar]
- 4.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 5.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158:563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- 7.Machein MR, Knedla A, Knoth R, Wagner S, Neuschl E, Plate KH. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557–1570. doi: 10.1016/S0002-9440(10)63413-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley CD, Olsen MW, Lund EL, Kristjansen PE. Angiogenic synergy of bFGF and VEGF is antagonized by Angiopoietin-2 in a modified in vivo Matrigel assay. Microvasc Res. 2004;68:161–168. doi: 10.1016/j.mvr.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Oliner J, Min H, Leal J, Yu D, Rao S, You E, Tang X, Kim H, Meyer S, Han SJ, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama T, Hatachi G, Wen CY, Yoshizaki A, Yamazumi K, Niino D, Sekine I. Expression and significance of Tie-1 and Tie-2 receptors, and angiopoietins-1, 2 and 4 in colorectal adenocarcinoma: immunohistochemical analysis and correlation with clinicopathological factors. World J Gastroenterol. 2005;11:964–969. doi: 10.3748/wjg.v11.i7.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakayama T, Yoshizaki A, Kawahara N, Ohtsuru A, Wen CY, Fukuda E, Nakashima M, Sekine I. Expression of Tie-1 and 2 receptors, and angiopoietin-1, 2 and 4 in gastric carcinoma; immunohistochemical analyses and correlation with clinicopathological factors. Histopathology. 2004;44:232–239. doi: 10.1111/j.0309-0167.2004.01817.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Cho CH, Hwang SJ, Choi HH, Kim KT, Ahn SY, Kim JH, Oh JL, Lee GM, Koh GY. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Liu YJ, Yu Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 2004;64:6119–6126. doi: 10.1158/0008-5472.CAN-04-1054. [DOI] [PubMed] [Google Scholar]
- 14.Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, McClain J, Copeland NG, Gilbert DJ, Jenkins NA, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure—an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 16.Boucher Y, Lee I, Jain RK. Lack of general correlation between interstitial fluid pressure and oxygen partial pressure in solid tumors. Microvasc Res. 1995;50:175–182. doi: 10.1006/mvre.1995.1051. [DOI] [PubMed] [Google Scholar]
- 17.Boucher Y, Leunig M, Jain RK. Tumor angiogenesis and interstitial hypertension. Cancer Res. 1996;56:4264–4266. [PubMed] [Google Scholar]
- 18.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 19.Solly K, Wang X, Xu X, Strulovici B, Zheng W. Application of Real-Time Cell Electronic Sensing (RT-CES) technology to cell-based assays. Assay Drug Dev Technol. 2004;2:363–372. doi: 10.1089/adt.2004.2.363. [DOI] [PubMed] [Google Scholar]
- 20.Kragh M, Hjarnaa PJ, Bramm E, Kristjansen PE, Rygaard J, Binderup L. In vivo chamber angiogenesis assay: an optimized Matrigel plug assay for fast assessment of antiangiogenic activity. Int J Oncol. 2003;22:305–311. [PubMed] [Google Scholar]
- 21.Rygaard K, Spang-Thomsen M. Quantitation and Gompertzian analysis of tumor growth. Breast Cancer Res Treat. 1997;46:303–312. doi: 10.1023/a:1005906900231. [DOI] [PubMed] [Google Scholar]
- 22.Kim KJ, Li B, Houck K, Winer J, Ferrara N. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7:53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 24.Fadnes HO, Reed RK, Aukland K. Interstitial fluid pressure in rats measured with a modified wick technique. Microvasc Res. 1977;14:27–36. doi: 10.1016/0026-2862(77)90138-8. [DOI] [PubMed] [Google Scholar]
- 25.Boucher Y, Jain RK. Microvascular pressure is the principal driving force for interstitial hypertension in solid tumors: implications for vascular collapse. Cancer Res. 1992;52:5110–5114. [PubMed] [Google Scholar]
- 26.Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6:4287–4291. [PubMed] [Google Scholar]
- 27.Asahara T, Chen D, Takahashi T, Fujikawa K, Kearney M, Magner M, Yancopoulos GD, Isner JM. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233–240. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 28.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 29.Presta LG, Chen H, O'Connor SJ, Chisholm V, Meng YG, Krummen L, Winkler M, Ferrara N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 30.Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 31.Ferrer FA, Miller LJ, Lindquist R, Kowalczyk P, Laudone VP, Albertsen PC, Kreutzer DL. Expression of vascular endothelial growth factor receptors in human prostate cancer. Urology. 1999;54:567–572. doi: 10.1016/s0090-4295(99)00156-9. [DOI] [PubMed] [Google Scholar]
- 32.Decaussin M, Sartelet H, Robert C, Moro D, Claraz C, Brambilla C, Brambilla E. Expression of vascular endothelial growth factor (VEGF) and its two receptors (VEG-R1-Flt1 and VEGF-R2-Flk1/KDR) in non-small cell lung carcinomas (NSCLCs): correlation with angiogenesis and survival. J Pathol. 1999;188:369–377. doi: 10.1002/(SICI)1096-9896(199908)188:4<369::AID-PATH381>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 33.Duff SE, Jeziorska M, Rosa DD, Kumar S, Haboubi N, Sherlock D, O'dwyer ST, Jayson GC. Vascular endothelial growth factors and receptors in colorectal cancer: implications for anti-angiogenic therapies. Eur J Cancer. 2006;42:112–117. doi: 10.1016/j.ejca.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 34.Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain RK, Suit HD, et al. Antivascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60:5565–5570. [PubMed] [Google Scholar]
- 35.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karmpaliotis D, Kosmidou I, Ingenito EP, Hong K, Malhotra A, Sunday ME, Haley KJ. Angiogenic growth factors in the pathophysiology of a murine model of acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2002;283:L585–L595. doi: 10.1152/ajplung.00048.2002. [DOI] [PubMed] [Google Scholar]