Abstract
In this study, we investigated the time course gene expression profile of preneoplastic nodules and hepatocellular carcinomas (HCC) to define the genes implicated in cancer progression in a resistant hepatocyte model. Tissues that included early nodules (1 month, ENT-1), persistent nodules (5 months, ENT-5), dissected HCC (12 months), and normal livers (NL) from adult rats were analyzed by cDNA arrays including 1185 rat genes. Differential genes were derived in each type of sample (n = 3) by statistical analysis. The relationship between samples was described in a Venn diagram for 290 genes. From these, 72 genes were shared between tissues with nodules and HCC. In addition, 35 genes with statistical significance only in HCC and with extreme ratios were identified. Differential expression of 11 genes was confirmed by comparative reverse transcription-polymerase chain reaction, whereas that of 2 genes was confirmed by immunohistochemistry. Members involved in cytochrome P450 and second-phase metabolism were downregulated, whereas genes involved in glutathione metabolism were upregulated, implicating a possible role of glutathione and oxidative regulation. We provide a gene expression profile related to the progression of nodules into HCC, which contributes to the understanding of liver cancer development and offers the prospect for chemoprevention strategies or early treatment of HCC.
Keywords: Hepatocarcinogenesis, transcriptomic, redox control, cancer markers, glutathione metabolism
Introduction
Hepatocellular carcinoma (HCC) is a cancer that is prevalent worldwide. Detection of serum α-fetoprotein and liver imaging techniques are the conventional methods used for the identification of this malignancy [1–3]. However, these techniques are not reliable for early diagnosis [4]. Early detection of HCC is an important issue because effective treatment of small tumors is possible by surgical resection [5]. The early stages of human and animal hepatocarcinogenesis are characterized by the presence of preneoplastic lesions, namely, nodules that could be useful for the identification of early tumor markers, for the study of liver cancer progression, and for chemopreventive strategies [6,7]. However, human precancerous lesions such as dysplasic nodules are rather difficult to obtain because of their small size, incidental detection, and coexistence with other liver pathologies [8]. Thus, the rat model for hepatocarcinogenesis has been proven important as a tool for the analysis of nodules and liver cancer progression. Chemically induced nodules in the rat liver share several morphologic, biochemical, and molecular characteristics with human dysplasic nodules [9]. In the resistant hepatocyte (RH) model described by Solt and Farber [10] and Farber and Sarma [11], a necrogenic dose of diethylnitrosamine (DEN) induces resistant hepatocytes (RHs) during initiation. These cells can be stimulated to develop rapidly into altered hepatocyte foci and nodules by a selection procedure in which the carcinogen 2-acetylaminofluorene (2-AAF) is administered in combination with partial hepatectomy (PH) [12,13]. With this regime, which induces rapid growth of resistant altered hepatocytes, visible nodules are formed synchronously—some of them displaying sufficient genomic damage and progress to HCC without any additional treatment with the carcinogen.
As with other cancers, HCC is caused by the accumulation of genetic alterations resulting in a distorted expression of thousands of genes. Hence, gene expression analysis with DNA microarray methodologies has been successfully used to study cancer development [14,15]. In this study, using a modified RH model, we investigated the time course of expression changes during the progression of nodules toward cancer using Atlas array membranes bearing 1185 well-typified rat genes. By comparing the expression data between tissues with nodules and HCC, we were able to provide a list of selectively expressed genes at different stages and those genes shared in both situations. We showed that some of the differentially regulated genes are involved in glutathione metabolism and redox control, agreeing with the proposition that altered cellular homeostasis may be a predisposing factor for disease progression in rat hepatocarcinogenesis.
Materials and Methods
Animals and Treatments
F344 rats weighing 180–200 g (UPEAL-Cinvestav, Mexico, DF, Mexico) were subjected to a 10-day carcinogen treatment. All experiments followed Institutional Animal Care and Use Committee Guidelines. Rats were initiated with an intraperitoneal dose of DEN (200 mg/kg; Sigma-Aldrich, Toluca, Mexico) and subjected to a modified selection regime 1 week later [6,16]. 2-AFF was administered by gavage at a dose of 25 mg/kg during three consecutive days, beginning on day 7 after initiation. On day 10, rats were subjected to PH. Animals were sacrificed by exsanguination under ether anesthesia at periods from 1 month after initiation up to 24 months. Livers were excised, washed in physiological saline solution, frozen in 2-methyl butane with liquid nitrogen or immersed in RNAlater (Sigma, St. Louis, MO), and stored at -80°C.
Histologic Analyses
Representative 20-µm thick sections from liver slices were stained for γ-glutamyl transpeptidase (GGT) activity, in accordance with Rutenburg et al. [17]. In addition, 5-µm sections were processed for routine histologic examination using hematoxylin and eosin (H&E) staining. For immunostaining, formalin-fixed paraffin specimens were blocked for 1 hour in 0.1% H2O2 in phosphate-buffered saline, pH 7.4. Subsequently, they were incubated with commercial monoclonal antibodies specific to glutathione S-transferase, placental form (GSTP; DakoCytomation, Glostrup, Denmark), or anti-cyclin D1 (Cell-Marque, Hotspring, AR) diluted 1:50 and 1:20, respectively, in blocking buffer overnight. After washing with phosphate-buffered saline, the primary antibody was detected using an avidin-biotin complex immunoperoxidase technique (Zymed Laboratories, Inc., Carlsbad, CA). No staining was observed when the primary antibody was substituted with mouse isotype control.
Liver Section, Enrichment of Nodular Tissue, and Tumor Dissection
Hepatocellular tumors with diameters larger than 5 mm were easily dissected, and their corresponding nontumorous (Nt) liver tissues were obtained. Small nodular lesions 0.5 to 3 mm in diameter were distinguished by their sharp grayish white color demarcation from the surrounding reddish brown liver. Then a stainless steel cork borer (internal diameter, 1 mm) was introduced into frozen tissues. In this way, between 15 and 25 nodules per liver were collected, pooled, and stored in RNAlater at -80°C. These samples were designated as enriched nodular tissues (ENTs). The increased presence of the hepatocarcinogenesis markers GSTP and GGT in ENT was verified by comparative reverse transcription-polymerase chain reaction (RT-PCR).
RNA Isolation, cDNA Synthesis, Labeling, and Purification
The total RNA of normal livers (NL), ENT of 1 month (ENT-1), ENT of 5 months (ENT-5), and individual HCC samples were obtained by tissue homogenization and extraction with Trizol (Life Technologies, Inc.). After DNase treatment, RNA was purified by phenol chloroform extraction. RNA quality and concentration were determined by capillary electrophoresis (RNA Nano LabChip; Agilent Technologies, Massy, France). For cDNA synthesis, 20 mg of RNA was reverse-transcribed for 1.5 hours at 42°C with 200 U of Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA); 6 µl of 5x first strand buffer (Invitrogen); 1 µmg of oligo(dT15); 50 µCi of [α-33P]dCTP (2500 Ci/mmol; Amersham Biosciences, Pittsburgh, PA); 5 µM dCTP; 0.8 mM each of dATP, dGTP, and dTTP; and 10 mM dithiothreitol, in a final volume of 30 µl. After 1 hour, 200 U of Superscript II reverse transcriptase was added to the reaction mixture. The resulting 33P-labeled cDNA was purified with MicroSpin S-200 HR columns (Amersham Biosciences), according to the manufacturer's instructions.
cDNA Microarray Membrane and Hybridization
Atlas rat toxicology cDNA expression 1.2 arrays (7860-1) were purchased from Clontech Laboratories, Inc. (Palto Alto, CA) The membranes contained 1176 known rat genes and 9 housekeeping control cDNA. The arrays were prehybridized with 15 ml of Church buffer [18] and 0.5 mg of denatured salmon testes DNA at 65°C for 2 hours, with continuous agitation. Then, 33P-labeled cDNA was added to the hybridization buffer at 65°C overnight. The membranes were washed at 65°C with 40 mM sodium phosphate buffer (pH 7.2) and 0.1% sodium dodecyl sulfate.
Imaging and Analysis
The membranes were then exposed to low-energy phosphorimage screens for 2 days. Images were acquired with a PhosphorImager 445 SI (Molecular Dynamics/Amersham Biosciences, Pittsburgh, PA). The spots were detected with XDotsReader (v. 1.8), and data were analyzed with the web service BioPlot (http://biopuce.insa-toulouse.fr/; Genopole-Toulouse, Toulouse, France). The intensity of each spot was corrected by subtracting the background; for normalization, the intensity of each spot was divided by the mean intensity of the nine housekeeping genes. From the RNA of rat livers (NL = 3; ENT-1 = 3; ENT-5 = 3; HCC = 3), 12 independent sets of data were obtained. Ratio was obtained from the average intensity (n = 3) of each gene compared with NL. Statistical data, such as P values from Student's t test and false discovery rate (FDR), were obtained with the BioPlot system. Genes with statistical significance (P ≤ .05 according to Student's t test) were considered differential genes and were classified as downregulated (< 1) or upregulated (> 1) genes according to the ratio. They were grouped and classified with the web service BioClust (http://biopuce.insatoulouse.fr/). Two hundred ninety differential genes detected in this study through their statistical values are listed in Table W1. The level of similarity of gene expression patterns was obtained by hierarchical clustering analysis, as determined by Euclidean distance and a complete linkage method using the web-available software Hierarchical Clustering Explorer (http://www.cs.umd.edu/hcil/hce/).
Comparative RT-PCR
RT-PCR was performed by a modification of the comparative PCR method [19]. Both cDNA synthesis and PCR were performed in one step (One Step RT-PCR System; Gibco Invitrogen Corp., Carlsbad, CA). Appropriate primers (0.2 µM each) and 1 µg of total RNA per 50 µl of reaction were used. The absence of DNA contamination was verified by PCR assay. Cycling parameters were as follows: 30 minutes at 45°C for cDNA synthesis, followed by 1 minute at 94°C; annealing for 45 seconds between 52°C and 60°C, followed by 1 minute at 72°C; and final elongation for 10 minutes at 72°C. An adequate number of cycles corresponding to exponential amplification was performed to avoid saturated products with a kinetic analysis of 20–35 cycles for each gene. Primer set sequences are shown in Table 1.
Table 1.
Primer Pairs Used for RT-PCR in This Study.
| Gene Name | Short Name | GenBank Accession Number | Forward and Reverse Primers (5′-3′) | Product Size (bp) |
| Glutathione S-transferase pi 1 | GSTP1 | X02904 | TGCCACCGTACACCATTGTGT; CAGCAGGTCCAGCAAGTTGTA | 479 |
| Glutamate-cysteine ligase, catalytic subunit | GCLC | J05181 | GCTGCATCGCCATTTTACCGAG; TGGCAACAGTCATTAGTTCTCCA | 883 |
| Glutamate cysteine ligase, modifier subunit | GCLM | L22191 | AGCTGGACTCTGTCATCATGG; TGGGTCATTGTGAGTCAGTAGC | 290 |
| Glutathione synthetase | GSS | L38615 | CACTATCTCTGCCAGCTTTGG; GTTCCTTTCCTTCTCCTGAGC | 211 |
| Annexin 5 | ANXA5 | M21730 | ATGGCTCTCAGAGGCACCGT; CGTGTTTCAGCTCGTAGGCG | 289 |
| Glutathione reductase | GSR | U73174 | CCATGTGGTTACTGCACTTCC; TTCTGGAACTCGTCCACTAGG | 171 |
| γ-Glutamyl transpeptidase | GGT1 | M33821 | CTCTGCATCTGGCTACCCAC; GGATGCTGGGTTGGAAGAGG | 418 |
| Cytochrome P450, subfamily IIC (CYP2CII) | CYP2C11 | J02657 | TCATTCCCAAGGGTACCAATG; GGAACAGATGACTCTGAATTCT | 664 |
| Sulfotransferase, estrogen-prefering | STE | M86758 | CTGGAGAGAGACCCATCAGC; TCATTTGCTGCTGGTAGTGC | 223 |
| Betaine-homocysteine methyltransferase | BHMT | AF038870 | GTCATGCAGACCTTCACTTTCTA; TAAGGCCTCGACTGCCCACACG | 315 |
| Regucalcin | RGN | D38467 | AGATGAACAAATCCCAGAT; ACCCTGCATAGGAATATGG | 305 |
| α-Actin | α-ACTIN | X80130 | CCAAGGCCAACCGCGAGAAGATGAC; AGGGTACATGGTGGTGCCGCCAGAC | 584 |
Results
Progression Stage in the Modified RH Model
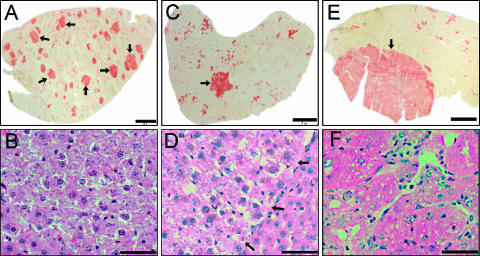
Hepatocyte nodules and tumor lesions were identified by determination of the marker GGT and by H&E staining (Figure 1). The amount of liver lesions expressing positive GGT activity during the progression stage is shown in Table 2. Numerous nodules up to 2 mm in diameter were uniformly stained for GGT activity at 1 month (Figure 1A). They contained clear eosinophilic cytoplasmic hepatocytes with minimal nuclear atypia (Figure 1B). The number of nodules showed a significant decrease from 1 to 5 months (Table 2), which is associated with the presence of remodeling and persistent nodules, as described by Enomoto and Farber [20]. Persistent nodules were detected mainly at 5 months, and they showed the largest areas (up to 3 mm2) with a uniform presence of GGT activity (Figure 1C). They had cytologic features similar to those of early nodules, but the increased size of the lesion compressed the surrounding NL (Figure 1D). HCC were found after 7 months, and with higher incidence at 12 months (Table 2). These lesions with variable histologic GGT activity had areas greater than 5 mm2 (Figure 1E). HCC exhibited increased thickness of the layers of hepatocytes, marked nucleoli, mitotic figures, nuclear atypia, and anisonucleosis (Figure 1F).
Figure 1.
Progression of hepatocyte nodules toward HCC in the modified RH model. Histologic determination of GGT activity in early nodules (arrows) of 1 month (A), intermediate persistent nodule (arrow) of 5 months (C), and HCC (arrow) of 12 months (E). The increased cytologic alterations and distorted hepatocyte rearrangement in the liver were visualized by H&E staining in an early nodule (B), in a persistent nodule (arrows indicate the borderline of a nodule) (D), and in HCC (F). Bars in (A), (C), and (E) correspond to 2 mm, whereas bars in (B), (D), and (F) correspond to 50 αm.
Table 2.
Quantitative Histologic Analysis of GGT-Positive Hepatocellular Lesions in Rats.
| Sacrifice (months) | Nodules/cm2 (Mean ± SD) | Persistent Nodules (%)* | Rats with HCC |
| 1 | 22.31 ± 3.74 | 1.85 ± 1.7 | 0/7 |
| 3 | 15.25 ± 1.80 | 3.89 ± 2.6 | 0/3 |
| 5 | 4.71† ± 1.77 | 14.61† ± 7.2 | 0/6 |
| 7 | 16.06 ± 7.34 | 1.99 ± 3.4 | 2/4 |
| 12 | 6/6 |
Uniformly stained nodules with area > 1 mm2.
Statistically different compared with the 1-month group.
Identification of Genes with Differential Expression in Hepatocellular Lesions
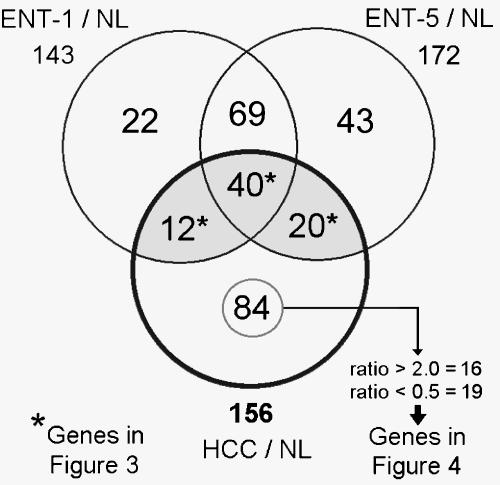
To investigate genes related to the progress of nodules toward HCC, the gene expression profiles of liver lesions samples (n = 3) were compared to NL profiles. The number of differential genes between ENT-1/NL, ENT-5/NL, and HCC/NL was 143, 172, and 156, respectively, corresponding to 290 genes. Venn diagram (Figure 2) was used to classify the 290 genes whose expression change was specific to or common in the comparisons. The complete data, including Venn diagram sections, gene names, functional classifications, ratios, and FDRs, are shown in Table W1. According to the main functions of the differential genes in Table W2, the highest proportion of selected genes was related to cellular metabolism (80 genes), followed by cell receptors and intracellular transducer genes (31 and 30 genes, respectively).
Figure 2.
Differentially expressed genes in liver lesions against NL. Venn diagram of 290 differential genes. Considering NL as reference, the differential genes were determined by statistical significance (P ≤ .05, Student's t test) in ENT-1, ENT-5, and HCC samples (n = 3 for each sample). From the intersection (*), 72 genes that were shared by ENT-1, ENT-5, and HCC are listed in Figure 3. From the 84 differential genes in HCC/NL, 19 highly downregulated (ratio ≤ 0.5) and 16 highly upregulated (ratio ≥ 2) genes are listed in Figure 4.
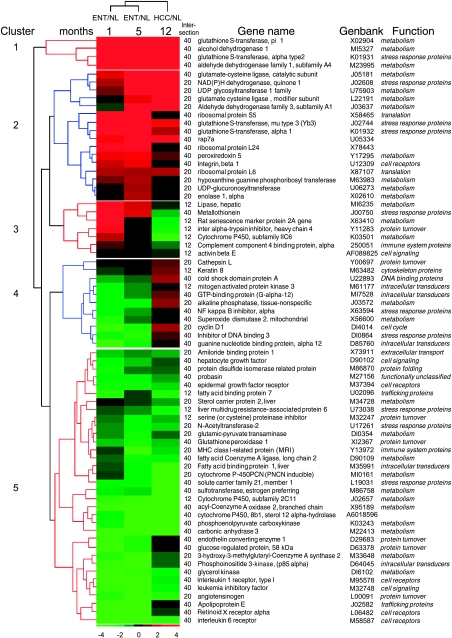
Differential genes with intersections between ENT and HCC were considered important because they could reflect the continuous process from preneoplasia to neoplasia. From the intersection of genes among the three comparisons, 72 genes shared between ENT and HCC were selected; 40 of these showed statistical significance in the three lesion development stages. These genes are displayed in Figure 3 according to ratio; their classification by hierarchical clustering indicates the different patterns of gene expression from early nodules to HCC. Analysis of clustered data revealed at least five behaviors in gene expression patterns. There are upregulated genes in clusters 1 and 2; the first cluster includes upregulated genes with a high ratio in three lesion development stages, such as the known hepatocarcinogenesis marker GST pi with ratios of ENT-1/NL = 13.59, ENT-5/NL = 21.89, and HCC/NL = 22.76, whereas upregulated genes in cluster 2 showed a high ratio in two lesion development stages (e.g., glutamate-cysteine ligase modifier; ENT-1/NL= 1.26, ENT-5/NL = 2.42, and HCC/NL= 4.59). Cluster 3 includes genes with transitions from upregulated levels in ENT to downregulated levels in HCC (e.g., metallothionein; ENT-1/NL = 5.08, ENT-5/NL = 2.29, and HCC/NL = 0.55). Cluster 4 includes genes with transitions from downregulated levels in ENT-1 to upregulated levels in HCC (e.g., cyclin D1; ENT-1/NL = 0.34, ENT-5/NL= 0.41, and HCC/NL = 2). Cluster 5 contains downregulated genes in three lesion development stages (e.g., CYP450-2C11; ENT-1/NL = 0.37, ENT-5/NL=0.34, and HCC/NL = 0.14).
Figure 3.
Gene expression patterns of 72 genes shared by ENT and HCC. As indicated in Figure 2, the genes from intersections 12*, 40*, and 20* were compiled. The labels of intersection in the Venn diagram, gene names, GenBank accession numbers, and main functions are shown. The genes were classified by hierarchical clustering, and the ratio average of each type of sample (columns) is presented in a matrix format. Green and red represent downregulation and upregulation, respectively, as indicated in the scale bar (log2 ratio-transformed scale). The dendrogram in blue or red on the left indicates the degree of similarity of selected genes. The clusters indicate the different behaviors of genes in the three types of sample.
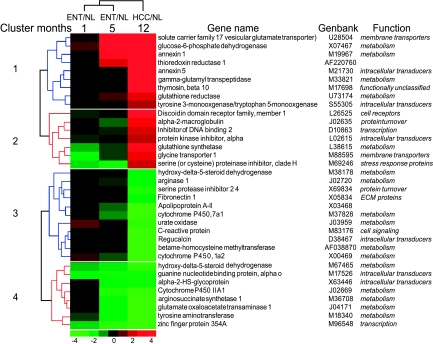
To extend the selection of important differential genes in hepatocarcinogenesis, from the 84 genes that showed statistical significance only in HCC, the highly upregulated genes (ratio ≥ 2) and the highly downregulated genes (ratio ≤ 0.5) were selected; the expression level of these 35 genes in the three lesion development stages is shown in Figure 4. Clustering analysis resulted in four gene clusters: cluster 1, highly upregulated genes in HCC with a tendency for upregulation in ENT (e.g., Annexin 5; ENT-1/NL = 1.39, ENT-5/NL = 1.43, and HCC/NL = 3.63); cluster 2, highly upregulated genes in HCC with a tendency for downregulation in ENT (e.g., protein kinase inhibitor α; ENT-1/NL = 0.72, ENT-5/NL = 0.94, and HCC/NL = 2.08); cluster 3, highly downregulated genes in HCC with a tendency for upregulation in ENT [e.g., betaine-homocysteine methyltransferase (BHMT); ENT-1/NL = 1.19, ENT-5/NL= 0.75, and HCC/NL = 0.28]; and, cluster 4, highly downregulated genes in HCC with a tendency for downregulation in ENT (e.g., cytochrome P450 IIA1; ENT-1/NL = 0.83, ENT-5/NL = 0.39, and HCC/NL = 0.34).
Figure 4.
Gene expression patterns of highly upregulated or downregulated genes in HCC. As indicated in Figure 2, genes with an expression ratio that had at least a two-fold difference were selected from the 84 genes that showed a statistical difference (P ≤ .05) only in the HCC/NL comparison. The genes were classified by hierarchical clustering as in Figure 3.
Immunohistologic Analysis of GSTP and Cyclin D1 in HCC
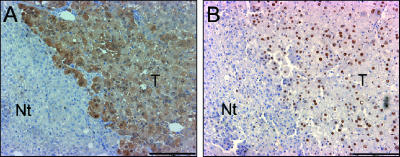
From the upregulated genes in HCC (Figure 3), the highest ratio (HCC/NL = 23) was found for the GSTP gene, which is the most important marker in rat hepatocarcinogenesis models; at the limits, the gene that is considered highly upregulated (HCC/NL = 2) is cyclin D1, which is an important oncogene involved in cell cycle regulation. To confirm the upregulation of these genes at the protein level, immunohistochemistry was performed in histologic serial sections of HCC (Figure 5). A specific increased level of GSTP in the cytoplasm of HCC cells was evident when compared to adjacent tissues (Figure 5A). In the same way, cyclin D1 was significantly increased in the nucleus of neoplastic hepatocytes (Figure 5B). These genes illustrate that upregulation for some genes at the mRNA level induces increased protein levels.
Figure 5.
Histologic confirmation of increased GSTP and cyclin D1 levels in HCC. Immunoperoxidase staining of GSTP (A) and cyclin D1 (B) in serial sections of HCC of a rat sacrificed 15 months after initiation. Scale bars, 200 µm.
Comparative RT-PCR Confirming Differential Gene Expression of Selected Genes
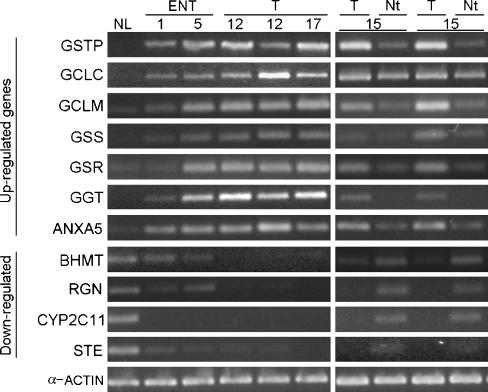
To validate cDNA array results, we used comparative RT-PCR for gene expression analysis. From the genes listed in Figures 3 and 4, four downregulated and seven upregulated selected genes (Table 1) were compared with mRNA levels in NL, ENT-1, ENT-5, HCC, and in the corresponding Nt tissues of two rats sacrificed at 15 months (Figure 6). There was a notable correspondence in the ratios obtained from cDNA arrays and the band level determined by RT-PCR. The 11 genes analyzed were consistent with their respective downregulation or upregulation in ENT and HCC with respect to NL. Because the surrounding Nt tissue is an important reference for histologic demarcation of tumor and marker discovery, we compared mRNA levels between these two samples. It is significant to note that there were higher levels of upregulated genes in tumors than in Nt tissues, and vice versa in downregulated genes. Thus, besides the validation of the altered expression of these genes, they could be important for the histologic distinction of tumors from adjacent Nt tissues.
Figure 6.
Confirmation of the differential expression of 11 genes by RT-PCR. Comparison of mRNA levels of 11 selected genes plus α-actin as control in NL, ENT-1, and ENT-5; individual tumors (T) at 12 and 17 months; and pairs of tumors (T) and corresponding Nt tissues at 15 months. Only for RT-PCR were RNA samples of NL and ENT pooled from three livers. Full gene names are shown in Table 1.
Discussion
Animal models of hepatocarcinogenesis recapitulate the underlying biology of liver tumorigenesis and have provided reliable data for understanding the cellular development of HCC in humans [21–23]. The progression stage in the RH model is characterized by the evolution from nodules to HCC without additional carcinogen treatment. The majority of nodules remodel or differentiate into a liver of normal appearance, whereas a few persistent nodules show spontaneous cell proliferation and size increase [20,24–26]. Considering that all rats with hepatic nodular lesions will present with HCC after 9 to 10 months [11], it is possible to hypothesize that nodular cells may have an altered genetic background that allows them to accumulate additional mutations and, after a selection process, to show autonomous cell proliferation. These genetic alterations should predispose nodules to undergoing a slow evolution to cancer; however, this issue remains unclear.
One positive feature of this model is its ability to distinguish a few persistent nodules from a large number of remodeling nodules and, thus, to allow a study of the nodule's cancer sequence [11]. According to our histologic analysis, the highest proportion of persistent nodules without HCC occurrence was found at 5 months. Although they were few in the liver, the increase in size of up to 3 mm in diameter allowed us to collect them easily from the frozen liver. Thus, we performed the study at three periods of progression: initial point, with tissues that included early nodules (1 month); intermediate point, with tissues that included persistent nodules (5 months); and end point, with well-developed HCC (12 months).
The evaluation of array results against NL, together with statistical analysis, allowed us to reveal differential gene expression patterns within each progression condition and to compare them. The known hepatocarcinogenesis markers such as α2-macroglobulin [27], GGT, and GSTP were upregulated, showing congruence of cDNA array results. With equal coherence, the oncogene cyclin D1 showed a two-fold increased mRNA level in array results and an increased protein presence in GSTP-positive cancer cells. Furthermore, differential gene expression was validated for 11 genes by comparative RT-PCR (four downregulated genes and seven upregulated genes). It is important to note that the expression of these genes was also compared in tumorous and corresponding adjacent Nt tissues of two rats sacrificed 15 months after DEN treatment, showing that tumorous tissues can be molecularly differentiable from surrounding tissues. The differential mRNA levels of the indicated genes in tumor and nontumor tissues could be useful for the development of additional tools for the histopathological evaluation and confirmation of HCC. There is a need for a noninvasive method for early HCC diagnosis; for this reason, we will perform additional studies focusing on gene products such as the secreted protein Annexin 5, which will lead to the identification of serological markers.
It is known that hepatocyte nodules are precursors for HCC [26]. With this in mind, we selected the genes that were statistically significant from preneoplasia to neoplasia; these 72 genes showed a wide pattern of expression courses from tissues with early nodules to liver cancer. Those genes with consistent upregulation (i.e., GCL, glutamate-cysteine ligase, and others) or with consistent downregulation (i.e., sulfotransferase, estrogen-preferring; CYP2C11; and others) from early nodules to HCC may form a fraction of an altered gene expression environment that predisposes nodules to progressing into HCC, and they would be considered as underlying genes involved in the progression stage of hepatocarcinogenesis. It was not surprising that, among these genes, GSTP was found because it is widely used as a marker in the basic analysis of chemical carcinogenesis [28,29]. Furthermore, other GST gene family members (i.e., α and µ) were upregulated with a similar course of expression, indicating perhaps the same transcriptional regulation or response to inducers [30].
The systems of transforming growth factor α and hepatocyte growth factor (HGF), and their receptors epidermal growth factor receptor (EGFR) and met proto-oncogene, respectively, are mitogenic for hepatocytes and have been suggested to contribute to HCC formation [31,32]. In our results, the four genes showed downregulated levels from early nodules to HCC, with ratios between 0.34 and 0.67 (Table W1). Our data are in agreement with previous reports that showed no increased presence of these gene products in rat preneoplastic and neoplastic hepatocytes [31,33,34]. In human HCC, expression of HGF and EGFR has shown great variability with respect to the pattern of histologic differentiation of HCC [35,36]. However, other growth factors showed upregulation in at least one lesion development stage, as follows (ENT-1, ENT-5, and HCC): fibroblast growth factor 10—4.09,4.24, and 1.37; insulin-like growth factor 1— 2.53,1.3, and 0.48; insulin-like growth factor binding protein 1 (Igfbp1)—2.46, 2.5, and 1.1; and Igfbp3—0.4, 0.6, and 2.07 (Table W1). The results from these growth factor studies have stimulated us to undertake additional investigations to understand the preferential cell proliferation of preneoplastic and neoplastic lesions.
There is strong evidence for the involvement of oxidative stress in hepatocarcinogenesis. Oxidative stress may trigger damage to cellular membranes and nuclear DNA, which result in lipid peroxidation and oxidative DNA damage, respectively. Here, by statistical selection, we have detected several differential genes involved in the control and regulation of oxidative stress: upregulated genes such as those for NAD(P)H dehydrogenase quinone 1, superoxide dismutase 2, mitochondrial alcohol dehydrogenase 1, peroxiredoxin, and thioredoxin reductase 1; downregulated genes such as those for carbonic anhydrase 3 and glutathione peroxidase; and genes with a transition from upregulated levels in preneoplasia to downregulated levels in cancer, such as that for metallothionein. The specific increase at the protein level of metallothionein in rat liver preneoplastic lesion has been demonstrated by Sawaki et al. [37], whereas downregulated expression in hepatocellular tumors has been described for human and mouse chemical carcinogenesis [38]. Although the participation of metallothionein in cell proliferation and carcinogenesis has been suggested [39], additional analysis should be performed to examine the possible correlation between its expression and preneoplastic/neoplastic transition.
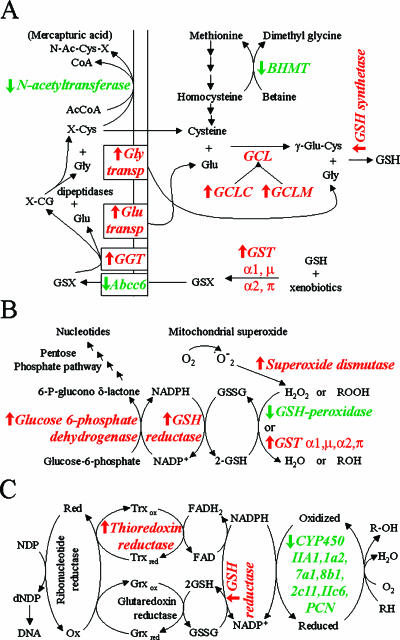
Many reports indicate that reduced glutathione (GSH) and its cooperating enzymes are important in neoplastic diseases and play a crucial role in defense against reactive oxygen species [40,41]. In rat chemical hepatocarcinogenesis models, increased levels of GSH, oxidized glutathione, GGT, and GSTP are often detected in preneoplastic hepatocyte nodules and hepatocellular tumors [42,43]. In our transcriptome analysis, we detected more than 10 genes that are involved in GSH metabolism. Figure 7 maps the modulation of some differential genes at mRNA levels in HCC in the metabolic pathways of GSH biosynthesis, amino acid recycling, antioxidation in GSH, and redox control for DNA synthesis. The upregulation of the GCLC, GCLM, and GSH synthetases essential for GSH biosynthesis could explain the increased levels of total glutathione in hepatocarcinogenesis. Moreover, the upregulation of GGT, glycine transporter 1 (Slc6a9), and solute carrier family 17-vesicular glutamate transporter member 1 (Slc17a1) could favor the catabolism of extracellular GSH and the recovery of glycine and glutamate, respectively. The downregulation of the liver multidrug resistance-associated protein 6 gene (Abcc6), which acts as a MgATP-dependent efflux pump that transports glutathione S-conjugates [44], could maintain the increased level of intracellular glutathione. One of the major determinants of the rate of GSH synthesis is the availability of cysteine [45]; N-acetyltransferase-2 catalyzes the N-acetylation of the cysteine conjugate (X-Cys), resulting in the formation of mercapturic acid, which is usually excreted in urine (its downregulated expression in hepatocellular tumors could be an adaptive response to maintaining the amount of cysteine essential for GSH biosynthesis). Another important source of cysteine comes from the trans-sulfuration pathway, which converts methionine to cysteine. Methionine can be resynthesized from homocysteine through the activity of BHMT; this gene was downregulated in tumors, favoring the idea that maintaining cysteine for GSH biosynthesis is an adaptive response of neoplastic cells.
Figure 7.
Mapping of some differentiable genes in cellular metabolic pathways. (A) Glutathione (GSH) biosynthesis and amino acid recycling. (B) Antioxidant function of GSH. (C) Role of GSH and NADPH in DNA synthesis and cytochrome oxidation. (↑) Upregulated differential genes (red). (↓) Downregulated differential genes (green). BHMT, betaine-homocysteine methyltransferase; GGT, γ-glutamyl transpeptidase; GCL, glutamate cysteine ligase; GST, glutathione S-transferase; Abcc6, liver multidrug resistance-associated protein 6.
GST gene products and GSH peroxidase 1 (Gpx1) share the ability to reduce organic peroxides through GSH oxidation. Nevertheless, contrary to GST genes, the Gpx1 gene was downregulated from early nodules to HCC, indicating that liver cancer may develop in a Gpx1-deficient condition. Reduced expression of this gene was reported in rat experimental HCC [46], and reduced activity was reported in human HCC [47,48]. The reduction of this important scavenger enzyme of toxic oxygen radicals exemplifies a shared phenomenon in HCC development in humans and rats.
Several reports and our data suggest that the altered gene expression of oxidative stress-related genes and GSH metabolism genes plays an important role in predisposing nodular hepatocytes to progression toward HCC. GSH level increases in human HCC and hepatocytes during active proliferation with respect to NL [45,49]. An increased level of GSH has been detected in a number of drug-resistant tumor cell lines and in tumor cells isolated from patients whose tumors are clinically resistant to drug therapy [50]. In human HCC, increased hepatic oxidative DNA damage has been reported on patients with HCC [51,52]. However, in the future, it will be important to determine the chronological causative participation of oxidative stress and GSH regulation in human and rat hepatocarcinogeneses.
Gene expression profiles provide a guide for the understanding of HCC development. Hierarchical clustering classification also allowed us to conclude that the pattern of gene expression is more similar between nodular samples at 1 and 5 months relative to HCC. A similar concept was found histologically in H&E staining (Figure 1), in which the cytologic features of early and persistent nodular hepatocytes are modestly altered with respect to neoplastic cells in cancer. Thus, gene profiles exemplify the continuous progression from nodules to HCC.
Numerous researchers have used microarrays to profile the gene expression pattern of human HCC, and they have found thousands of altered genes [15,22,27,53]. However, among these genes, it is difficult to discern which are critical for HCC development and are not just a consequence of increased genomic instability. It is known that liver cancer, as with other solid malignancies, displays a high number of genetic alterations and huge heterogeneity in gene expression profiles. For this reason, it is essential to differentiate the important genes implicated in liver tumorigenesis with respect to those genes that are not related to cancer development or are considered as biologic noise.
The gene expression patterns associated with human HCC progression have been addressed by a comparison of early HCC and nodule-in-nodule-type HCC (advanced HCC within early HCC) [53]. Heat shock protein 70 (HSP70) was identified as a marker of early HCC. Coincidentally, we found increased levels of the HSP70-8 in ENT-1 (ratio = 2.79), ENT-5 (ratio = 2.99), and HCC (ratio = 1.51) (Table W1). Lee et al. [22] have revealed two subclasses of HCC patients, characterized by the expression profiles of a limited number of genes that accurately predict the length of survival. Some of these genes with downregulated levels [such as regucalcin (RGN), insulin receptor (Insr), interleukin 6 receptor (II6r), enoyl coenzyme A hydratase short chain 1 (Echs1), androgen receptor (Ar), and acyl coenzyme A oxidase 2 branched chain (Acox2)] were also downregulated in rat HCC (Table W1), whereas upregulated genes [such as tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein eta polypeptide (Ywhah); serine (or cysteine) proteinase inhibitor clade H, member 1 (Serpinh1); ribosomal proteins S3, S5, and S9 (Rps3, Rps5, and Rps9); nucleophosmin 1 (Npm1); fatty acid binding protein 5 epidermal (Fabp5); and cold shock domain protein A (Csda)] were increased in both human and rat HCC. The potential of the RH model, together with DNA microarray technology, has allowed us to reveal gene expression changes in rat HCC that could be relevant to human HCC.
To increase the screening of global gene expression during hepatocarcinogenesis, we are now expanding our study to high-density oligonucleotide microarrays. In the present work studying 1185 rat genes, it was possible to identify a gene expression profile that is related to the progression of rat hepatocarcinogenesis, which suggests altered cellular homeostasis as a predisposing factor for the evolution of nodules toward cancer. Furthermore, data may provide opportunities to find new markers for rat liver preneoplastic nodules and target genes for chemoprevention or early treatment of HCC.
Supplementary Material
Acknowledgements
We are grateful to J. Fernandez, M. Flores, R. Leyva, and R. Gaxiola for technical support at the Animal House.
Abbreviations
- HCC
hepatocellular carcinoma
- DEN
diethylnitrosamine
- 2-AAF
2-acetylaminofluorene
- GSTP
glutathione S-transferase, placental form
- GGT
γ-glutamyl transpeptidase
Footnotes
This work was supported by CONACYT grant 39525-M, ECOS-ANUIES grant M02-S01, and a fellowship from CONACYT (JIPC 144549).
This article refers to supplementary material, which is designated by “W” (i.e., Table W1, Figure W1) and is available online at www.bcdecker.com.
References
- 1.Plentz RR, Tillmann HL, Kubicka S, Bleck JS, Gebel M, Manns MP, Rudolph KL. Hepatocellular carcinoma and octreotide: treatment results in prospectively assigned patients with advanced tumor and cirrhosis stage. J Gastroenterol Hepatol. 2005;20:1422–1428. doi: 10.1111/j.1440-1746.2005.03959.x. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15:948–963. doi: 10.1002/hep.1840150532. [DOI] [PubMed] [Google Scholar]
- 3.Hussain SM, Zondervan PE, I. Jzermans JN, Schalm SW, de Man RA, Krestin GP. Benign versus malignant hepatic nodules: MR imaging findings with pathologic correlation. Radiographics. 2002;22:1023–1036. doi: 10.1148/radiographics.22.5.g02se061023. (discussion, 1037–1029) [DOI] [PubMed] [Google Scholar]
- 4.Saffroy R, Pham P, Lemoine A, Debuire B. Molecular biology and hepatocellular carcinoma: current status and future prospects. Ann Biol Clin (Paris) 2004;62:649–656. [PubMed] [Google Scholar]
- 5.Choi BI. The current status of imaging diagnosis of hepatocellular carcinoma. Liver Transpl. 2004;10:S20–S25. doi: 10.1002/lt.20038. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco-Legleu CE, Marquez-Rosado L, Fattel-Fazenda S, Arce-Popoca E, Perez-Carreon JI, Villa-Trevino S. Chemoprotective effect of caffeic acid phenethyl ester on promotion in a medium-term rat hepatocarcinogenesis assay. Int J Cancer. 2004;108:488–492. doi: 10.1002/ijc.11595. [DOI] [PubMed] [Google Scholar]
- 7.Marquez-Rosado L, Trejo-Solis MC, Garcia-Cuellar CM, Villa-Trevino S. Celecoxib, a cyclooxygenase-2 inhibitor, prevents induction of liver preneoplastic lesions in rats. J Hepatol. 2005;43:653–660. doi: 10.1016/j.jhep.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto T, Kaneda K, Hirohashi K, Kinoshita H, Sakurai M. Sinusoidal capillarization and arterial blood supply continuously proceed with the advance of the stages of hepatocarcinogenesis in the rat. Jpn J Cancer Res. 1996;87:442–450. doi: 10.1111/j.1349-7006.1996.tb00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pascale RM, Simile MM, De Miglio MR, Muroni MR, Calvisi DF, Asara G, Casabona D, Frau M, Seddaiu MA, Feo F. Cell cycle deregulation in liver lesions of rats with and without genetic predisposition to hepatocarcinogenesis. Hepatology. 2002;35:1341–1350. doi: 10.1053/jhep.2002.33682. [DOI] [PubMed] [Google Scholar]
- 10.Solt D, Farber E. New principle for the analysis of chemical carcinogenesis. Nature. 1976;263:701–703. [Google Scholar]
- 11.Farber E, Sarma DS. Hepatocarcinogenesis: a dynamic cellular perspective. Lab Invest. 1987;56:4–22. [PubMed] [Google Scholar]
- 12.Solt DB, Cayama E, Tsuda H, Enomoto K, Lee G, Farber E. Promotion of liver cancer development by brief exposure to dietary 2-acetylaminofluorene plus partial hepatectomy or carbon tetrachloride. Cancer Res. 1983;43:188–191. [PubMed] [Google Scholar]
- 13.Rotstein J, Macdonald PD, Rabes HM, Farber E. Cell cycle kinetics of rat hepatocytes in early putative preneoplastic lesions in hepatocarcinogenesis. Cancer Res. 1984;44:2913–2917. [PubMed] [Google Scholar]
- 14.Kim JW, Wang XW. Gene expression profiling of preneoplastic liver disease and liver cancer: a new era for improved early detection and treatment of these deadly diseases? Carcinogenesis. 2003;24:363–369. doi: 10.1093/carcin/24.3.363. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LH, Ji JF. Molecular profiling of hepatocellular carcinomas by cDNA microarray. World J Gastroenterol. 2005;11:463–468. doi: 10.3748/wjg.v11.i4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez-Perez Y, Carrasco-Legleu C, Garcia-Cuellar C, Perez-Carreon J, Hernandez-Garcia S, Salcido-Neyoy M, Aleman-Lazarini L, Villa-Trevino S. Oxidative stress in carcinogenesis. Correlation between lipid peroxidation and induction of preneoplastic lesions in rat hepatocarcinogenesis. Cancer Lett. 2005;217:25–32. doi: 10.1016/j.canlet.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Rutenburg AM, Kim H, Fischbein JW, Hanker JS, Wasserkrug HL, Seligman AM. Histochemical and ultrastructural demonstration of gamma-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- 18.Church GM, Gilbert W. Genomic sequencing. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brass N, Heckel D, Meese E. Comparative PCR: an improved method to detect gene amplification. Biotechniques. 1998;24:22–24. 26. doi: 10.2144/98241bm02. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto K, Farber E. Kinetics of phenotypic maturation of remodeling of hyperplastic nodules during liver carcinogenesis. Cancer Res. 1982;42:2330–2335. [PubMed] [Google Scholar]
- 21.Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–346. doi: 10.1038/ng0802-339. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A., Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 23.Feitelson MA, Pan J, Lian Z. Early molecular and genetic determinants of primary liver malignancy. Surg Clin North Am. 2004;84:339–354. doi: 10.1016/S0039-6109(03)00226-3. [DOI] [PubMed] [Google Scholar]
- 24.Tatematsu M, Nagamine Y, Farber E. Redifferentiation as a basis for remodeling of carcinogen-induced hepatocyte nodules to normal appearing liver. Cancer Res. 1983;43:5049–5058. [PubMed] [Google Scholar]
- 25.Farber E. Pre-cancerous steps in carcinogenesis. Their physiological adaptive nature. Biochim Biophys Acta. 1984;738:171–180. doi: 10.1016/0304-419x(83)90002-1. [DOI] [PubMed] [Google Scholar]
- 26.Farber E. Experimental induction of hepatocellular carcinoma as a paradigm for carcinogenesis. Clin Physiol Biochem. 1987;5:152–159. [PubMed] [Google Scholar]
- 27.Sukata T, Uwagawa S, Ozaki K, Sumida K, Kikuchi K, Kushida M, Saito K, Morimura K, Oeda K, Okuno Y, et al. alpha(2)-Macroglobulin: a novel cytochemical marker characterizing preneoplastic and neoplastic rat liver lesions negative for hitherto established cytochemical markers. Am J Pathol. 2004;165:1479–1488. doi: 10.1016/s0002-9440(10)63406-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendrich S, Campbell HA, Pitot HC. Quantitative stereological evaluation of four histochemical markers of altered foci in multistage hepatocarcinogenesis in the rat. Carcinogenesis. 1987;8:1245–1250. doi: 10.1093/carcin/8.9.1245. [DOI] [PubMed] [Google Scholar]
- 29.Higashi K, Hiai H, Higashi T, Muramatsu M. Regulatory mechanism of glutathione S-transferase P-form during chemical hepatocarcinogenesis: old wine in a new bottle. Cancer Lett. 2004;209:155–163. doi: 10.1016/j.canlet.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 31.Hu Z, Evarts RP, Fujio K, Omori N, Omori M, Marsden ER, Thorgeirsson SS. Expression of transforming growth factor alpha/epidermal growth factor receptor, hepatocyte growth factor/c-met and acidic fibroblast growth factor/fibroblast growth factor receptors during hepatocarcinogenesis. Carcinogenesis. 1996;17:931–938. doi: 10.1093/carcin/17.5.931. [DOI] [PubMed] [Google Scholar]
- 32.Schiffer E, Housset C, Cacheux W, Wendum D, Desbois-Mouthon C, Rey C, Clergue F, Poupon R, Barbu V, Rosmorduc O. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–314. doi: 10.1002/hep.20538. [DOI] [PubMed] [Google Scholar]
- 33.Huitfeldt HS, Skarpen E, Lindeman B, Becher R, Thrane EV, Schwarze PE. Differential distribution of Met and epidermal growth factor receptor in normal and carcinogen-treated rat liver. J Histochem Cytochem. 1996;44:227–233. doi: 10.1177/44.3.8648082. [DOI] [PubMed] [Google Scholar]
- 34.Imai T, Masui T, Nakanishi H, Inada K, Kobayashi K, Nakamura T, Tatematsu M. Expression of hepatocyte growth factor and c-met mRNAs during rat chemically induced hepatocarcinogenesis. Carcinogenesis. 1996;17:19–24. doi: 10.1093/carcin/17.1.19. [DOI] [PubMed] [Google Scholar]
- 35.D'Errico A, Fiorentino M, Ponzetto A, Daikuhara Y, Tsubouchi H, Brechot C, Scoazec JY, Grigioni WF. Liver hepatocyte growth factor does not always correlate with hepatocellular proliferation in human liver lesions: its specific receptor c-met does. Hepatology. 1996;24:60–64. doi: 10.1002/hep.510240112. [DOI] [PubMed] [Google Scholar]
- 36.Kira S, Nakanishi T, Suemori S, Kitamoto M, Watanabe Y, Kajiyama G. Expression of transforming growth factor alpha and epidermal growth factor receptor in human hepatocellular carcinoma. Liver. 1997;17:177–182. doi: 10.1111/j.1600-0676.1997.tb00803.x. [DOI] [PubMed] [Google Scholar]
- 37.Sawaki M, Enomoto K, Hattori A, Tsuzuki N, Sawada N, Mori M. Elevation of metallothionein level in preneoplastic lesions during chemical hepatocarcinogenesis of the Fischer 344 rat. Toxicol Lett. 1999;108:55–61. doi: 10.1016/s0378-4274(99)00127-7. [DOI] [PubMed] [Google Scholar]
- 38.Waalkes MP, Diwan BA, Rehm S, Ward JM, Moussa M, Cherian MG, Goyer RA. Down-regulation of metallothionein expression in human and murine hepatocellular tumors: association with the tumornecrotizing and antineoplastic effects of cadmium in mice. J Pharmacol Exp Ther. 1996;277:1026–1033. [PubMed] [Google Scholar]
- 39.Cherian MG, Howell SB, Imura N, Klaassen CD, Koropatnick J, Lazo JS, Waalkes MP. Role of metallothionein in carcinogenesis. Toxicol Appl Pharmacol. 1994;126:1–5. doi: 10.1006/taap.1994.1083. [DOI] [PubMed] [Google Scholar]
- 40.Denda A, Tang Q, Tsujiuchi T, Tsutsumi M, Amanuma T, Murata Y, Tamura K, Horiguchi K, Nakae D, Konishi Y. Effects of oxidative stress induced by redox-enzyme modulation on the progression stage of rat hepatocarcinogenesis. Carcinogenesis. 1993;14:95–101. doi: 10.1093/carcin/14.1.95. [DOI] [PubMed] [Google Scholar]
- 41.Koike K, Miyoshi H. Oxidative stress and hepatitis C viral infection. Hepatol Res. 2005;34:65–73. doi: 10.1016/j.hepres.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 42.Lu SC, Huang ZZ, Yang H, Tsukamoto H. Effect of thioacetamide on the hepatic expression of gamma-glutamylcysteine synthetase subunits in the rat. Toxicol Appl Pharmacol. 1999;159:161–168. doi: 10.1006/taap.1999.8729. [DOI] [PubMed] [Google Scholar]
- 43.Mauriz JL, Linares P, Macias RI, Jorquera F, Honrado E, Olcoz JL, Gonzalez P, Gonzalez-Gallego J. TNP-470 inhibits oxidative stress, nitric oxide production and nuclear factor kappa B activation in a rat model of hepatocellular carcinoma. Free Radic Res. 2003;37:841–848. doi: 10.1080/1071576031000136577. [DOI] [PubMed] [Google Scholar]
- 44.Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD. Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6) Cancer Res. 2002;62:6172–6177. [PubMed] [Google Scholar]
- 45.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J. 1999;13:1169–1183. [PubMed] [Google Scholar]
- 46.Lertprasertsuke N, Shinoda M, Takekoshi S, Yoshimura S, Watanabe K. Suppression of messenger ribonucleic acid for glutathione peroxidase in chemically induced rat hepatocellular carcinoma and its biological significance. Tokai J Exp Clin Med. 1990;15:285–292. [PubMed] [Google Scholar]
- 47.Casaril M, Gabrielli GB, Dusi S, Nicoli N, Bellisola G, Corrocher R. Decreased activity of liver glutathione peroxidase in human hepatocellular carcinoma. Eur J Cancer Clin Oncol. 1985;21:941–944. doi: 10.1016/0277-5379(85)90112-9. [DOI] [PubMed] [Google Scholar]
- 48.Corrocher R, Casaril M, Bellisola G, Gabrielli G, Hulpe M, Garofoli E, Nicoli N. Reduction of liver glutathione peroxidase activity and deficiency of serum selenium in patients with hepatocellular carcinoma. Tumori. 1986;72:617–619. doi: 10.1177/030089168607200613. [DOI] [PubMed] [Google Scholar]
- 49.Huang ZZ, Chen C, Zeng Z, Yang H, Oh J, Chen L, Lu SC. Mechanism and significance of increased glutathione level in human hepatocellular carcinoma and liver regeneration. FASEB J. 2001;15:19–21. doi: 10.1096/fj.00-0445fje. [DOI] [PubMed] [Google Scholar]
- 50.Mulcahy RT, Untawale S, Gipp JJ. Transcriptional upregulation of gamma-glutamylcysteine synthetase gene expression in melphalan-resistant human prostate carcinoma cells. Mol Pharmacol. 1994;46:909–914. [PubMed] [Google Scholar]
- 51.Schwarz KB, Kew M, Klein A, Abrams RA, Sitzmann J, Jones L, Sharma S, Britton RS, Di Bisceglie AM, Groopman J. Increased hepatic oxidative DNA damage in patients with hepatocellular carcinoma. Dig Dis Sci. 2001;46:2173–2178. doi: 10.1023/a:1011958814371. [DOI] [PubMed] [Google Scholar]
- 52.Jungst C, Cheng B, Gehrke R, Schmitz V, Nischalke HD, Ramakers J, Schramel P, Schirmacher P, Sauerbruch T, Caselmann WH. Oxidative damage is increased in human liver tissue adjacent to hepatocellular carcinoma. Hepatology. 2004;39:1663–1672. doi: 10.1002/hep.20241. [DOI] [PubMed] [Google Scholar]
- 53.Chuma M, Sakamoto M, Yamazaki K, Ohta T, Ohki M, Asaka M, Hirohashi S. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.