Abstract
The influence of the fMRI (functional Magnetic Resonance Imaging) scanning environment on working memory and long-term memory performance was investigated. We predicted that performance would be impaired on memory tasks in the distracting fMRI environment relative to laboratory performance. Results indicated that both young and old adults showed performance decrements in the scanning environment compared to the laboratory for a long term memory task, but not for a passive working memory task, consistent with the idea that divided attention costs occur for more difficult tasks. In addition, elderly adults were disproportionately impaired by the scanning environment on the long-term memory task, congruent with the finding that divided attention costs at encoding are larger for older than younger adults. The findings suggest that performance may be changed by the scanning environment and that, in some circumstances, the fMRI environment may have a disproportionate effect on cognitive performance of older adults.
Keywords: Cognition, fMRI, Aging, Working Memory, Long-term Memory
1. INTRODUCTION
Despite the growth of functional magnetic resonance imaging (fMRI) as a tool in cognitive neuroscience research, the impact of the fMRI scanning environment on cognitive behavior is poorly understood. Cognitive performance may be impaired when tasks are completed in an MR scanner against a backdrop of loud scanning sequences, motion-restriction, and claustrophobic conditions. These sources of interference may be similar to performing tasks under divided attention conditions. Performing a concurrent forced-choice reaction time task during encoding reduces memory performance (Anderson, Craik, & Naveh-Benjamin, 1998), but even tasks with seemingly low cognitive demands can impact performance. For example, making saccadic eye movements impairs judgments of object orientation (Irwin & Brockmole, 2004) and walking around an irregularly-shaped track leads to impoverished memory encoding (Lindenberger, Marsiske, & Baltes, 2000). These data suggest that the noisy, restrictive environment of the scanner might disrupt cognitive performance. The only previous study to address these questions (Mikhelashvili-Browner, Yousern, Mandir, Calhoun, Wu, Oguz, & Vaughan, 2002) found that the scanning environment did not affect simple reaction time for middle aged or elderly adults, but simple reaction time is the most basic of cognitive tasks. It may be that the effects of the scanner will not be evidenced until more demanding tasks are used.
The idea that deleterious effects of environment will appear only for more effortful tasks is consistent with prior research on working memory. When tasks require little executive function, sufficient cognitive resources are available to handle distractions; as task demands increase, distractions take a larger toll. For example, Klein & Boals (2001) found that aversive life events interacted with working memory performance, with amount of stress increasingly positively correlated with performance at higher capacity levels.
However, effort may not be the only determinant of which tasks are vulnerable to impairments due to scanning environment. Based on findings illustrating that different forms of distraction exert disparate effects on performance (Kim, Kim, & Chun, 2005), the scanning environment may not impair all types of tasks equally. We hypothesize that the distraction of the scanning environment should only impair performance when cognitive tasks draw on the same brain areas that mitigate the effects of distraction. Prior research has shown that dual-task costs occur when cognitive mechanisms are shared across tasks (Irwin & Brockmole, 2004; Meyer & Kieras, 1997).
Another issue we address in the present research is whether the cognitive costs of scanning are greater for elderly than young adults. Some studies show older adults experience more interference from a secondary task than younger adults during word encoding (Lindenberger et al., 2000; Park, Smith, Dudley, & Lafronza, 1989), suggesting that their cognitive performance may be more susceptible to disruption by the scanning environment than the performance of young adults. Given evidence that age differences may be magnified on more difficult cognitive tasks relative to easier conditions (Earles, Kersten, Mas, & Miccio, 2004), such impairments may arise in the scanning environment only for more difficult tasks. However, the literature is somewhat mixed, with some studies finding divided attention produces similar absolute costs to memory performance for young and elderly, but disproportionate reaction time slowing for the secondary task for elderly (Anderson et al., 1998). More general forms of distraction, such as everyday stress, may add to overall age-related decrements in episodic encoding (VonDras, Powless, Olson, Wheeler, & Snudden, 2005), and Earles and colleagues suggest that anxiety underlies elderly adults’ greater impairment on difficult tasks (Earles & Kersten, 1998; Earles et al., 2004).
In the present research, we compared the behavioral performance of older and younger participants in laboratory studies to participants in fMRI studies performing the identical tasks. The fMRI version of the task in Experiment 1 had two components: subjects encoded pictures and retained them in working memory while in the scanner, and then later, recognition performance was assessed outside of the scanner. This design permits us to assess whether encoding in the scanner affects immediate working memory, as well as long-term memory, relative to a group who encoded materials out-of-the-scanner. In addition, the working memory task involved two conditions, allowing us to examine whether the effects of scanning environment play a larger role for more difficult tasks, and whether difficulty interacts with scanning environment to produce larger age deficits. Experiment 2 examines long-term recognition when both encoding and retrieval occurred in the scanner compared to out of the scanner.
2. Experiments 1a & 1b
2.1 RESULTS & DISCUSSION
The first study included both a working memory and a long-term memory (recognition) task. Separate analyses were conducted on the data from the two memory stages. Young and elderly who participated in either the scanner or the laboratory were compared to assess the relative effects of the scanning environment on memory performance and response bias.
2.1.1 Experiment 1a: Working Memory Analysis
For the working memory data, A’ scores, a nonparametric measure of discrimination based on hit and false alarm rates (Snodgrass & Corwin, 1988; Stanislaw & Todorov, 1999), are displayed in Figure 1. These were calculated separately for the Visual and Maintenance trials, and subjected to a 2 × 2 × 2 mixed analysis of variance (ANOVA), with Age (Young/Elderly) and Environment (Laboratory/fMRI) as between subjects variables and Condition (Visual/Maintenance) as a within subject variable. Of primary interest, neither the main effect nor any of the interactions involving Environment approached significance (Fs < 1). Notably, there was no support for an interaction of Age and Environment, (F (1, 46) = .02, p > .80). This suggests that the fMRI environment exerted no significant effect on performance. We should note that the only significant effects were a main effect of Age (F (1, 46) = 4.58, p < .04), with young performing better than elderly participants (respective marginal means of .91 and .89), and of Condition (F (1, 46) = 12.43, p < .002), with better performance on the visual trials than the maintenance trials (marginal means of .92 vs. .88). The scores reported here do not even approach ceiling performance, as an A’ score of .92 represents, for example, a hit rate of .92 and a false alarm rate of .21.
Figure 1.
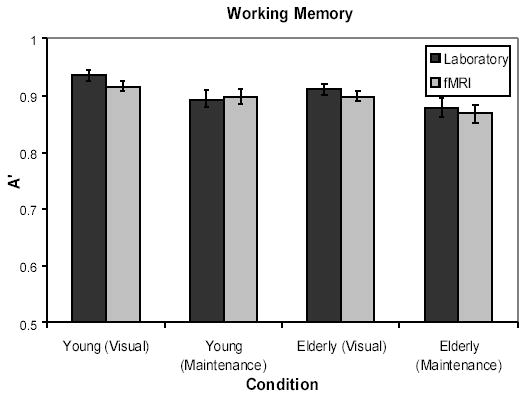
Graph of working memory performance for laboratory and fMRI participants for Experiment 1a.
2.1.2 Experiment 1a: Working Memory Response Bias
The response bias measure C was calculated to compare tendencies to respond “yes” or “no” across conditions and populations using a 2×2×2 mixed ANOVA with Age (Young/Elderly) and Environment (Laboratory/fMRI) as between subjects variables and Condition (Visual/Maintenance) as a within subject variable. For the measure C, a negative score signifies a tendency to respond “yes” while a positive score signifies a tendency to respond “no” (Stanislaw & Todorov, 1999). Unlike the recognition data, response bias was influenced by the environment. There was a main effect of Environment (F (1, 46) = 4.51, p < .04), with more liberal responding in the fMRI environment (M = −.52) than the laboratory environment (M = −.18). There was also a main effect of Condition (F (1, 46) = 66.10, p < .001), with a more liberal bias for the Visual condition (M = −.66) than the Maintenance condition (M = −.04). However, both of these main effects were qualified by an interaction of Environment × Condition, (F (1, 46) = 8.55, p < .006), that occurred because there was an across-the-board liberal response bias in only the Visual condition. The high standard deviations suggest the results may be unreliable, but if reliable, the interactions suggest that the fMRI environment induced a more liberal response bias in some conditions. Means are presented in Table 1. We also note that there was a significant main effect of Age (F (1, 46) = 13.09, p < .002), with young responding more liberally than elderly (marginal means of −.64 vs. −.06), a common finding in the aging literature.
Table 1.
Comparison of response bias measures.
| Young -fMRI | Young -lab | Elderly -fMRI | Elderly -lab | |
|---|---|---|---|---|
| Study 1: Working memory | ||||
| Visual condition | −1.03 (.76) | −.89 (.72) | −.39 (.59) | −.31 (.46) |
| Maintenance condition | −.71 (.70) | .08 (.57) | .06 (.48) | .39 (.65) |
| Study 1: Long-term memory | ||||
| Visual condition | .81 (.43) | .75 (.90) | .64 (.76) | .30 (.40) |
| Maintenance condition | .84 (.41) | .48 (.63) | .55 (.54) | .32 (.36) |
| Study 2: Long-term memory | .71 (.42) | .93 (.58) | .42 (.48) | .69 (.53) |
2.2.1 Experiment 1b: Long-term Memory Analysis
The working memory task also served as incidental encoding of the pictures into long-term memory. Long-term memory was assessed outside of the scanner for the fMRI group, meaning that any differences between the laboratory and fMRI groups resulted from the fMRI environment during encoding. A’ scores were calculated using high-confidence hits and false alarms to the lures matched to the Visual and Maintenance condition and then were subjected to a 2 × 2 × 2 mixed ANOVA, with Age (Young/Elderly) and Environment (Laboratory/fMRI) as between subjects variables and Condition (Visual/Maintenance) as a within subject variable. Consistent with the working memory data, the main effects of Age (F (1, 46) = 6.31, p < .02), and Condition (F (1, 46) = 5.01, p < .03), were significant, with young recognizing pictures more accurately than elderly (marginal means .82 and .77) and performance on Visually-encoded trials better than on Maintenance trials (.80 and .78). In contrast to the working memory data, the main effect of Environment was highly significant (F (1, 46) = 14.90, p < .001), with poorer performance in the fMRI scanning condition compared to the laboratory condition (marginal means of .75 for fMRI vs. .83 for laboratory). The interaction of Environment with Condition was also significant (F (1, 46) = 4.61, p < .04), such that encoding pictures in the scanner affected performance on the more difficult Maintenance trials more than the Visual trials.
There was a marginal interaction of Age × Environment × Condition (F (1, 46) = 2.78, p =.10). To interpret this predicted trend for a three-way interaction, young and elderly were analyzed separately. For the elderly, the main effect of Environment was highly significant (F (1, 24) = 20.33, p < .001) and the interaction of Environment × Condition was also significant (F (1, 24) = 5.46, p < .03). In contrast, the young adults showed only a trend for the main effect of Environment (F (1, 22) = 2.40, p < .14) but no interaction of Environment × Condition (F (1, 22) = .19, p > .65). See Figure 2.
Figure 2.
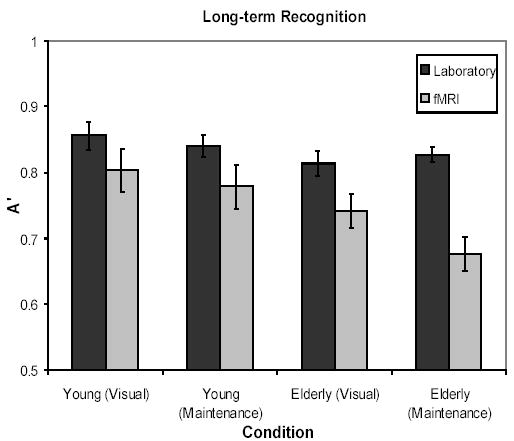
Graph of long-term memory performance for laboratory and fMRI participants for Experiment 1b.
2.2.2 Experiment 1b: Long-term Memory Response Bias
The response bias measure C was subjected to a 2×2×2 mixed ANOVA with Age (Young/Elderly) and Environment (fMRI/lab) as between subject variables, and Condition (Visual/Maintenance) as a within subject variable. This analysis revealed a marginal main effect of Environment (F (1, 46) = 2.83, p = .10), with more stringent responding in the magnet than in the laboratory (marginal means .71 and .46). These data stand in contrast to the working memory data which showed a more liberal bias for responding in the magnet. None of the other main effects or interactions reached significance. Means and standard deviations are presented in Table 1.
2.3 Discussion
The results of the first experiment suggest that, under some conditions, the scanning environment can impair performance relative to laboratory environments. During the working memory task (Experiment 1a) the scanning environment did not impair performance on visual comparison judgments, or a passive maintenance task. In contrast, the fMRI environment disrupted long-term recognition of pictures encoded in the scanner, particularly when the pictures were encoded during the more difficult maintenance condition. The results of the long-term memory comparison suggest that the impairment from environment could be larger for older adults, particularly in the more difficult maintenance condition. Interestingly, these differences in long-term memory emerge despite equivalent performance for laboratory and fMRI participants in the working memory task. Because the working memory task also served as the encoding task for long-term memory, this suggests that information was processed equally well across these groups in working memory during the initial presentation, but the additional processing needed to encode the information into long-term memory was not as successful for the subjects who encoded in the scanner.
In contrast to the memory data, comparisons of response bias suggest that the scanning environment impacted responding for both working memory and long-term memory, albeit in different ways. The scanning environment was associated with more liberal responding for working memory, but more conservative responding for the long-term memory. Although the biases operate in different directions, both suggest that the scanning environment is associated with a shift in the willingness to endorse a representation as veridical.
3. Experiment 2
One factor that could explain the divergent results for Experiments 1a and 1b is a mismatch between the encoding (in the scanner) and recognition (out of the scanner) environment for fMRI subjects in Experiment 1b. Encoding specificity research (Tulving & Thompson, 1973) reveals that memory can be impaired when information is encoded in one environment but recognition is tested in a different environment. Because pictures in Experiment 1b were encoded in the scanner and tested in a laboratory environment for the fMRI group, perhaps effects of fMRI environment actually reflect the mismatch between encoding and retrieval conditions, a difference not present for the laboratory group. Experiment 2 uses another data set in which pictures were encoded and retrieved in the scanner for the fMRI group to address this confound, and tests whether the results of Experiment 1b replicate. Data from Experiment 2 consisted solely of long-term memory recognition data, with no manipulation of encoding conditions (as was the case in Experiment 1).
3.1 RESULTS & DISCUSSION
3.1.1 Recognition Data Analysis
A’ scores, calculated using high confidence responses, were subjected to a 2 × 2 univariate ANOVA, with Age (Young/Elderly) and Environment (Laboratory/fMRI) as between-subject variables. As shown in Figure 3, there was a main effect of Environment (F (1, 44) = 4.26, p <.05), with laboratory participants performing better than fMRI participants. The main effect of Age was also significant (F (1, 44) = 18.31, p <.001), with young performing better than elderly. Age did not interact with Environment (F < 1).
Figure 3.
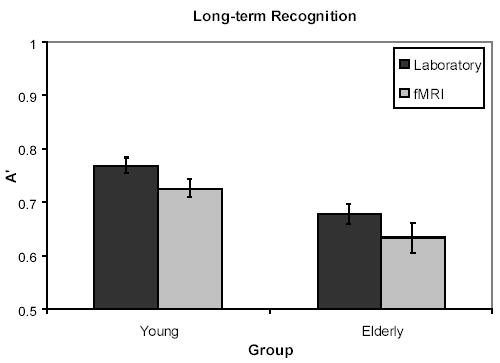
Graph of long-term memory performance for laboratory and fMRI participants for Experiment 2.
3.1.2 Response Bias Data Analysis
A 2×2 univariate analysis of variance, with between-subject variables of Age (Young/Elderly) and Environment (Lab/fMRI), was conducted on the response bias measure C. There was only a trend for a main effect of Environment (F (1, 44) = 2.73, p = .10), such that participants in the laboratory tended to adopt a more stringent response criterion (“no” bias) than participants in the scanner (marginal means of .81 vs. 57). There was a marginal main effect of Age (F (1, 44) = 3.41, p < .08), with young adults (M = .82) exhibiting a more stringent (“no”) response criterion than elderly adults (M = .55), but the interaction of Age × Environment did not approach significance (F < 1). See Table 1.
3.2 Discussion
As in Experiment 1b, long-term recognition was worse in the scanning environment than the laboratory environment. This finding suggests that the impairments identified in Experiment 1b are not explained by simple encoding specificity effects that resulted from a change in the testing environment between encoding (in the scanner) and recognition (out of the scanner). Although this replication provides more credence for the finding that the fMRI environment can impair performance on some tasks, the finding that elderly may be more impaired in the fMRI environment than young adults did not replicate. A number of factors could explain this discrepancy, chiefly that the laboratory participants were not matched on cognitive variables in Experiment 2, which could occlude the detection of differences between the laboratory and fMRI groups. It is also possible that presenting the item for only two seconds in the maintenance condition (Experiment 1b) led to impoverished encoding for the elderly in the scanner. However, the results of Experiment 1b cannot be directly compared to those of Experiment 2 because the number of encoded items, encoding interval, and timing of the trials varied substantially across the two studies.
Although the scanning environment only marginally affected response bias, it is important to note that it tended to shift the response bias in an opposite direction than that found in Experiment 1b. In the present study, scanning environment was associated with a marginally less stringent response criterion, whereas the scanning environment tended to shift the response bias in a marginally more liberal direction in Experiment 1b. This could be explained by Maintenance condition, which was present in Experiment 1b but not Experiment 2. Response bias to scene recognition tended to be more impacted by the scanning environment in the Maintenance condition relative to the Visual condition, which is most analogous to the encoding condition in Experiment 2.
4. GENERAL DISCUSSION
The main finding in this study is that the fMRI environment decreased performance in young and elderly adults on a long-term recognition task, but not on an immediate working memory task. The finding of impaired long-term memory performance replicated across two studies, in which recognition was tested outside of the scanner (Experiment 1b), as well as inside the scanner (Experiment 2). This pattern suggests that the results are not due to a simple mismatch between encoding and retrieval environments. Furthermore, the scanning environment also can affect response bias, shifting participants’ relative conservative or liberal threshold to endorse an item as previously encountered. These criterion shifts reveal another way in which prior behavioral literature or behavioral piloting in the laboratory may not directly map onto performance in the scanner.
Of interest is the suggestion from Experiment 1b that the deleterious effects of the fMRI environment on recognition memory are greater for older adults than for younger adults, particularly for difficult tasks. This finding is consistent with studies that indicate older adults are more prone to dual-task costs than younger adults (Baltes & Lindenberger, 1997; Hasher & Zacks, 1988; Lindenberger & Baltes, 1994; Lindenberger et al., 2000), and that divided attention during encoding affects the recognition performance of old more than young (Anderson et al., 1998; Park et al., 1989). However tantalizing such a finding may be, further research is need to further establish the presence of such an effect, particularly given the failure to replicate this effect in Experiment 2. Larger sample sizes, careful matching of participants, and manipulating levels of difficulty (as in Experiment 1b) will provide greater precision in identifying disproportionate effects of the scanning environment for older adults.
These findings pose pragmatic questions regarding the interpretation of fMRI data. For example, are there cases in which the scanning environment changes behavior on tasks that are well-explored in laboratories, and if so, do a subset of the neural activations reflect the demands of the scanning environment? These questions could be compounded for between-group comparisons, particularly for the study of aging, should the disproportionate effect of scanning on older adults’ cognitive performance replicate in future studies. It is possible that some of the additional networks recruited by older adults in neuroimaging studies could conceivably be a response to the magnet environment rather than to the demands of the cognitive task. This seems possible because dual task performance implicates dorsolateral prefrontal, anterior cingulate, and intra-parietal regions (D’Esposito, Detre, Alsop, Shin, Atlas, & Grossman, 1995; Dreher & Grafman, 2003), the same regions where increased recruitment is seen in older adults. One solution is to provide older adults with practice in a mock scanning environment that could reduce the impact of the fMRI environment on task performance because training diminishes dual-tasks costs in young and elderly (Kramer, Larish, & Strayer, 1995).
Based on these findings, we speculate that tasks that rely on dorsolateral prefrontal cortex will be most prone to performance decrements due to the fMRI environment. Maintenance of information in working memory relies chiefly upon ventrolateral prefrontal regions (Park et al., 2003; Rypma & D’Esposito, 2000) and the working memory task did not show magnet sensitivity. In contrast, the scanning environment did impair long term memory performance, which relies more on dorsolateral prefrontal cortex (e.g., Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Ranganath, Johnson & D’Esposito, 2003). Dorsolateral prefrontal cortex also plays an increased role for encoding under divided attention conditions (Anderson, Iidaka, Cabeza, Kapur, McIntosh, & Craik, 2000). Furthermore, older adults recruit dorsolateral prefrontal cortex for successful encoding of pictures more than younger adults (Gutchess et al., 2005), consistent with the possibility that the scanning environment disproportionately impairs encoding by older adults. Although these studies cannot conclusively demonstrate the locus of the divided attention effects for the fMRI environment, it seems promising that tasks drawing heavily upon dorsolateral prefrontal cortex will be impaired by the scanning environment.
In addition to robustly addressing between groups differences and the role of dorsolateral prefrontal cortex in scanner costs, future studies may benefit from random assignment of participants to either the fMRI or laboratory setting. Although eligibility restrictions limit the pool of potential fMRI participants substantially for older adults, this should be particularly promising for younger adults. Future studies may also have the ability to disambiguate the contribution of multiple features unique to the fMRI environment, such as noise, motion restriction, and anxiety. Mock scanners allow for greater manipulation of these factors as well as relaxation of eligibility criteria, in contrast to the more restricted nature of a true fMRI environment.
In conclusion, scanning environment impaired encoding of pictures into long-term memory but we found little evidence that scanning environment impairs performance on a low-effort working memory task. Performance impairments from the fMRI environment may be most severe when cognitive tasks place high demand on neural resources that draw upon dorsolateral prefrontal areas, or for subject populations who are most prone to dual-task costs.
5. EXPERIMENTAL PROCEDURES
5.1 Participants
For Experiment 1, 24 young adults and 26 elderly adults were included in the study. Half of the participants in each age group participated in the fMRI study1 (Park, Welsh, Marshuetz, Gutchess, Mikels, Polk, Noll, & Taylor, 2003), and half in a laboratory study. Participants in the two groups were matched on age, education, and Shipley vocabulary (Shipley, 1986).
For Experiment 2, 14 young adults and 13 elderly adults, drawn from Gutchess et al. (2005), were included in the fMRI group, and 12 young adults and 9 elderly adults were included in the laboratory group. Two additional elderly were excluded from the laboratory condition due to failure to follow task instructions. In this study, laboratory participants were not matched to the functional MRI participants; laboratory participants were younger than fMRI participants (F (1, 44) = 4.72, p <.05) but had significantly less education (F (1, 44) = 4.52, p <.05), lower vocabulary scores (F (1, 44) = 5.66, p <.05), and lower Digit Comparison performance (F (1, 44) = 106.70, p <.001). These differences are not a concern for the comparison of performance across laboratory and fMRI environments because we predicted the less-skilled laboratory participants would exhibit better memory performance than the fMRI participants, whose performance would be impaired by the fMRI environment.
Participant characteristics are displayed in Table 2. Written consent was provided for all studies according to the requirements of the University of Michigan Medical and Behavioral Sciences Institutional Review Boards.
Table 2.
Participant characteristics
| Young | Elderly | |||
|---|---|---|---|---|
| fMRI | lab | fMRI | lab | |
| Study 1 | ||||
| Age | 20.58 (.90) | 20.42 (1.08) | 67.00 (4.42) | 69.92 (5.39) |
| Gender | 7M, 5F | 5M, 7F | 6M, 7F | 6M, 7F |
| Education (yrs) | 14.75 (.62) | 14.92 (.87) | 15.31 (1.97) | 14.75 (2.60) |
| Shipley | 32.67 (2.61) | 32.67 (2.61) | 35.23 (2.24) | 35.08 (2.53) |
| N | 12 | 12 | 13 | 13 |
| Study 2 | ||||
| Age | 21.00 (2.00) | 19.74 (1.10) | 70.00 (3.44) | 68.00 (3.19) |
| Gender | 7M, 7F | 5M, 7F | 7M, 6F | 3M, 6F |
| Education (yrs) | 14.96 (1.67) | 13.00 (1.13) | 15.12 (2.33) | 14.83 (1.87) |
| Shipley | 32.71 (3.15) | 29.25 (3.72) | 34.62 (3.95) | 32.78 (4.60) |
| N | 14 | 12 | 13 | 9 |
5.2 Procedures
5.2.1 Experiment 1 Procedure
Participants studied 96 color photographs of outdoor scenes, with each followed by a picture fragment probe to assess maintenance of the picture in working memory. Half of the pictures were presented for six seconds (Visual Condition) and followed by a picture fragment probe. Subjects made a yes/no decision as to whether the fragment was part of the picture that they had just studied. The other half of the pictures were presented for 2 seconds, followed by a blank four second interval during which the participant maintained a visual image of the picture in working memory before the probe was presented (Maintenance Condition). See Figure 4 and Park et al. (2003) for details about the study design. Laboratory participants were tested sitting at computers, while fMRI participants were tested lying supine during actual scan acquisition. Otherwise, the working memory task was identical. Approximately 20 minutes after encoding, long-term memory was assessed outside of the scanner for all subjects. Subjects made recognition judgments for 168 pictures: 96 pictures encoded during the working memory task and 72 distractor pictures. There were three types of distractors: one-third matched in content, color, and composition to the Visual Condition pictures, one-third matched to the Maintenance condition, and one-third entirely novel distractors. Participants pressed a button to denote whether they had encoded each picture previously: Yes-high confidence, Yes-low confidence, or No. The tasks were presented with PsyScope (Cohen, MaxWhinney, Flatt, & Provost, 1993).
Figure 4.
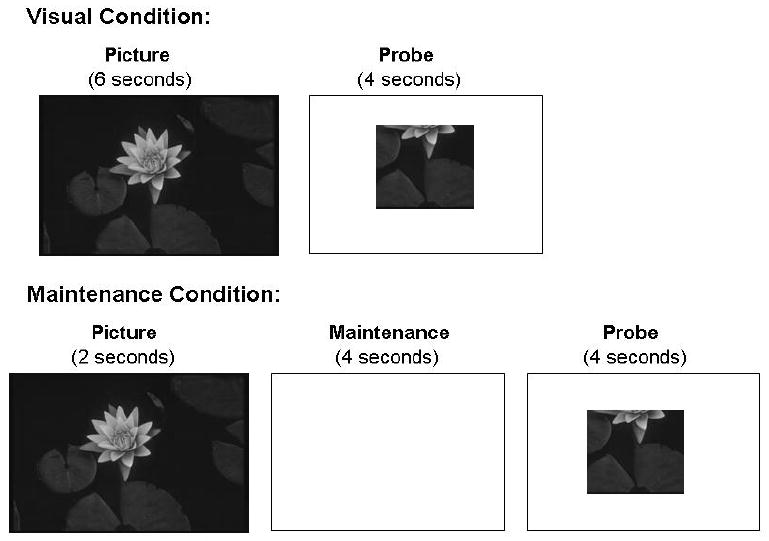
Task from Experiment 1 (see Park et al., 2003).
5.2.2 Experiment 2 Procedure
One hundred ninety-four pictures were encoded for 4-seconds each, interspersed with fixation trials. After 15 minutes, recognition was tested on 388 pictures (194 targets and 194 similar lures). For additional procedural details, see Gutchess et al. (2005). Critically, recognition memory for the fMRI participants was assessed in the scanner, in contrast to the long-term recognition test in Experiment 1. E-Prime software (Psychology Software Tools, Pittsburgh, PA) was used to present the task.
Acknowledgments
Research was supported by National Institute on Aging Neuroscience and Neuropsychology Program Grant R01 AGO6265-15.
Footnotes
Additional participants above and beyond those included in the fMRI paper (Park et al., 2003) are included in the present analysis. This is because those participants who piloted the fMRI study or were removed from the fMRI analyses due to excessive motion or normalization failure have complete sets of behavioral, but not fMRI, data.
References
- Anderson ND, Craik FIM, Naveh-Benjamin M. The attentional demands of encoding and retrieval in younger and older adults: 1. Evidence from divided attention costs. Psychol Aging. 1998;13:405–423. doi: 10.1037//0882-7974.13.3.405. [DOI] [PubMed] [Google Scholar]
- Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FIM. The effects of divided attention on encoding and retrieval related brain activity: A PET study of younger and older adults. J Cog Neurosci. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the life span: A new window to the study of cognitive aging? Psychol Aging. 1997;12:12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JDE. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behav Res Meth Ins C. 1993;25:257–271. [Google Scholar]
- D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995;378:279–281. doi: 10.1038/378279a0. [DOI] [PubMed] [Google Scholar]
- Dreher JC, Grafman J. Dissociating the roles of the rostral anterior cingulate and the lateral prefrontal cortices in performing two tasks simultaneously or successively. Cereb Cortex. 2003;13:329–339. doi: 10.1093/cercor/13.4.329. [DOI] [PubMed] [Google Scholar]
- Earles JL, Kersten AW. Influences of age and perceived activity difficulty on activity recall. J Gerontol B-Psychol. 1998;53B:P324–328. doi: 10.1093/geronb/53b.5.p324. [DOI] [PubMed] [Google Scholar]
- Earles JL, Kersten AW, Mas BB, Miccio DM. Aging and memory for self-performing tasks: Effects of task difficulty and time pressure. J Gerontol B-Psychol. 2004;59B:P285–293. doi: 10.1093/geronb/59.6.p285. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Welsh RC, Hedden T, Bangert A, Minear M, Liu L, Park DC. Aging and the neural correlates of successful picture encoding: Frontal activations compensate for decreased medial temporal activity. J Cog Neurosci. 2005;17:84–96. doi: 10.1162/0898929052880048. [DOI] [PubMed] [Google Scholar]
- Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and new view. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. Academic Press; San Diego: 1988. pp. 193–225. [Google Scholar]
- Irwin DE, Brockmole JR. Suppressing where but not what: The effect of saccades on dorsal- and ventral-stream visual processing. Psychol Sci. 2004;15:467–473. doi: 10.1111/j.0956-7976.2004.00703.x. [DOI] [PubMed] [Google Scholar]
- Kim SY, Kim MS, Chun MM. Concurrent working memory load can reduce distraction. Proc Natl Acad Sci USA. 2005;102:16524–16529. doi: 10.1073/pnas.0505454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K, Boals A. The relationship of life event stress and working memory capacity. Appl Cogn Psychol. 2001;15:565–579. [Google Scholar]
- Klingberg T. Concurrent performance of two working memory tasks: Potential mechanisms of interference. Cereb Cortex. 1998;8:593–601. doi: 10.1093/cercor/8.7.593. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Larish JF, Strayer DL. Training for attentional control in dual task settings: A comparison of young and old adults. J Exp Psychol Appl. 1995;1:50–76. [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: A strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Lindenberger U, Marsiske M, Baltes PB. Memorizing while walking: Increase in dual-task costs from young adulthood to old age. Psychol Aging. 2000;15:417–436. doi: 10.1037//0882-7974.15.3.417. [DOI] [PubMed] [Google Scholar]
- Meyer DE, Kieras DE. A computational theory of executive cognitive processes and multiple-task performance: I. Basic mechanisms. Psych Rev. 1997;104:3–65. doi: 10.1037/0033-295x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Mikhelashvili-Browner N, Yousern DM, Mandir AS, Calhoun VD, Wu C, Oguz KK, Vaughan CL. Correlation of reaction time in and out of the functional MR unit. Acad Radiol. 2002;9:513–519. doi: 10.1016/s1076-6332(03)80327-6. [DOI] [PubMed] [Google Scholar]
- Park DC, Smith AD, Dudley WN, Lafronza VN. Effects of age and a divided attention task presented during encoding and retrieval on memory. J Exp Psychol Learn. 1989;15:1185–1191. doi: 10.1037//0278-7393.15.6.1185. [DOI] [PubMed] [Google Scholar]
- Park DC, Welsh RC, Marshuetz C, Gutchess AH, Mikels J, Polk TA, Noll DC, Taylor SF. Working memory for complex scenes: Age differences in frontal and hippocampal activations. J Cog Neurosci. 2003;15:1122–1134. doi: 10.1162/089892903322598094. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Johnson MK, D’Esposito M. Prefrontal activity associated with working memory and episodic long-term memory. Neuropsychologia. 2003;41:378–389. doi: 10.1016/s0028-3932(02)00169-0. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. Isolating the neural mechanisms of age-related changes in human working memory. Nat Neurosci. 2000;3:509–515. doi: 10.1038/74889. [DOI] [PubMed] [Google Scholar]
- Shipley WC. Shipley Institute of Living Scale. Western Psychological Services; Los Angeles: 1986. [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav Res Meth Ins C. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Tulving E, Thompson DM. Encoding specificity and retrieval processes in episodic memory. Psych Rev. 1973;80:352–373. [Google Scholar]
- VonDras DD, Powless MR, Olson AK, Wheeler D, Snudden AL. Differential effects of everyday stress on the episodic memory test performances of young, midlife, and older adults. Aging Ment Health. 2005;9:60–70. doi: 10.1080/13607860412331323782. [DOI] [PubMed] [Google Scholar]


