Abstract
The need to discover and develop new antimalarial therapeutics is severe. The annual mortality attributed to malaria, currently approximately 2.5 million, is increasing due primarily to widespread resistance to currently used drugs. One strategy to identify new treatment alternatives for malaria is to examine libraries of diverse compounds for the possible identification of novel scaffolds. Beginning with libraries of drug or drug-like compounds is an ideal starting point because, in the case of approved drugs, substantial pharmacokinetic and toxicologic data should be available for each compound series. We have employed a high throughput screen of the MicroSource Spectrum and Killer collections, a library of known drugs, bioactive compounds, and natural products. Our screening assay identifies compounds that inhibit growth of Plasmodium falciparum cultured in human erythrocytes. We have identified 36 novel inhibitors of P. falciparum, of which 19 are therapeutics, and five of these drugs exhibit effective 50% inhibitory concentrations within similar ranges to therapeutic serum concentrations for their currently indicated uses: propafenone, thioridazine, chlorprothixene, perhexiline and azlocillin. The findings we report here indicate that this is an effective strategy to identify novel scaffolds and therefore aid in antimalarial drug discovery efforts.
Keywords: malaria, drug discovery, biological screening
INTRODUCTION
Malaria is a devastating disease with an annual morbidity of 300-500 million people and annual mortality of over one million. The majority of deaths result from pediatric cases in developing countries (1). Plasmodium falciparum is the single-celled eukaryotic parasite responsible for the most lethal form of human malaria, cerebral malaria. The parasite is transmitted to humans by Anopheles mosquitoes. Endemic regions encompass approximately 40% of the global human population. The socioeconomic impact of malaria in endemic regions is estimated at a depressed annual growth rate of 1.5% over a 25 year period, as compared with other countries (2).
The current state of antimalarial chemotherapeutics is particularly bleak for those living in malaria endemic regions of the world due to the low economic incentive for drug development and the rise of resistant strains. Chloroquine resistant strains of P. falciparum are now common in most malaria endemic regions (1), exacerbating the need for novel, cheap alternatives. Unfortunately, the success rate for new chemotherapeutics to move into clinical use is extremely low. This is true for any new drug, however the situation is significantly worse for antimalarial therapeutics (3). Only one new antimalarial chemotherapeutic, malarone, developed by GlaxoWellcome, received FDA approval for clinical use in the last decade.
With the cost of new drug discovery exceeding $750M per new chemical entity (4), novel therapeutics for diseases endemic to the third world would seem out of reach. If academia is to play a role in the discovery and development of drugs for socially imperative but financially challenging conditions, new development paradigms must evolve. Since the cost of creating, maintaining and screening large compound libraries is high, we have chosen the alternative approach of screening a smaller, focused compound library. We used a high-throughput cell-based assay that quantifies parasite growth cultured in human erythrocytes to screen the MicroSource Spectrum and Killer Collections. Together, these make up a library of 2,160 known drugs, bioactive compounds, and natural products. This approach aims to leverage the extensive work that has already been done with the known drugs to ensure their viability as human therapies. Another potentially advantageous aspect to screening this library of diverse compounds is the possibility of identifying novel scaffolds to optimize that are entirely unrelated to known antimalarial drugs.
In the MicroSource Spectrum and Killer Collections we have identified 19 drugs with previously unreported growth inhibitory activity for P. falciparum at micromolar or submicromolar concentrations. A companion article in this Journal reports the results of screening the same compound collection against T. brucei (5). Several compounds inhibit both P. falciparum and T. brucei. We discuss these broader spectrum antiparasitic agents and the novel antimalarial inhibitors that will merit further study. Particularly exciting, is the discovery that five drugs from the collection have anti-P. falciparum growth activity in similar ranges to current therapeutic serum concentrations. Follow up assays of compounds related to these lead molecules suggests initial structure activity relationships.
MATERIALS AND METHODS
MicroSource Collection
The MicroSource collection, as referred to in this work, is the MicroSource Spectrum (2,000 compounds) and Killer (160 compounds) collections. This library is made up of known drugs, bioactives and natural products. The compounds were obtained from the Bay Area Screening Center (BASC) at the California Institute for Quantitative Biology (QB3) (San Francisco, California). BASC originally obtained these compounds from MicroSource Discovery, Inc. Compounds in neat DMSO were delivered in 96-well polypropylene conical plates. All compounds were diluted to 100 μL in 10% DMSO in 1X PBS. 5 μL of compound in 10% DMSO were transferred to 200 μL of parasite culture for the growth inhibition assay, yielding a final DMSO concentration of 0.24%. P. falciparum growth is highly sensitive to DMSO, thus requiring this low concentration.
Plasmodium falciparum inhibition assay
Growth inhibition of P. falciparum cultures was quantifed using a fluorescent-active cell sorting (FACS) assay ((6,7)). Briefly, cultures of P. falciparum (3D7 (8) and W2 (9) strains) were grown in purified human erythrocytes and RPMI 1640 media supplemented with 0.25 % Albumax II (GIBCO, Life Technologies, San Diego, California, United States), 2 g/L sodium bicarbonate, 0.1 mM hypoxanthine, 25 mM HEPES (pH 7.4), and 50 μg/L gentamycin, at 37°C, 5% O2, and 6% CO2. Synchronous cultures (0.8% ring-stage, 0.5% hematocrit) were plated onto 96-well tissue-culture plates (Falcon) and cultured in the presence of compounds (0.24% final DMSO concentration).
After a 72 hr growth period, cultures were fixed for 1 hr at RT in 1% paraformaldehyde and stained with 50 nM YOYO-1 (Molecular Probes) for approximately 24 hr in the dark at RT. Samples were analyzed on a Becton-Dickinson LSR2 fitted with a microplate reader. Uninfected erythrocytes were used to determined background autofluorescence. Parasite growth in each sample was determined relative to infected erythrocytes without test compound. Chloroquine was used as a control to monitor the quality of the assay.
Initial and follow up screens were performed at final drug concentrations of 10 μM, 1 μM and 100 nM. Dose response curves were generated with 9-point, serially diluted concentration schemes: 6 μM, 3 μM, 1 μM, 600 nM, 300 nM, 100 nM, 60 nM, 30 nM, 10 nM. Effective 50% inhibitory concentrations were determined using Graphpad Prism software.
Analysis of inhibition data
All data relevant to the project (plate and compound information, screening data, annotation info, etc) was stored in multiple tables in a mySQL database (v. 4.1.7). Data was manipulated and analyzed using protocols written in Pipeline Pilot 4.5.2 (Scitegic, Inc). Our protocols automated the process of joining experimental data to compound information, flagging suspicious plates based on low Z-prime and Z-factors, extracting compounds with statistically significant activity, annotating hits with additional information (i.e., chemical similarity to known bioactive compounds, known genotoxic/cytotoxic molecules, or available compounds, and profiles from ADME models), and generating preliminary structure-activity relationships. The results from this analysis, available as a searchable html hierarchy, guided subsequent rounds of screening.
RESULTS
The MicroSource Spectrum and Killer Collections (referred to hereafter as the MicroSource collection) make up a library of 2,160 known drugs, bioactive compounds and natural products. We screened the entire MicroSource collection against two representative laboratory strains of P. falciparum: 3D7 (drug-sensitive (8)) and W2 (drug-resistant (9)). The growth inhibition assay we use consists of a three-day incubation of each compound with P. falciparum cultured in human erythrocytes, followed by detection and quantification of parasite growth via flow cytometry, as detailed in the Methods & Materials Section (6,7). Industrial high throughput screening efforts normally use compound concentrations of 1 to 10 μM for cell-based assays. We performed our initial screen at 10 μM final concentration. The quality of the results was found to be quite high, with a median Z-prime factor of 0.75 (Z-prime is a measure of assay quality, with a maximum of 1.0 indicating an ideal assay, and > 0.5 indicating a good, reliable assay(10)). More than 50% of the compounds in this library exhibited some amount of malaria growth inhibition, presumably due to the nature of the library.
Compounds with Z-score greater than or equal to 1.65, as determined by comparison to negative controls (corresponding to a p-value less than or equal to 0.05), are considered statistically significant hits in biological screening assays. We initially used this cutoff to determine the hit list for active P. falciparum inhibitors in our screen. However, this cutoff included only compounds with 100% inhibition activity against 3D7 and W2. We therefore relaxed the cutoff to a Z-score of 1.50 (corresponding to a p-value less than or equal to 0.067), which then included 126 compounds with inhibition activity greater than 97.5% against 3D7 and 228 compounds with inhibition activity greater than 93.7% against W2. 103 compounds inhibited both 3D7 and W2 P. falciparum strains, making a total of 251 compounds for further investigation. This subset of compounds was screened again at lower concentrations of 1 μM and 100 nM.
72 compounds exhibited significant activity (defined here as greater than 70% growth inhibition relative to control) at 1 μM and 19 compounds exhibited significant activity at 100 nM. The 72 compounds with greater than 70% growth inhibition relative to control at 1 μM were flagged for further investigation (see Supplemental Table 1). Literature searches were conducted to determine current therapeutic uses, toxicity information, and any prior identification of antimalarial activity. Compounds within this set of 72 that lacked literature evidence of anti-P. falciparum activity were then investigated for dose response behavior.
Supplemental Table 1.
Compounds with greater than 70% inhibition activity against Plasmodium falciparum at 1 μM.
| Compound | Current Use | Antimalarial Activity |
|---|---|---|
| 3,7-dihydroxyflavone | ||
| amphotericin B | anti-fungal | (33) |
| acivicin | antibiotic, anti-neoplastic | (34) |
| aclacinomycin A1 | antineoplastic | |
| acriflavinium hydrochloride | antiinfective | |
| actinomycin D | antineoplastic | (23) |
| aklavine hydrochloride | antibiotic, antineoplastic | |
| alexidine hydrochloride | antibacterial | |
| amodiaquine | anti-malarial | (14) |
| angolensin | natural product | |
| anisomycin | antiprotozoal, antifungal | (24) |
| atovaquone | antipneumocystic, antimalarial | (18) |
| avermectin B1 (ivermectin) | antibiotic, anti-thelmintic | (35) |
| azlocillin | antibiotic | |
| bebeerine | antimalarial, muscle relaxant | |
| benzalkonium chloride | topical anti-infective | |
| benzethonium chloride | topical anti-infective, antiseptic | |
| cadmium acetate | ||
| celastrol | antineoplastic, anti-inflamatory | (36) |
| cetrimonium | topical antiseptic, disinfectant | |
| cetylpyridinium chloride | topical anti-infective | |
| chloroquine | anti-malarial | |
| chlorprothixene | anti-psychotic, anti-histamine | |
| ciclopiroxolamine | topical anti-fungal | |
| cinchonidine | antimalarial | (16) |
| cinchonine | antimalarial | (15) |
| coralyne chloride | natural product | |
| cycloheximide | antibiotic | (23,24) |
| cyclosporin A | natural product: immunosuppressant | (37-39) |
| cytochalasin R | (40,41) | |
| deoxygedunin | natural product | |
| dequalinium chloride | anti-bacterial | |
| dihydroartemisinin | antimalarial, anti-inflammatory | (19) |
| dihydrocelastrol derivative | immuosuppressive, anti-inflammatory | |
| dihydroergotamine | vasoconstrictor, antimigraine | |
| dihydrogambogic acid | natural product | |
| emetine | natural product: anti-amebic, anti-proliferative | |
| gambogic acid | natural product: anti-inflammatory, anti-carcinomal | |
| gentian violet | anti-bacterial, anthelmintic | (42) |
| heudelottin C | natural product | |
| homidium bromide | antiprotozoal | |
| hycanthone | anthelmintic, antischistomal | |
| hydroquinidine | antimalarial | |
| hydroxychloroquine | antimalarial, lupus suppressant | (13) |
| hydroxyprogesterone | progestogen | |
| lasolacid sodium | antibiotic | (43) |
| lycorine | natural product: mucolytic | (44) |
| mefloquine | antimalarial | (17) |
| methotrexate | antineoplastic, antirheumatic | (25) |
| methylbenzethonium chloride | topical antiseptic | |
| mitomycin C | antineoplastic | |
| mitoxantrone | antineoplastic | |
| monensin sodium | natural product: antibiotic | (43) |
| pararosaniline pamoate | anti-Schistosomal | |
| pentamidine | anti-trypanasomal, antiprotozoal | (26,27) |
| perhexiline maleate | coronary vasodilator | |
| propafenone | antiarrhythmic | |
| puromycin | antineoplastic, antiprotozoal | (24) |
| pyrimethamine | antimalarial | (13) |
| quinacrine | antimalarial | (20) |
| quinidine | antiarrhythmic, antimalarial | (12) |
| quinine | antimalarial | (11) |
| rhodomyrtoxin B | ||
| rutilantinone | antibiotic, anti-neoplastic | |
| salinomycin | antibiotic | (45) |
| selamectin | veterinary antiparasitic | |
| suloctidil | vasodilator | |
| tannic acid | nonspecific enzyme/receptor blocker | |
| tetrandrine | calcium/potassium channel blocker | (21,22) |
| thimerosal | anti-infective, preservative | (46) |
| thioridazine | anti-psyochtic | |
| tilirone | antiviral |
13 of the 72 active inhibitors of P. falciparum were previously known antimalarial therapeutic agents. These compounds served as an excellent internal validation of our screening assay, as we were blinded to the identities of the compounds. The known antimalarial therapeutics we identified were all active at 100 nM and included: quinine (11), quinidine (12), chloroquine and hydroxychloroquine (13), amodiaquine (14), cinchonine (15), cinchonidine (16), mefloquine (17), bebeerine, atovaquone (18), pyrimethamine (13), dihydroartemisinin (19) and quinacrine (20). Six more members of the MicroSource Collection inhibited P. falciparum growth at a concentration of 100 nM. These compounds have been studied previously and confirmed to have antimalarial activity: tetrandrine (21,22), actinomycin D (23), anisomycin (24), puromycin (24), methotrexate (25), and pentamidine (26,27). 17 additional known inhibitors of P. falciparum were identified with significant activity at a concentration of 1 μM.
To our knowledge, 36 compounds have not been studied previously for antimalarial activity and inhibited P. falciparum growth at 1 μM. 19 of these compounds were known therapeutics. This set included known antibiotics, topical anti-infectives, natural products, a known anti-trypanasomal, anti-neoplastics, vasodilators and anti-psychotics. Effective 50% inhibitory concentration values (EC50’s) of these novel antimalarial inhibitors are listed in Table 1.
Table 1.
Representative EC50 values and distribution of MicroSource antimalarial inhibitors with optical, intraenous and oral delivery routes.
| Compound | 3D7 (μM) | W2(μM) |
|---|---|---|
| Topical (9) |
||
| acriflavinium | 0.03 | 0.04 |
| benzalkonium | 0.2 | 0.3 |
| benzethonium | 0.3 | 0.2 |
| cetrimonium | 0.9 | 0.6 |
| cetylpyridinium | 0.4 | 0.5 |
| ciclopiroxolamine | 1.2 | 0.7 |
| dequalinium | 0.01 | 0.04 |
| methylbenzethonium | 0.3 | 0.2 |
| tannic acid | 1.6 | 1.3 |
| Intravenous (5) |
||
| aclarubicin (aclacinomycin A1) | 0.4 | 0.4 |
| azlocillin | 5.1 | 2.5 |
| dihydroergotamine | 3.0 | 3.0 |
| hydroxyprogesterone | 1.0 | 5.4 |
| mitoxantrone | 0.1 | 0.1 |
| Oral (7) | ||
| chlorprothixene | 1.7 | 1.0 |
| dihydroergotamine | 3.0 | 3.0 |
| hycanthone | 1.9 | 0.3 |
| hydroxyprogesterone | 1.0 | 5.4 |
| perhexiline | 1.1 | 0.6 |
| propafenone | 1.0 | 0.2 |
| thioridazine | 2.6 | 1.9 |
DISCUSSION
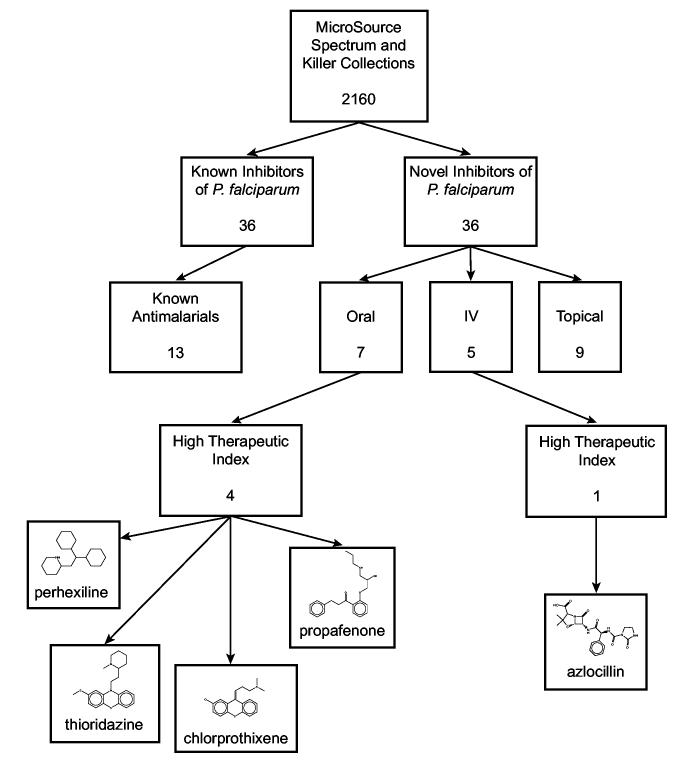
One advantage to screening a library such as the MicroSource Collection, which contains already approved drugs, is the potential to identify novel inhibitors of P. falciparum that have been tested and formulated for safe therapeutic use in human. Of course, not all FDA-approved drugs are appropriate for antimalarial treatment for many reasons. However, drug delivery route is one simple filter to differentiate out potential scaffolds for further optimization. We therefore group the novel inhibitors of P. falciparum by delivery route, excluding those that are simply natural products and bioactives, not therapeutics, and those that are not human therapies. The novel inhibitors that meet these conditions consist of nine topical, five intravenous (IV) and seven orally administered therapeutics (some of these are available in multiple delivery methods, see Table 1). A flowchart following the distribution of antimalarial activity in the MicroSource Collection is shown in Figure 1.
Figure 1.
Distribution of antimalarial inhibition activity in the MicroSource Spectrum and Killer Collections. 36 novel compounds exhibited ≥70% growth inhibition of P. falciparum cultures at 1 μM final concentration. Of these 36 novel antimalarial compounds, nine are topical therapeutics, five are intravenous therapeutics, and seven are oral therapeutics. Five drugs are identified with a high therapeutic index; 50% effective inhibitory concentrations in the same range as therapeutic serum levels in patients.
Inhibitors that are Topical Therapeutics
Several novel inhibitors of P. falciparum are topically administered therapeutics. These compounds are mainly comprised of antibiotics, anti-infectives, antiseptics and anti-fungal drugs. Table 1 shows representative EC50 values for these inhibitors. A number of these compounds inhibit P. falciparum growth in concentrations of the low hundreds of nanomolar range. However, topical treatments are inappropriate as potential antimalarial therapeutics, since the required antimalarial activity must take place within the bloodstream.
Inhibitors that are Intravenous Therapies
Intravenous therapies are better candidates for antimalarial treatment than topical drugs in that they have undergone clinical testing within the bloodstream, and therefore may make better starting point scaffolds. In particular, IV formulations of antimalarials are important for treatment of acute severe cerebral malaria and are part of the WHO product profile. However, IV administration does not satisfy the need for antimalarial therapeutics to be easily accessible for large populations for routine therapy or prophylaxis.
Active compounds with an IV administration route include an antibiotic, a vasoconstrictor, a progestogen and two antineoplastic therapies. The fact that two of these (dihydroergotamine and hydroxyprogesterone) are available not only in IV form, but also oral delivery, shows that the possibility exists to pursue these further. The effective therapeutic ranges for these two compounds are unfortunately orders of magnitude lower than the EC50 values observed here against cultured malaria (28), however this is not the case for azlocillin. The EC50 values for azlocillin are approximately 5 and 2 μM against 3D7 and W2, respectively. While the raw values seem high, these inhibitory concentrations are well within the range of minimum inhibitory concentrations of azlocillin for the treatment of severe infections (4-16 μM) (28).
Mitoxantrone is an antineoplastic with particularly good inhibition activity: approximately 100 nM EC50 values against both strains of P. falciparum. However, therapeutic serum levels are unknown for this antineoplastic (28). The same is true for aclarubin.
Inhibitors that are Oral Therapies
Novel inhibitors of P. falciparum that are oral therapeutics are ideal for potential antimalarial drug development. These compounds have already been clinically tested and formulated for the preferred delivery method. Seven members of the MicroSource Collection fall under this category. Two of these drugs are anti-psychotics (chlorprothixene and thioridazine), one is a vasoconstrictor (dihydroergotamine) and one is a vasodilator (perhexiline), one is an anhelmintic (hycanthone), one is a progestogen (hydroxyprogesterone), and one is an antiarrythmic (propafenone). The EC50 values for these drugs against drug sensitive and drug resistant strains of P. falciparum are shown in Table 1.
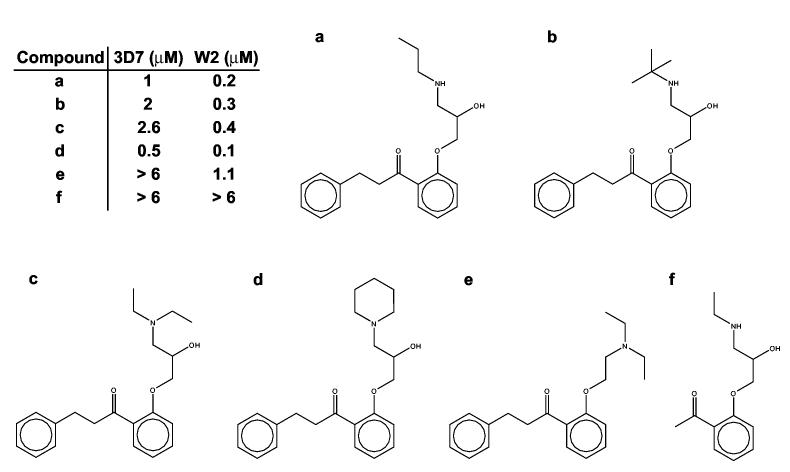
None of the seven orally administered drugs has remarkably low effective 50% inhibitory concentrations against these two strains of the parasite. However, the EC50 values of some of these drugs fall into the therapeutic range (detected in serum levels) for their indicated therapies. Propafenone, with submicromolar EC50 values against 3D7 and W2, is one of these drugs. Therapeutic serum levels are extremely variable from patient to patient, and effective treatments cover a concentration range of 0.1 - 8.0 μM (28). The dangers of cardiotoxicity and seizures associated with propafenone overdose require administration to be initiated under hospital supervision (28). However, the negative side effects associated with propafenone may not be associated with the attributes that could make it an effective inhibitor of malaria growth, and optimization may prove fruitful. As an initial probe of the potential for propafenone optimization, we tested five analogs of propafenone (Chembridge, Inc.). These compounds and their EC50 values are shown in Figure 2. The wide range of EC50’s in this small set of propafenone analogs increases the likelihood that these compounds are making specific interactions, and not merely inhibiting growth by non-specific interactions. In addition, one compound is twice as potent as propafenone (EC50 = 0.5 and 0.1 μM for 3D7 and W2, respectively). This preliminary data is very promising for selectivity, and even further optimization of the antimalarial effects of the propafenone series.
Figure 2.
The oral antiarrythmic drug propafenone (A) and five analogs of propafenone. The structures and EC50 values of this series suggest that P. falciparum growth inhibition is accomplished by specific interactions, and that optimization of antimalarial activity is possible.
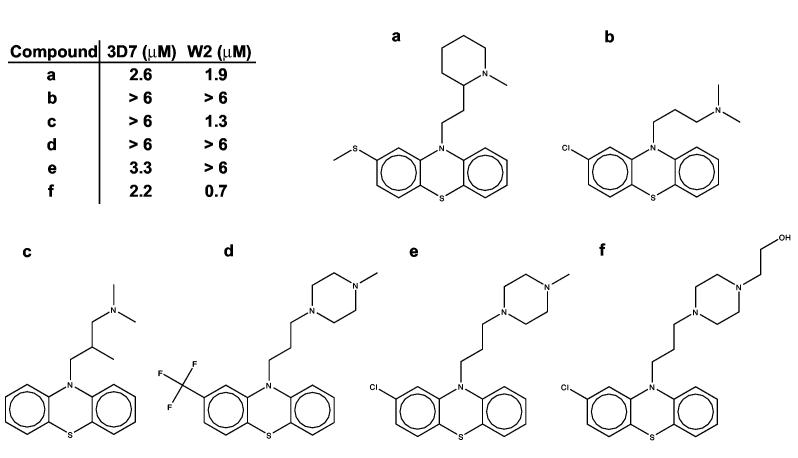
Thioridazine is another drug with similar therapeutic and effective 50% inhibitory concentration ranges against P. falciparum. Phenothiazines (thioridazine) and thioxanthenes (chlorprothixene) are antimicrobials that have traditionally been used to manage psychoses, however various members of these classes, including chlorpromazine, have been shown effective in killing P. falciparum cultures (29-31). The therapeutic range of thioridazine in serum is difficult to estimate, due to the large variations from patient to patient, as with propafenone. However, one source reports an estimated therapeutic range of roughly 0.5 -6.4 μM (32). The antimalarial activity demonstrated against cultured P. falciparum is well within this concentration range. However, the onset of toxic effects due to thioridazine is indicated at serum levels greater than 2.5 μM, which is close to the EC50 values against the two strains of malaria tested here. A preliminary test of five analogs of thioridazine (Figure 3) shows a range of EC50 values, and thus like propafenone, a real possibility for optimization. The major difficulty in development of thioridazine or chlorprothixene analogs lies in the need to decrease the CNS effects while improving antimalarial effects. The antipsychotic therapeutic range of chlorprothixene is approximately 60-600 nM (28), which is close to the EC50 values of 1-2 μM against strains of P. falciparum.
Figure 3.
The oral anti-psychotic drug thioridazine (A) and five analogs of thioridazine. Like propafenone, the structures and EC50 values of this series suggest that P. falciparum growth inhibition is accomplished by specific interactions, and that optimization of antimalarial activity is possible.
Perhexiline is the final orally delivered therapeutic in this set with comparable therapeutic and effective 50% inhibitory concentration ranges. Therapeutic dosing results in serum levels of 0.4-1.5 μM (28), and the EC50 values against P. falciparum strains 3D7 and W2 are roughly 1.0 and 0.6 μM, respectively.
The therapeutic dosing ranges for hydroxyprogesterone, dihydroergotamine and hycanthone are all orders of magnitude lower than the activity observed against P. falciparum.
Inhibitors Common to P. falciparum and T. brucei
18 compounds in the MicroSource collection inhibit growth of both P. falciparum and T. brucei at 1 μM final concentration (see companion article (5)). These compounds are listed in Supplemental Table 2. Therapeutics that are efficacious against both of these species are interesting as possible broad spectrum antiparasitic agents. This is important due to the fact that the regions of the world where these parasites are endemic do indeed overlap, and dual infections are plausible and sometimes likely. Therapies that are able to treat both of theses diseases would be particularly attractive to those afflicted.
Supplemental Table 2.
Antimalarial inhibitors identified in this stuty with activity against T. brucei.
| Compound | Current use |
|---|---|
| aclacinomycin A1 | anti-neoplastic |
| acriflavinium hydrochloride | anti-infective |
| actinomycin D | anti-neoplastic |
| alexidine hydrochloride | antibacterial |
| anisomycin | antiprotozoal, antifungal |
| dequalinium chloride | anti-bacterial |
| dihydrocelastrol derivative | immunosuppressive, anti-inflammatory |
| emetine | natural product: anti-amebic, anti-proliferative |
| gambogic acid | natural product: anti-inflammatory, anti-carcinomal |
| gentian violet | anti-bacterial, anthelmintic |
| lycorine | natural product: mucolytic |
| mitomycin C | anti-neoplastic |
| monensin sodium | natural product: antibiotic |
| pararosaniline pamoate | anti-schistosomal |
| pentamidine | anti-trypanasomal, antiprotozoal |
| puromycin hydrochloride | antineoplastic, antiprotozoal |
| rutilantinone | antibiotic, anti-neoplastic |
| thimerosal | anti-infective, preservative |
CONCLUSIONS AND FUTURE DIRECTIONS
Whole cell screening of diverse compound libraries is an essential part of the malaria drug discovery effort. The results of our high throughput growth inhibition screen are encouraging and show that progress may be made through this kind of approach. Within this one set of 2,160 compounds, we have identified 36 novel inhibitors of P. falciparum, of which 19 are known drugs. Five of these drugs exhibit growth inhibition activity against cultured malaria in the same concentration range as exhibited in patient serum levels at therapeutic dosing for their indicated uses, and two of these drugs, propafenone and thioridazine, show preliminary evidence of specific inhibition with potential for scaffold optimization.
The preliminary identification of novel inhibitors of antimalarial growth in vitro is promising and strongly supports the high throughput screening of additional compound libraries. The capacity we regularly screen with our flow cytometric assay is roughly 500-1000 compounds per week with a single concentration pass against two strains of malaria. This is far from high throughput by industry standards. The throughput capacity of screening large libraries could certainly be increased by the incorporation of additional robotics into our system and possibly switching to a faster plate fluorescence platform. Regardless, the screening of more libraries with diverse scaffolds is an important strategy to continue exploring.
Another direction to explore in future work is the transcriptome response to the antimalarial inhibitors identified in this work. Transcriptome response to these known drugs will aid in the determination of novel pathways to target for drug design in the P. falciparum genome. Combination drug therapies are imperative for effective antimalarials due to the high efficiency of the parasite to develop mechanisms of resistance to drug currently in use. Continuation of the kind of library screening that we have presented here and transcriptome analysis of proposed therapeutics are potentially effective strategies to help fight the worsening malaria plight.
ACKNOWLEDGMENTS
JLW acknowledges funding from a Giannini Family Foundation Fellowship. AAS acknowledges funding from the Burroughs Wellcome Fund. APL, RKG and JLD acknowledge support by NIAID U01 AI53862.
The authors thank ZBM, AMB and JHM for excellent discussions, and AC, MU and JW for assistance with compound plate preparations.
REFERENCES
- 1. (2005), World Health Organization.
- 2.Sachs J, Malaney P. Nature. 2002;1415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 3.Rosenthal PJ. J Exp Biol. 2003;206:3735–3744. doi: 10.1242/jeb.00589. [DOI] [PubMed] [Google Scholar]
- 4. (2005), Tufts Center for the Study of Drug Development.
- 5.Mackey ZB, Baca AM, Mallari JP, Apsel B, Shelat AS, Hansell EJ, Chiang PK, Wolff B, Guy KR, Williams J, McKerrow JH. Chemical Biology and Drug Design. 2006 doi: 10.1111/j.1747-0285.2006.00389.x. in press. [DOI] [PubMed] [Google Scholar]
- 6.Barkan D, Ginsburg H, Golenser J. Int J Parasitol. 2000;30:649–653. doi: 10.1016/s0020-7519(00)00035-7. [DOI] [PubMed] [Google Scholar]
- 7.Madrid PB, Wilson NT, DeRisi JL, Guy RK. J Comb Chem. 2004;6:437–442. doi: 10.1021/cc0340473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walliker D, Quakyi IA, Wellems TE, McCutchan TF, Szarfman A, London WT, Corcoran LM, Burkot TR, Carter R. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 9.Oduola AM, Weatherly NF, Bowdre JH, Desjardins RE. Exp Parasitol. 1988;66:86–95. doi: 10.1016/0014-4894(88)90053-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang JH, Chung TD, Oldenburg KR. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 11.Handfield-Jones RP. Ann Trop Med Parasitol. 1949;43:345–348. doi: 10.1080/00034983.1949.11685420. [DOI] [PubMed] [Google Scholar]
- 12.White NJ, Looareesuwan S, Warrell DA, Chongsuphajaisiddhi T, Bunnag D, Harinasuta T. Lancet. 1981;2:1069–1071. doi: 10.1016/s0140-6736(81)91275-7. [DOI] [PubMed] [Google Scholar]
- 13.Hoekenga MT. Am J Trop Med Hyg. 1954;3:833–838. doi: 10.4269/ajtmh.1954.3.833. [DOI] [PubMed] [Google Scholar]
- 14.Hoekenga MT. J Am Med Assoc. 1952;149:1369–1371. doi: 10.1001/jama.1952.02930320009003. [DOI] [PubMed] [Google Scholar]
- 15.Sowunmi A, Salako LA, Laoye OJ, Aderounmu AF. Trans R Soc Trop Med Hyg. 1990;84:626–629. doi: 10.1016/0035-9203(90)90127-z. [DOI] [PubMed] [Google Scholar]
- 16.Barennes H, Kahiatani F, Pussard E, Clavier F, Meynard D, Njifountawouo S, Verdier F. Trans R Soc Trop Med Hyg. 1995;89:418–421. doi: 10.1016/0035-9203(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 17.Trenholme CM, Williams RL, Desjardins RE, Frischer H, Carson PE, Rieckmann KH, Canfield CJ. Science. 1975;190:792–794. doi: 10.1126/science.1105787. [DOI] [PubMed] [Google Scholar]
- 18.Chiodini PL, Conlon CP, Hutchinson DB, Farquhar JA, Hall AP, Peto TE, Birley H, Warrell DA. J Antimicrob Chemother. 1995;36:1073–1078. doi: 10.1093/jac/36.6.1073. [DOI] [PubMed] [Google Scholar]
- 19.Looareesuwan S, Wilairatana P, Vanijanonta S, Pitisuttithum P, Viravan C, Kraisintu K. Ann Trop Med Parasitol. 1996;90:21–28. doi: 10.1080/00034983.1996.11813022. [DOI] [PubMed] [Google Scholar]
- 20.Boroda C. West Afr Med J. 1955;4:144–148. [PubMed] [Google Scholar]
- 21.Ye ZG, Van Dyke K. Biochem Biophys Res Commun. 1989;159:242–248. doi: 10.1016/0006-291x(89)92429-7. [DOI] [PubMed] [Google Scholar]
- 22.Ye ZG, Van Dyke K, Castranova V. Biochem Biophys Res Commun. 1989;165:758–765. doi: 10.1016/s0006-291x(89)80031-2. [DOI] [PubMed] [Google Scholar]
- 23.Geary TG, Jensen JB. Am J Trop Med Hyg. 1983;32:221–225. doi: 10.4269/ajtmh.1983.32.221. [DOI] [PubMed] [Google Scholar]
- 24.Ekong RM, Kirby GC, Patel G, Phillipson JD, Warhurst DC. Biochem Pharmacol. 1990;40:297–301. doi: 10.1016/0006-2952(90)90691-d. [DOI] [PubMed] [Google Scholar]
- 25.Elslager EF, Jacob P, Johnson J, Werbel LM, Worth DF, Rane L. J Med Chem. 1978;21:1059–1070. doi: 10.1021/jm00208a010. [DOI] [PubMed] [Google Scholar]
- 26.Stead AM, Bray PG, Edwards IG, DeKoning HP, Elford BC, Stocks PA, Ward SA. Mol Pharmacol. 2001;59:1298–1306. doi: 10.1124/mol.59.5.1298. [DOI] [PubMed] [Google Scholar]
- 27.Bray PG, Barrett MP, Ward SA, de Koning HP. Trends Parasitol. 2003;19:232–239. doi: 10.1016/s1471-4922(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 28. Thompson Micromedex, Greenwood Village, Colorado (Edition expires 2005)
- 29.Amaral L, Viveiros M, Kristiansen JE. Trop Med Int Health. 2001;6:1016–1022. doi: 10.1046/j.1365-3156.2001.00804.x. [DOI] [PubMed] [Google Scholar]
- 30.Kristiansen JE, Jepsen S. Acta Pathol Microbiol Immunol Scand [B] 1985;93:249–251. doi: 10.1111/j.1699-0463.1985.tb02884.x. [DOI] [PubMed] [Google Scholar]
- 31.Basco LK, Le Bras J. Antimicrob Agents Chemother. 1992;36:209–213. doi: 10.1128/aac.36.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. (2001), Bio-Reference Laboratories, Inc.
- 33.Hatabu T, Takada T, Taguchi N, Suzuki M, Sato K, Kano S. Antimicrob Agents Chemother. 2005;49:493–496. doi: 10.1128/AAC.49.2.493-496.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vilmont M, Azoulay M, Frappier F. Ann Parasitol Hum Comp. 1990;65:162–166. doi: 10.1051/parasite/1990654162. [DOI] [PubMed] [Google Scholar]
- 35.Nasveld P, Russell B, Kotecka B, Rieckmann K. Southeast Asian J Trop Med Public Health. 2003;34:552–553. [PubMed] [Google Scholar]
- 36.Figueiredo JN, Raz B, Sequin U. J Nat Prod. 1998;61:718–723. doi: 10.1021/np9704157. [DOI] [PubMed] [Google Scholar]
- 37.Biswas S, Saxena QB, Upender M. Indian J Malariol. 1991;28:1–8. [PubMed] [Google Scholar]
- 38.Peterson MR, Hall DR, Berriman M, Nunes JA, Leonard GA, Fairlamb AH, Hunter WN. J Mol Biol. 2000;298:123–133. doi: 10.1006/jmbi.2000.3633. [DOI] [PubMed] [Google Scholar]
- 39.Kumar R, Adams B, Musiyenko A, Shulyayeva O, Barik S. Mol Biochem Parasitol. 2005;141:163–173. doi: 10.1016/j.molbiopara.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Dieckmann-Schuppert A, Franklin RM. Cell Biol Int Rep. 1989;13:411–418. doi: 10.1016/0309-1651(89)90135-5. [DOI] [PubMed] [Google Scholar]
- 41.Dieckmann-Schuppert A, Franklin RM. Cell Biol Int Rep. 1989;13:207–214. doi: 10.1016/0309-1651(89)90067-2. [DOI] [PubMed] [Google Scholar]
- 42.Yang SL, Di Santi SM, Amato Neto V, Moreira AA, Pinto PL, Boulos M, Campos R, de Sant’Ana EJ, Shiroma M. Rev Inst Med Trop Sao Paulo. 1988;30:17–20. doi: 10.1590/s0036-46651988000100003. [DOI] [PubMed] [Google Scholar]
- 43.Gumila C, Ancelin ML, Jeminet G, Delort AM, Miquel G, Vial HJ. Antimicrob Agents Chemother. 1996;40:602–608. doi: 10.1128/aac.40.3.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sener B, Orhan I, Satayavivad J. Phytother Res. 2003;17:1220–1223. doi: 10.1002/ptr.1346. [DOI] [PubMed] [Google Scholar]
- 45.Mehlhorn H, Ganster HJ, Raether W. Zentralbl Bakteriol Mikrobiol Hyg [A] 1984;256:305–313. [PubMed] [Google Scholar]
- 46.Zidovetzki R, Sherman IW, Prudhomme J, Crawford J. Parasitology. 1994;108(Pt 3):249–255. doi: 10.1017/s0031182000076095. [DOI] [PubMed] [Google Scholar]