Abstract
Previously, we reported that dietary choline influences development of the hippocampus in fetal rat brain. It is important to know whether similar effects of choline occur in developing fetal mouse brain because interesting new experimental approaches are now available using several transgenic mouse models. Timed-pregnant mice were fed choline-supplemented (CS), control (CT) or choline-deficient (CD) AIN-76 diet from embryonic day 12 to 17 (E12–17). Fetuses from CD dams had diminished concentrations of phosphocholine and phosphatidylcholine in their brains compared with CT or CS fetuses (P < 0.05). When we analyzed fetal hippocampus on day E17 for cells with mitotic phase–specific expression of phosphorylated histone H3, we detected fewer labeled cells at the ventricular surface of the ventricular zone in the CD group (14.8 ± 1.9) compared with the CT (30.7 ± 1.9) or CS (36.6 ± 2.6) group (P < 0.05). At the same time, we detected more apoptotic cells in E17 hippocampus using morphology in the CD group (11.8 ± 1.4) than in CT (5.6 ± 0.6) or CS (4.2 ± 0.7) group (P < 0.05). This was confirmed using terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin anti-digoxigenin fluorescein conjugate antibody nick end-labeling (TUNEL) and activated caspase-3 immunoreactivity. We conclude that the dietary availability of choline to the mouse dam influences progenitor cell proliferation and apoptosis in the fetal brain. J. Nutr. 133: 3614–3618, 2003.
Keywords: choline, phosphatidylcholine, brain development, mice, hippocampus
Maternal dietary choline supplementation in rats results in lifelong cognitive and memory enhancement in the offspring. The underlying physiologic and biochemical bases for these lifelong alterations are just beginning to be understood. Certain anatomical regions of the hippocampus (e.g., dentate gyrus) play critical roles in learning and acquisition of memory. In rats, prenatal choline supplementation increased the sensitivity of CA1 hippocampal neurons to stimulation of long-term potentiation (LTP)4 (1), and increased working spatial memory (2–6), whereas prenatal choline deficiency increased the threshold for LTP and retarded temporal processing (7). We previously reported that, in rats, these physiologic and behavioral changes may be related to neuroanatomical changes in regions of fetal brain hippocampus and basal forebrain that are known to regulate memory (8–10). The recent development of genetically modified mice that have defects in phosphatidylcholine (PtdCho) synthesis (11) and in folate metabolism (12) could enhance the elucidation of the underlying mechanisms for the choline effect on brain development. However, mouse metabolism and brain development differ substantially from those of rats, and it has not yet been proven that maternal dietary choline influences fetal brain development in mice.
The neuroanatomical development of the hippocampus is dictated by the tight regulation of proliferation in progenitor-type stem cells [mainly within two relatively thin layers of tissue lining the primitive ventricular cavities; the ventricular zone (vz) and the subventricular zone (svz) (13)], and their subsequent migration and differentiation to form specialized regions of the hippocampus (14). In the developing fetal rat hippocampus, changing the level of choline in the maternal diet altered neurogenesis (8,9). These effects were mediated in part by altered expression of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors (CDKI), proteins that inhibit cell cycling (10). Choline deficiency in fetal rat brain on embryonic day 18 (E18) reduces cell proliferation and increases apoptosis (8,9). Here we report that choline deficiency reduces cell proliferation and increases apoptosis in developing mouse hippocampus on day E17.
MATERIALS AND METHODS
Animals
Timed-pregnant C57 BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME) and used in all experiments. The day after mating was considered day E1. The mice were housed individually in cages in a temperature-controlled room at 24°C and exposed to a 12-h light:dark cycle. In all experiments, unless otherwise indicated, the mice consumed AIN-76A purified diet (15) with 1.1 g/kg choline chloride replacing 2 g/kg choline bitartrate (ingredients, g/kg: casein, 200.00; DL-methionine, 3.00; cornstarch, 150.00; sucrose, 500.90; cellulose, 50.00; corn oil, 50.00; salt mix #200000, 35.00; vitamin mix #300050, 10.00) (Dyets, Bethlehem, PA). The AIN-76A diet was chosen because it was used in all of the earlier published studies on the effects of choline on rat brain development (8–10). The mice consumed their experimental diet and water ad libitum until d 12 of gestation. On E12, the pregnant mice were randomly assigned to one of the three feeding groups (n = 6/group). The choline-deficient (CD) group received the AIN-76A diet containing 0.0 g/kg choline chloride, the control (CT) group received the AIN-76A diet containing 1.1 g/kg choline chloride and the choline-supplemented (CS) group received the AIN-76A diet containing 4.95 g/kg choline chloride. These pregnant mice received their special diets from the morning of day E12 until the morning of day E17.
Tissue collection
On day E17, pregnant mice were anesthetized with a single subcutaneous injection of 0.03 mL ketamine (100 g/L) and 0.02 mL xylazine (20 g/L) (Henry Schein, Melville, NY) and kept on a heating pad to maintain body temperature. The uterine horns were exposed by a midline abdominal incision and the fetuses were removed individually for perfusion. The chest cavity of the fetus was opened and, for in situ fixation, ~2 mL of perfusion fixative containing 4% formaldehyde and 0.2% glutaraldehyde (Polysciences, Warrington, PA) were injected into the fetal heart. The fetuses were decapitated and the fetal skull was opened for postfixation overnight. The heads were stored overnight in the perfusion fixative and later in 0.1 mol/L phosphate buffer. The fetal brains were embedded in paraffin and 5-μm coronal serial sections were cut and applied to glass slides for histological and immunohistochemical assays. Because there is a posterior to anterior gradient of neurogenesis in fetal mouse brain, the paraffin sections were reviewed at the time of sectioning to ensure that they included anatomically reproducible areas of the hippocampus as defined by a standard atlas of the developing brain (16).
The residual fetal bodies were kept on ice for later use for sex determination. Within each feeding group, the dam livers and at least one pup per dam were collected and used to determine choline and its metabolite concentrations. For biochemical analyses, fetuses were not perfused, and brains and livers were collected and quick frozen in liquid nitrogen.
Assessment of mitosis
The mitotic and synthetic zone of the vz adjacent to the lateral ventricle is the region of fetal hippocampus from which neuronal and glial type cells originate (17). The cells at the ventricular surface of the vz of the developing hippocampus include progenitor cells that have exited S phase and entered the mitotic phase of the cell cycle. To determine whether mitosis in fetal hippocampus was altered by maternal dietary choline availability, coronal sections were probed for phosphorylated histone H3 (phospho-histone H3), the core protein of the nucleosome. Phosphorylation of histone H3 is a specific marker for mitosis (18), occurring at the end of prophase, an event that is essential for the maintenance of mitosis-associated chromosome condensation (19). Slides were depar-affinized in fresh xylene for 3 steps of 20 min, then rehydrated in absolute ethanol for 15 min, 70% ethanol with 0.25% ammonium for 1 h (20) and 50% ethanol for 15 min. This was followed by three washes for 5 min in PBS with 0.1% Tween 20 (PBST; Sigma, St. Louis, MO). Antigen retrieval was performed for 20 min in 20 μmol/L proteinase K (Sigma) and for 40 min in 10 g/L sodium borohydrate (Sigma). Nonspecific sites were blocked with 2% non-immune goat serum (Chemicon, Temecula, CA) in PBST. Brain sections were incubated in rabbit anti-phospho-Histone H3 antibody (Upstate, Lake Placid, NY) at a concentration of 2 mg/L in blocking buffer, overnight at 4°C. Sites of phospho-histone H3 expression were detected using a Cy3 conjugated goat anti-rabbit IgG (Chemicon) at 1:500 dilution, for 2 h at room temperature. 4′,6-Diamidino-2-phenylindole (DAPI, Sigma), 0.1 mg/L for 20 min, was used to counterstain nuclear DNA. Sections were mounted using 80% Tris-buffered glycerol, pH 7.0 (21) and a #1 thickness coverglass. Images were acquired using a Zeiss Confocal Laser Scanning Microscope LSM 210 (Carl Zeiss, Thornwood, NY) as described below. The number of phospho-histone H3 labeled cells was measured at the ventricular surface of the vz beginning at the junction of the hippocampus and choroid plexus (hippocampal wedge), and extending toward the cortical vz. Cells were counted at a final magnification of 200X in four hippocampal hemispheres from two consecutive serial sections and the values were averaged to obtain a single value/hippocampal region for each mouse. Calibrated 50X magnification images of the same regions were used to measure the length of the hippocampal vz with an internal macro of NIH Image program version 1.61.
Assessment of apoptosis
We used a combination of classical apoptotic morphology, active caspase-3 immunoreactivity and terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin anti-digoxigenin fluorescein conjugate antibody nick end-labeling (TUNEL) to detect apoptotic cells in fetal brain. Apoptotic cells exhibit chromatin condensation and hypersegmentation of nuclear chromatin within shrunken cells, changes that are characteristic of end-stage apoptosis (22). These apoptotic cells were detected hemilaterally in each of the selected sections after hematoxylin and eosin staining using a light microscope as described previously (9). Apoptotic indices for the fetal mouse brain hippocampus of the different mouse groupings are presented as the number of apoptotic cells/section of the hippocampal hemispheres. Four hippocampal hemispheres from two consecutive serial sections were averaged to obtain single hemilateral value/hippocampal section/mouse. For double labeling of TUNEL and active caspase-3, the DNA terminal labeling, conducted according to the manufacturer’s protocol (S 7111 Apoptag Plus Fluorescein In Situ Apoptosis Detection Kit, Serologicals, Norcross, GA), was followed by overnight incubation with the primary antibody to cleaved (active) caspase-3 (Asp175) (Cell Signaling Technology, Beverly, MA). Then, fluorescein-antidigoxigenin conjugate and goat rhodamine-anti-rabbit IgG (Calbiochem, San Diego, CA) were applied for 2 h at room temperature to display the TUNEL-positive nuclei and active caspase-3. DAPI (0.1 mg/L for 20 min) was used to counterstain nuclear DNA. The TUNEL and active caspase-3 positive cells were identified and scored by a trained observer (C.N.C.), who was unaware of the maternal diet grouping, based upon the presence of green fluorescent nuclear staining for TUNEL and red fluorescent nuclear staining for active caspase-3. Stained nuclei were usually but not always condensed, and intense blue fluorescent chromatin was often visible inside, consistent with fragmented DNA.
Image analysis
For apoptosis studies using morphological criteria, image acquisition was performed on a Nikon FXA microscope (Nikon, Garden City, NY) equipped with an Optronics TEC-470 CCD Video Camera System (Optronics Engineering, Goleta, CA) and the public domain NIH Image program version 1.61 as described previously (10).
For the mitosis assessment, TUNEL and active caspase-3 evaluation, the image analysis of fetal brain slices was performed on a Zeiss Confocal Laser Scanning Microscope LSM 210 (Carl Zeiss) equipped with an Optronics DEI 750 low light level integrating CCD camera (Optronics Engineering) connected to an Apple Macintosh G3 computer utilizing a Scion CG7 image capture card, for digital image capture of standard and epifluorescence images, and the public domain NIH Image program version 1.61. All images were captured by using 5, 20 or 40X objectives and fluorescent filters optimized for observing DAPI (blue), fluorescein isothiocyanate (green) and rhodamine-Cy3 conjugates (red) signals, respectively. Images obtained from the same field with different fluorescent probes were subsequently overlapped or merged.
Analysis of brain and hepatic choline metabolite concentrations
Choline and its metabolites, glycerophosphocholine (GPCho), phosphocholine (PCho) and PtdCho, were analyzed using GC/MS as previously described (23).
Statistics
Statistical differences among group means for mitotic cells [phospho-histone H3(+) cells/region of interest in one section], apoptotic cell number (apoptotic cells/hippocampal section per mouse), and choline and its metabolites in liver and whole brain were assessed as follows. We tested that variances were equal using O’Brien, Brown-Forsythe, Levene, and Bartlett tests (JMP Version 3; SAS Institute, Cary, NC). For observed significance probabilities > 0.05 (considered evidence of equal variances across the levels), we used comparisons for each pair using Student’s t test, for all pairs using Tukey-Kramer Honestly Significant Difference test, and comparison with control using Dunnett’s Method as indicated in the figures. If the tests of equal variances revealed that the group variances were significantly different (the probability of obtaining by chance alone an F-value larger than the one calculated if, in reality, the variances are equal across all levels), the Welch ANOVA for the means was used in place of the usual ANOVA (the Welch statistic is based on the usual ANOVA F-test: however, the means have been weighted by the reciprocal of the sample variances of the group means) (JMP Version 3; SAS Institute). Differences were considered significant at P < 0.05.
RESULTS
Choline and its metabolites in dam liver and fetal liver and brain
After 5 d of CD or CS diet intake by pregnant dams, we detected some significant differences from the CT group in concentrations of choline and its metabolites in dam livers and fetal livers and brains (Table 1). In livers of CD dams, all choline metabolite concentrations were lower than in CT dams, whereas in livers of CS dams, concentrations of choline, GPCho and PCho were greater than in controls. In livers of CD fetuses, choline, GPCho and PCho concentrations were lower than in CT fetuses. PCho and PtdCho concentrations were lower in brain of CD fetuses than in controls.
TABLE 1.
Choline and its metabolites in fetal mouse brain and liver, and in dam liver of timed-pregnant mice fed choline-supplemented (CS), control (CT) or choline-deficient (CD) AIN-76 diet from embryonic day 12 to 17 (E12–17)1,2
| Cho | GPCho | PCho | PtdCho | |
|---|---|---|---|---|
| ———nmol/g——— | μmol/g | |||
| Dam liver | ||||
| CD | 15.6 ± 0.8* | 206.8 ± 8.0* | 96.5 ± 5.5* | 14.7 ± 0.8* |
| CT | 26.1 ± 3.2 | 233.9 ± 12.0 | 136.7 ± 5.5 | 19.7 ± 1.0 |
| CS | 74.0 ± 11.7* | 387.8 ± 49.1* | 844.4 ± 110.1* | 19.8 ± 0.7 |
| Fetal liver E17 | ||||
| CD | 144.9 ± 7.9* | 451.9 ± 34.8* | 723.3 ± 130.8* | 9.0 ± 0.3 |
| CT | 221.9 ± 16.0 | 709.2 ± 62.7 | 1027.6 ± 140.2 | 9.4 ± 0.4 |
| CS | 250.9 ± 5.5 | 772.1 ± 50.8 | 1188.7 ± 194.0 | 9.6 ± 0.2 |
| Fetal brain E17 | ||||
| CD | 217.8 ± 20.9 | 603.6 ± 34.3 | 3399.2 ± 192.2* | 12.0 ± 0.3* |
| CT | 226.1 ± 18.3 | 617.2 ± 36.5 | 3938.4 ± 197.1 | 13.2 ± 0.3 |
| CS | 242.5 ± 21.5 | 609.3 ± 44.8 | 3999.1 ± 170.6 | 12.9 ± 0.4 |
Values are means ± SEM, n = 6–7 dams and 4–7 pups from at least 3 dams.
Different from control, P < 0.05 (ANOVA, Dunnett’s test).
Cho, choline; GPCho, glycerophosphocholine; PCho, phosphocholine; PtdCho, phosphatidylcholine.
Mitosis
We measured the accumulation of phospho-H3-labeled cells at the ventricular surface of the hippocampal vz, adjacent to the fimbria (Fi) (Fig. 1B, C), primordial dentate gyrus (DG) and Ammon’s horn (AH) (Fig. 1B, D). On day E17, a significantly lower proportion of the cells at the ventricular surface of the hippocampal vz were phospho-H3-labeled in the CD group compared with the CT or CS groups. When analyzed by single regions, the CD group had fewer phospho-H3-labeled cells in all areas (Fi, DG, AH) compared with the CT group; however, the CS group had more phospho-H3-labeled cells in one region (Fi) than the CT group (Fig. 1A).
FIGURE 1.
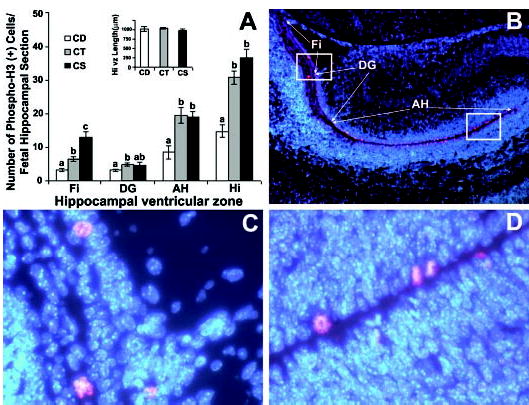
Maternal dietary choline deficiency in timed-pregnant mice fed choline-supplemented (CS), control (CT) or choline-deficient (CD) AIN-76 diet from embryonic day 12 to 17 (E12–17) decreases mitosis in embryonic mice on day E17 at the ventricular surface of the ventricular zone of the hippocampus. All mice were killed on E17 and coronal sections were prepared from the brains of fetuses from each group for the analysis of mitosis using the mitosis-specific marker anti-phospho-histone H3 as described in Materials and Methods. The DAPI nuclear DNA counterstaining is blue, whereas the Cy3 conjugated secondary antibody bound to the anti-phospho-histone H3 (Ser10) primary antibody stains red. Panel A: In CD fetal hippocampus compared with CT, there were fewer phospho-histone H3 positive cells at the ventricular surface of the ventricular zone adjacent to fimbria (Fi), dentate gyrus (DG) and Ammon’s Horn (AH), and this was reflected in the calculated values for the whole hippocampal section length of ventricular zone (Hi). Compared with the CT group, the CS group had a higher incidence of phospho-histone H3-labeled cells at the ventricular surface of the ventricular zone only in the fimbria. The graph insert shows the equivalence of the sections in terms of total hippocampal ventricular zone length. Values are means ± SEM of at least 6 pups per group from 6 dams. Means without a common letter differ, P < 0.05 (for each pair using Student’s t test, for all pairs using Tukey-Kramer HSD test, and comparison with control using Dunnett’s Method). Panel B shows a representative fetal hippocampus at a magnification of 50X with the regions of interest marked. Panels C and D are 400X magnifications of the boxed regions in panel B, and show representative labeled cells in the hippocampal regions.
Apoptosis
In the hippocampus on day E17, apoptotic cells were located mainly in the regions of developing fimbria and dentate gyrus (Fig. 2B). The number of apoptotic cells counted using morphological criteria (Fig. 2C), TUNEL or activated caspase-3 immunoreactivity (Fig. 2D) in the whole hippocampal area was more than 100% higher in the CD group than in the CT and CS groups (P < 0.01) (Fig. 2A).
FIGURE 2.
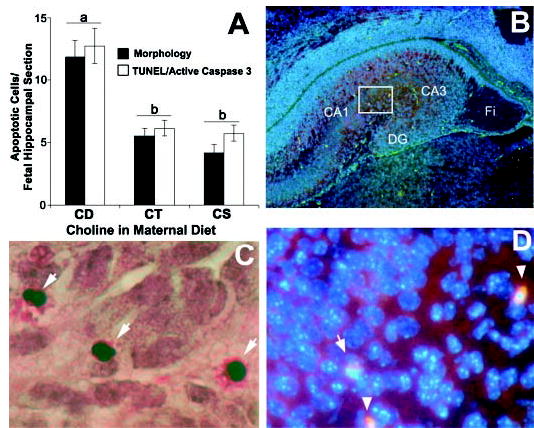
Maternal dietary choline deficiency in timed-pregnant mice fed choline-supplemented (CS), control (CT) or choline-deficient (CD) AIN-76 diet from embryonic day 12 to 17 (E12–17) increases apoptosis in day E17 mouse hippocampus. Coronal sections were prepared from the brains of day E17 fetuses from each diet group and were analyzed for apoptosis using a combination of classical apoptotic morphology, active caspase-3 immunoreactivity and TUNEL (terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin anti-digoxigenin fluorescein conjugate antibody nick end-labeling). Consecutive serial sections were used for TUNEL and active (cleaved) caspase-3 staining as described in Materials and Methods. Panel A: The graph shows apoptotic cell counts using morphological criteria (means ± SEM, n = 10–12 pups/group from at least 5 dams), and TUNEL-cleaved caspase-3 immunostaining (means ± SEM, n = 6 pups/group from 3 different dams). Means without common letters differ, P < 0.05 (for each pair using Student’s t test, for all pairs using Tukey-Kramer HSD test, and comparison with control using Dunnett’s Method). Panel B: A 50X magnification image of the right hippocampal hemisphere showing green TUNEL-positive cells distributed in the cortical plate (CA1, CA2,3), dentate gyrus (DG) and fimbria (Fi). Panel C: A representative image at 400X magnification is shown; the arrows point to cells showing apoptotic morphology. Panel D: Higher power view of the boxed area in panel B obtained by screening the fluorescent images for observing DAPI (blue, nuclear counterstaining), fluorescein isothiocyanate (green, positive TUNEL), and rhodamine (red, activated caspase-3); spherical TUNEL and activated caspase-3 positive nuclei are indicated by arrowheads. Normal nuclei stain blue.
DISCUSSION
Dietary choline intake by pregnant mice during d 12–17 of gestation influenced fetal brain development. On day E17, fewer hippocampal cells had phosphorylated histone-3 in CD compared with CT and CS brains (Fig. 1), consistent with decreased progenitor cell proliferation in the choline-deprived fetal hippocampus. CD more than doubled apoptosis rates in the day E17 hippocampus (Fig. 2). These results suggest that as in developing rat hippocampus, choline availability plays an important role in regulating different phases of the cell cycle and apoptosis in developing mouse hippocampus.
Previous studies in rats showed that maternal dietary choline supplementation increases the size of the cell body of cholinergic neurons (24) and decreases choline acetyltransferase activity in brain (25), whereas choline deficiency decreases cholinergic activity in the offspring’s brain (26,27). Neurogenesis and the timing of commitment to neuronal differentiation are thought to be essential for the acquisition of spatial learning, long-term memory and temporal processing (reviewed above). We suggest that the changes we describe in hippocampal cell proliferation and survival underlie these functional effects.
A complex family of proteins regulates cell division in neural progenitor cells. Members of the Ink and Kip families of CDKI slow progression through the cell cycle. We showed previously that feeding a CD diet increased, whereas CS decreased, the expression of p15Ink4b and p27Kip1 CDKI in the developing rat hippocampus (10). Increased expression of CDKI results in cell cycle arrest and decreased entry into the mitotic phase due to activation of G1 or G2 cell cycle checkpoint responses (28). Our observations in CD mice could be explained by increased expression of CDKI.
We described previously how choline availability modulates apoptosis signaling pathways in the hepatocytes, PC12 cell and cultured fetal hippocampal neuron (8,9,29,30). Apoptosis plays a critical role in determining the size of neuronal subpopulations, and thus, morphogenesis, in developing brain regions (31). Newly generated cells in the fetal hippocampus can reenter the cell cycle (i.e., remain as stem cells), commit to differentiation or undergo apoptosis. Previous studies in developing rat brain demonstrated that CD increased, whereas CS decreased, apoptosis in day E18–20 hippocampus (9). Here we show that apoptosis in the hippocampus of developing mice, like rats, is increased by maternal dietary choline deprivation.
It is interesting that maternal dietary choline deficiency did not alter choline concentrations but rather decreased PCho and PtdCho concentrations in the fetal mouse brain (Table 1). We reported previously that PCho is the storage form for choline in tissues and is the first indicator of diminished choline status in liver tissue (32). PtdCho is an important component of membranes, and changes in brain concentrations could be mediating changes in apoptosis and cell proliferation (29,33). Choline metabolism is closely interrelated to methyl group and folate metabolism (34), and changes in methylation of genes or histones also could be the mechanisms of action (35).
The demonstration of a choline effect on the development of the mouse brain is important because it makes possible new studies using new mouse models that have been created with genetic manipulations in enzymes of methyl group metabolism including methylenetetrahydrofolate reductase (36), methionine adenosyltransferase (37), cystathionine β-synthase (38) and phosphatidylethanolamine methyltransferase (39). We reported recently that methylenetetrahydrofolate reductase knockout mice and phosphatidylethanolamine methyltransferase knockout mice become choline deficient (40,41). These mouse models could be very helpful in unraveling how choline modulates neurogenesis in fetal brain. The studies we report here show that the mouse replicates many of the choline-mediated changes previously described in rats and suggest that use of transgenic mouse models would be appropriate in future investigations.
Footnotes
Presented in part at Neuroscience 2002, November 2002, Orlando, FL [Zeisel, S. H., Craciunescu, C. N., Mar, M.-H. & Albright, C. D. (2002) Maternal dietary choline availability alters the neurogenesis in fetal mouse brain hippocampus.].
Funded by grants from the National Institutes of Health (AG09525, DK55865). Support for this work was also provided by grants from the NIH to the UNC Clinical Nutrition Research Unit (DK56350) and Center for Environmental Health Susceptibility (ES10126).
Abbreviations used: AH, Ammon’s horn; CA1, CA2, CA3, hippocampal regions with numbered designations (Ammon’s horn); CD, choline-deficient diet; CDKI, cyclin-dependent kinase inhibitors; CS, choline-supplemented diet; CT, control diet; DAPI, 4′,6-diamidino-2-phenylindole, nuclear DNA staining; DG, hippocampal region of dentate gyrus; E, embryonic (gestation) day; Fi, fimbria; GPCho, glycerophosphocholine; LTP, long-term potentiation; p15Ink4B and p27Kip1, CDKI with appropriately named designations; PCho, phosphocholine; PtdCho, phosphatidylcholine; svz, subventricular zone of the developing hippocampus; TUNEL, terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-digoxigenin anti-digoxigenin fluorescein conjugate antibody nick end-labeling; vz, ventricular zone of the hippocampus.
References
- 1.Jones JP, Meck W, Williams CL, Wilson WA, Swartzwelder HS. Choline availability to the developing rat fetus alters adult hippocampal long-term potentiation. Brain Res Dev Brain Res. 1999;118:159–167. doi: 10.1016/s0165-3806(99)00103-0. [DOI] [PubMed] [Google Scholar]
- 2.Meck WH, Smith RA, Williams CL. Organizational changes in cholinergic activity and enhanced visuospatial memory as a function of choline administered prenatally or postnatally or both. Behav Neurosci. 1989;103:1234–1241. doi: 10.1037//0735-7044.103.6.1234. [DOI] [PubMed] [Google Scholar]
- 3.Meck W, Williams C. Perinatal choline supplementation increases the threshold for chunking in spatial memory. Neuroreport. 1997;8:3053–3059. doi: 10.1097/00001756-199709290-00010. [DOI] [PubMed] [Google Scholar]
- 4.Meck W, Williams C. Characterization of the facilitative effects of perinatal choline supplementation on timing and temporal memory. Neuroreport. 1997;8:2831–2835. doi: 10.1097/00001756-199709080-00005. [DOI] [PubMed] [Google Scholar]
- 5.Tees RC. The influences of rearing environment and neonatal choline dietary supplementation on spatial learning and memory in adult rats. Behav Brain Res. 1999;105:173–188. doi: 10.1016/s0166-4328(99)00074-1. [DOI] [PubMed] [Google Scholar]
- 6.Tees RC. The influences of sex, rearing environment, and neonatal choline dietary supplementation on spatial and nonspatial learning and memory in adult rats. Dev Psychobiol. 1999;35:328–342. doi: 10.1002/(sici)1098-2302(199912)35:4<328::aid-dev7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 7.Meck W, Williams C. Simultaneous temporal processing is sensitive to prenatal choline availability in mature and aged rats. Neuroreport. 1997;8:3045–3051. doi: 10.1097/00001756-199709290-00009. [DOI] [PubMed] [Google Scholar]
- 8.Albright CD, Tsai AY, Friedrich CB, Mar MH, Zeisel SH. Choline availability alters embryonic development of the hippocampus and septum in the rat. Brain Res Dev Brain Res. 1999;113:13–20. doi: 10.1016/s0165-3806(98)00183-7. [DOI] [PubMed] [Google Scholar]
- 9.Albright CD, Friedrich CB, Brown EC, Mar MH, Zeisel SH. Maternal dietary choline availability alters mitosis, apoptosis and the localization of TOAD-64 protein in the developing fetal rat septum. Brain Res Dev Brain Res. 1999;115:123–129. doi: 10.1016/s0165-3806(99)00057-7. [DOI] [PubMed] [Google Scholar]
- 10.Albright CD, Mar MH, Friedrich CB, Brown EC, Zeisel SH. Maternal choline availability alters the localization of p15Ink4B and p27Kip1 cyclin-dependent kinase inhibitors in the developing fetal rat brain hippocampus. Dev Neurosci. 2001;23:100–106. doi: 10.1159/000048701. [DOI] [PubMed] [Google Scholar]
- 11.Walkey CJ, Donohue LR, Bronson R, Agellon LB, Vance DE. Disruption of the murine gene encoding phosphatidylethanolamine N-methyltransferase. Proc Natl Acad Sci USA. 1997;94:12880–12885. doi: 10.1073/pnas.94.24.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J, Jr, Mar MH, Zeisel SH, Castro C, Garrow T, Rozen R. Homocysteine-betaine interactions in a murine model of 5, 10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–514. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 13.Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1969;166:257–261. doi: 10.1002/ar.1091660214. [DOI] [PubMed] [Google Scholar]
- 14.Caviness VS, Jr, Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- 15.American Institute of Nutrition. Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J Nutr. 1977;107:1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 16.Jacobowitz D, Abbott L. CRC Press; Boca Raton, FL: 1997. Chemoarchitectonic Atlas of the Developing Mouse Brain. [Google Scholar]
- 17.Altman J, Bayer SA. Mosaic organization of the hippocampal neuroepithelium and the multiple germinal sources of dentate granule cells. J Comp Neurol. 1990;301:325–342. doi: 10.1002/cne.903010302. [DOI] [PubMed] [Google Scholar]
- 18.Shibata K, Inagaki M, Ajiro K. Mitosis-specific histone H3 phosphorylation in vitro in nucleosome structures. Eur J Biochem. 1990;192:87–93. doi: 10.1111/j.1432-1033.1990.tb19199.x. [DOI] [PubMed] [Google Scholar]
- 19.Hans F, Dimitrov S. Histone H3 phosphorylation and cell division. Oncogene. 2001;20:3021–3027. doi: 10.1038/sj.onc.1204326. [DOI] [PubMed] [Google Scholar]
- 20.Baschong W, Suetterlin R, Laeng RH. Control of autofluorescence of archival formaldehyde-fixed, paraffin-embedded tissue in confocal laser scanning microscopy (CLSM) J Histochem Cytochem. 2001;49:1565–1572. doi: 10.1177/002215540104901210. [DOI] [PubMed] [Google Scholar]
- 21.Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW. Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem. 1999;47:229–236. doi: 10.1177/002215549904700211. [DOI] [PubMed] [Google Scholar]
- 22.Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- 23.Pomfret EA, daCosta K, Schurman LL, Zeisel SH. Measurement of choline and choline metabolite concentrations using high-pressure liquid chromatography and gas chromatography-mass spectrometry. Anal Biochem. 1989;180:85–90. doi: 10.1016/0003-2697(89)90091-2. [DOI] [PubMed] [Google Scholar]
- 24.Loy R, Heyer D, Williams CL, Meck WH. Choline-induced spatial memory facilitation correlates with altered distribution and morphology of septal neurons. Adv Exp Med Biol. 1991;295:373–382. doi: 10.1007/978-1-4757-0145-6_21. [DOI] [PubMed] [Google Scholar]
- 25.Cermak JM, Blusztajn JK, Meck WH, Williams CL, Fitzgerald CM, Rosene DL, Loy R. Prenatal availability of choline alters the development of acetylcholinesterase in the rat hippocampus. Dev Neurosci. 1999;21:94–104. doi: 10.1159/000017371. [DOI] [PubMed] [Google Scholar]
- 26.Holler T, Berse B, Cermak J, Diebler M, Blusztajn J. Differences in the developmental expression of the vesicular acetylcholine transporter and choline acetyltransferase in the rat brain. Neurosci Lett. 1996;212:107–110. doi: 10.1016/0304-3940(96)12808-1. [DOI] [PubMed] [Google Scholar]
- 27.Holler T, Cermak J, Blusztajn J. Dietary choline supplementation in pregnant rats increases hippocampal phospholipase D activity of the offspring. FASEB J. 1996;10:1653–1659. doi: 10.1096/fasebj.10.14.9002559. [DOI] [PubMed] [Google Scholar]
- 28.Leshem Y, Halevy O. Phosphorylation of pRb is required for HGF-induced muscle cell proliferation and is p27kip1-dependent. J Cell Physiol. 2002;191:173–182. doi: 10.1002/jcp.10089. [DOI] [PubMed] [Google Scholar]
- 29.Yen CL, Mar MH, Zeisel SH. Choline deficiency-induced apoptosis in PC12 cells is associated with diminished membrane phosphatidylcholine and sphingomyelin, accumulation of ceramide and diacylglycerol, and activation of a caspase. FASEB J. 1999;13:135–142. [PubMed] [Google Scholar]
- 30.Holmes-McNary M, Baldwin J, Zeisel SH. Opposing regulation of choline deficiency-induced apoptosis by p53 and NF-κB. J Biol Chem. 2001;276:41197–41204. doi: 10.1074/jbc.M010936200. [DOI] [PubMed] [Google Scholar]
- 31.Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. doi: 10.1016/s0166-2236(00)01581-2. [DOI] [PubMed] [Google Scholar]
- 32.Pomfret EA, da Costa K, Zeisel SH. Effects of choline deficiency and methotrexate treatment upon rat liver. J Nutr Biochem. 1990;1:533–541. doi: 10.1016/0955-2863(90)90039-n. [DOI] [PubMed] [Google Scholar]
- 33.Jackowski S. Coordination of membrane phospholipid synthesis with the cell cycle. J Biol Chem. 1994;269:3858–3867. [PubMed] [Google Scholar]
- 34.Zeisel SH. Choline: an essential nutrient for humans. Nutrition. 2000;16:669–671. doi: 10.1016/s0899-9007(00)00349-x. [DOI] [PubMed] [Google Scholar]
- 35.Niculescu MD, Zeisel SH. Diet, methyl donors and DNA methylation: interactions between dietary folate, methionine and choline. J Nutr. 2002;132:2333S–2335S. doi: 10.1093/jn/132.8.2333S. [DOI] [PubMed] [Google Scholar]
- 36.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 37.Lu SC, Alvarez L, Huang ZZ, Chen L, An W, Corrales FJ, Avila MA, Kanel G, Mato JM. Methionine adenosyltransferase 1A knockout mice are predisposed to liver injury and exhibit increased expression of genes involved in proliferation. Proc Natl Acad Sci USA. 2001;98:5560–5565. doi: 10.1073/pnas.091016398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Mali-now MR, Maeda N. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci USA. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Waite KA, Cabilio NR, Vance DE. Choline deficiency-induced liver damage is reversible in Pemt(−/−) mice. J Nutr. 2002;132:68–71. doi: 10.1093/jn/132.1.68. [DOI] [PubMed] [Google Scholar]
- 40.Schwahn BC, Chen Z, Laryea MD, Wendel U, Lussier-Cacan S, Genest J, Jr, Mar MH, Zeisel SH, Castro C, Garrow T, Rozen R. Homocysteine-betaine interactions in a murine model of 5, 10-methylenetetrahydrofolate reductase deficiency. FASEB J. 2003;17:512–514. doi: 10.1096/fj.02-0456fje. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Song J, Mar MH, Edwards LJ, Zeisel SH. Phosphatidylethanolamine N-methyltransferase (PEMT) knockout mice have hepatic steatosis and abnormal hepatic choline metabolite concentrations despite ingesting a recommended dietary intake of choline. Biochem J. 2003;370:987–993. doi: 10.1042/BJ20021523. [DOI] [PMC free article] [PubMed] [Google Scholar]


