To the Editor: Since low-frequency variants in the base-excision-repair gene MYH (MIM 604933) were first demonstrated to confer a recessive colorectal cancer (CRC) risk,1,2 there has been speculation of an additional dominant effect.3,4 In a recent article, Farrington et al.5 described the results of screening a series of 2,239 CRC cases and 1,845 controls for germline variants in the human homolog of the Escherichia coli muty gene (MYH). In whites, Y165C and G382D are the principal disease-causing variants of MYH. Among the cases, Farrington et al.5 detected 46 monoallelic carriers of these variants (14 Y165C heterozygotes and 32 G382D heterozygotes) and 11 biallelic carriers (8 G382D homozygotes and 3 compound heterozygotes), with corresponding frequencies among the controls of 28 monoallelic carriers and 0 biallelic carriers. They employed the method of Hugot et al.6 to estimate the genotype relative risk (GRR) associated with biallelic and monoallelic variant carriers. Their study confirmed the well-established increased risk of CRC in biallelic carriers of variants in MYH (GRR=92.65; 95% CI 41.60–213.20). Although they did not find a statistically significant increased risk for monoallelic variant carriers (GRR=1.35; 95% CI 0.92–2.07), the authors suggested that MYH heterozygosity might be associated with an elevated CRC risk—primarily in later life—after they arbitrarily restricted their analysis to cases of CRC diagnosed after age 55 years (GRR=1.68; 95% CI 1.07–2.95).
We believe that such a conclusion may be premature. First, the GRR calculation approach employed is not necessary for assessment of risk associated with heterozygote variant carriers. Moreover, other analytical approaches, including the standard asymptotic approach and exact approaches, yield 95% CIs that do not exclude an odds ratio (OR) of 1. Second, the type of stratification employed raises the issue of post hoc analysis. Third, we have similarly determined the frequencies of Y165C and G382D in a large case-control study and find no evidence that monoallelic MYH variant status influences CRC risk.
Our analysis was based on a series of 2,561 patients with histologically confirmed colorectal adenocarcinomas (1,474 males and 1,087 females; mean ± SD age at diagnosis 61 ± 11.4 years) ascertained through an ongoing initiative at the Institute of Cancer Research/Royal Marsden Hospital National Health Service Trust. We previously reported MYH results for a subset of 358 of these cases.4 A total of 2,695 control individuals (836 males and 1,859 females; mean ± SD age 59 ± 10.9 years) were the spouses of patients with malignancies, recruited as part of the National Cancer Research Network Trial (1999–2002), the Royal Marsden Hospital Trust/Institute of Cancer Research Family History and DNA Registry (1999–2004), the National Study of Colorectal Cancer Genetics Trial (2004), or the UK Study of Breast Cancer Genetics, all established within the United Kingdom. None of the controls had a personal history of malignancy at the time of ascertainment. All cases and controls were British whites, and there were no obvious differences in the demography of cases and controls in terms of place of residence within the United Kingdom. Blood samples were obtained with informed consent and ethics review board approval, in accordance with the tenets of the Declaration of Helsinki. Genotyping of Y165C and G382D was performed using customized Illumina Sentrix Bead Arrays in accordance with the manufacturer’s protocols. Assay validation was conducted using TaqMan and by direct sequencing of a subset of samples.
Among the patients with CRC, we identified 4 individuals with biallelic variants (1 G382D homozygote and 3 compound heterozygotes) and 53 with monoallelic variants (38 G382D heterozygotes and 15 Y165C heterozygotes). Among controls, no biallelic variants were identified, but 57 monoallelic variant carriers were identified (40 with G382D and 17 with Y165C). For each SNP, genotype distributions among controls did not deviate significantly from Hardy-Weinberg equilibrium (exact P=1.0). These frequencies of MYH variants are comparable to those documented in other populations—specifically those from the United Kingdom–based series reported by Farrington et al.5 (P=.17 for controls; P=.97 for cases) (table 1)—and translate to risks of 5.57 (95% CI 0.69–∞) and 0.98 (95% CI 0.66–1.46) associated with biallelic and monoallelic carrier status, respectively. Stratification of the data by 10-year age bands provided no evidence that risk associated with monoallelic carrier status was influenced by age (P=.13). Furthermore, after the data were partitioned, as by Farrington et al.,5 risks associated with early- and late-onset disease were comparable (for age at onset ⩽55 years, OR 0.83; 95% CI 0.42–1.51; for age at onset >55 years, OR 1.03; 95% CI 0.66–1.58).
Table 1. .
Summary of Published Case-Control Studies of the Relationship between MYH Variants Y165C and G382D and Risk of CRC
| Cases |
Controls |
||||||
| Study and Ethnicity |
Place of Study | n | No. (%) of Monoallelic Carriers | No. (%) of Biallelic Carriers | n | No. (%) of Monoallelic Carriers | No. (%) of Biallelic Carriers |
| Croitoru et al.3,a | Ontario | 1,238 | 32 (2.6) | 9 (.7) | 1,255 | 21 (1.7) | 0 (0) |
| Farrington et al.5,b | Scotland | 2,217 | 46 (2.1) | 11 (.5) | 1,822 | 28 (1.5) | 0 (0) |
| Enholm et al.8,c | Finland | 1,003 | 5 (.5) | 4 (.4) | 424 | 0 (0) | 0 (0) |
| Kambara et al.9,d | Brisbane, Australia | 92 | 2 (2.2) | 0 (0) | 53 | 1 (1.9) | 0 (0) |
| Wang et al.10,e | Minnesota | 444 | 10 (2.3) | 2 (.5) | 313 | 4 (1.3) | 0 (0) |
| Peterlongo et al.11,f: | |||||||
| All ethnicities | New York | 555 | 4 (.7) | 2 (.4) | 918 | 7 (.8) | 0 (0) |
| White | New York | 244 | 4 (1.6) | 2 (.8) | 366 | 6 (1.6) | 0 (0) |
| Jewish | New York | 266 | 0 (0) | 0 (0) | 450 | 0 (0) | 0 (0) |
| Zhou et al.12,g | Sweden | 438 | 6 (1.4) | 0 (0) | 469 | 3 (.6) | 0 (0) |
| Present study: | |||||||
| White | United Kingdom | 2,561 | 53 (2.1) | 4 (.2) | 2,695 | 57 (2.1) | 0 (0) |
Cases were aged 20–74 years; study included age- and sex-matched population controls.
Of the cases, 872 were <55 years old at diagnosis; study included age- and sex-matched population-based controls.
Study included unselected cases (mean ± SD age at diagnosis 67.2 ± 12.1 years) and blood-donor controls.
Mean ± SD age of cases was 69.1 ± 10.6 years; study included blood-donor controls.
Of the cases, 116 were <50 years old at diagnosis; controls were taken from among individuals undergoing screening colonoscopy with no evidence of adenomatous polyps.
Mean age of cases was 62.2 years; study included population-based controls matched by age (mean age 55.0 years), ethnicity, and religious group.
Study included sporadic CRC cases and blood-donor controls.
To further explore the possibility that monoallelic variant status might affect CRC, we applied a kin-cohort approach to compare risks in the 14,668 first-degree relatives of carriers and noncarriers. Data on history of any type of cancer, including age at diagnosis as well as vital status and current age or age at death, were collected for parents, siblings, and offspring by a previously validated questionnaire. Fourteen (4.3%) of the 324 relatives of variant carriers and 431 (3.0%) of the 14,344 relatives of noncarriers had received a diagnosis of CRC. Age-specific cumulative CRC distributions in first-degree relatives were estimated using a marginal-likelihood approach,7 and bootstrap estimates for the hazard ratios (HRs) were used to calculate 95% CIs. The HR generated from this analysis for CRC associated with monoallelic variant status was 1.74 (95% CI 0.62–3.60).
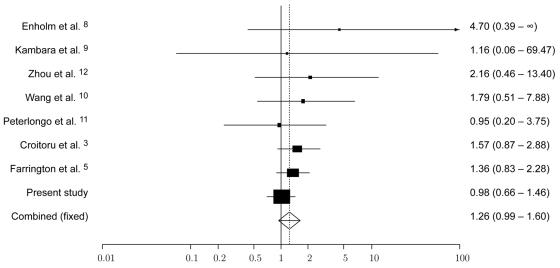
To date, seven published studies have reported the frequency of Y165C and G382D MYH variants in CRC cases and controls3,5,8–12 (table 1 and fig. 1). Collectively, these provide information on the frequency of MYH variants in 8,546 cases and 7,949 controls. To further quantify the risks associated with MYH status, we performed a pooled analysis of these published studies with our data. ORs were calculated for each study by use of exact logistic regression, since five of the studies contained <5 individuals in a single category. Meta-analysis was conducted using standard methods for combining estimates of ORs based on the weighted sum of the log estimates, with the inverse of the variance of the estimate as the weight. An exact conditional-likelihood approach13 was used to obtain a 95% CI for the pooled OR. There was no significant evidence of heterogeneity between studies (Cochran’s Q=3.74; P=.81); however, we used both fixed- and random-effects models to combine study results. Under the fixed-effects model, the pooled OR for monoallelic carrier status was 1.26 (95% CI 0.99–1.60), whereas, under the random-effects model, the pooled OR was 1.24 (95% CI 0.98–1.59). The risk associated with biallelic carrier status is not finite because there is no representation in controls, but an exact approach yields a lower 95% confidence bound of 7.39 for the risk estimate. Alternatively, a naive approach based on the convention of adding 0.5 to each empty cell generates a risk estimate of 6.06 (95% CI 2.02–18.19). Although this analysis provides robust evidence that carriers of biallelic MYH variants are at a significantly increased risk of CRC, the data do not indicate a statistically significant excess of MYH carriers among CRC cases compared with among controls. Our estimate of the risk associated with monoallelic carrier status is, in fact, likely to be inflated, since we restricted our analysis to the pathogenic MYH variants Y165C and G382D and since some individuals heterozygous for these variants may carry additional pathogenic variants. Hence, it is likely that additional, apparently heterozygous cases will, in reality, be compound heterozygotes. For example, in the studies by Croitoru et al.3 and Farrington et al.,5 some cases heterozygous for Y165C or G382D carried additional rare variants (three cases and one case, respectively). With our analysis adjusted for these observations, the pooled OR associated with monoallelic variant status is 1.23 (95% CI 0.96–1.58).
Figure 1. .
Funnel plot of OR of CRC risk associated with monoallelic Y165C and G382D MYH variants, under a fixed-effects model. Studies are plotted in order of decreasing variance of the log(OR). Horizontal lines represent 95% CIs. Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond and broken line represent the overall summary estimate, with the 95% CI given by the width of the diamond. The unbroken vertical line is at the null value (OR 1.0).
Our analysis of quantifying the CRC risk associated with carriers of monoallelic MYH variants illustrates an inherent problem in studying low-penetrance variants. By definition, such alleles are not associated with large risks. If the population frequency of an at-risk genotype is low (i.e., <2%), then exceptionally large studies are required to estimate precisely the risks. For example, to detect comprehensively the relative risk of 1.2 would require 22,000 cases and 22,000 controls. In conclusion, we believe that the assertion that monoallelic carrier status for MYH variants confers an elevated risk of CRC is unsupported on the basis of current data.
Web Resource
The URL for data presented herein is as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for MYH) [PubMed]
References
- 1.Al-Tassan N, Chmiel NH, Maynard J, Fleming N, Livingston AL, Williams GT, Hodges AK, Davies DR, David SS, Sampson JR, Cheadle JP (2002) Inherited variants of MYH associated with somatic G:C→T:A mutations in colorectal tumors. Nat Genet 30:227–232 10.1038/ng828 [DOI] [PubMed] [Google Scholar]
- 2.Sieber OM, Lipton L, Crabtree M, Heinimann K, Fidalgo P, Phillips RK, Bisgaard ML, Orntoft TF, Aaltonen LA, Hodgson SV, Thomas HJ, Tomlinson IP (2003) Multiple colorectal adenomas, classic adenomatous polyposis, and germ-line mutations in MYH. N Engl J Med 348:791–799 10.1056/NEJMoa025283 [DOI] [PubMed] [Google Scholar]
- 3.Croitoru ME, Cleary SP, Di Nicola N, Manno M, Selander T, Aronson M, Redston M, Cotterchio M, Knight J, Gryfe R, Gallinger S (2004) Association between biallelic and monoallelic germline MYH gene mutations and colorectal cancer risk. J Natl Cancer Inst 96:1631–1634 [DOI] [PubMed] [Google Scholar]
- 4.Fleischmann C, Peto J, Cheadle J, Shah B, Sampson J, Houlston RS (2004) Comprehensive analysis of the contribution of germline MYH variation to early-onset colorectal cancer. Int J Cancer 109:554–558 10.1002/ijc.20020 [DOI] [PubMed] [Google Scholar]
- 5.Farrington SM, Tenesa A, Barnetson R, Wiltshire A, Prendergast J, Porteous M, Campbell H, Dunlop MG (2005) Germline susceptibility to colorectal cancer due to base-excision repair gene defects. Am J Hum Genet 77:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G (2001) Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411:599–603 10.1038/35079107 [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee N, Wacholder S (2001) A marginal likelihood approach for estimating penetrance from kin-cohort designs. Biometrics 57:245–252 10.1111/j.0006-341X.2001.00245.x [DOI] [PubMed] [Google Scholar]
- 8.Enholm S, Hienonen T, Suomalainen A, Lipton L, Tomlinson I, Karja V, Eskelinen M, Mecklin JP, Karhu A, Jarvinen HJ, Aaltonen LA (2003) Proportion and phenotype of MYH-associated colorectal neoplasia in a population-based series of Finnish colorectal cancer patients. Am J Pathol 163:827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kambara T, Whitehall VL, Spring KJ, Barker MA, Arnold S, Wynter CV, Matsubara N, Tanaka N, Young JP, Leggett BA, Jass JR (2004) Role of inherited defects of MYH in the development of sporadic colorectal cancer. Genes Chromosomes Cancer 40:1–9 10.1002/gcc.20011 [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Baudhuin LM, Boardman LA, Steenblock KJ, Petersen GM, Halling KC, French AJ, Johnson RA, Burgart LJ, Rabe K, Lindor NM, Thibodeau SN (2004) MYH mutations in patients with attenuated and classic polyposis and with young-onset colorectal cancer without polyps. Gastroenterology 127:9–16 10.1053/j.gastro.2004.03.070 [DOI] [PubMed] [Google Scholar]
- 11.Peterlongo P, Mitra N, Chuai S, Kirchhoff T, Palmer C, Huang H, Nafa K, Offit K, Ellis NA (2005) Colorectal cancer risk in individuals with biallelic or monoallelic mutations of MYH. Int J Cancer 114:505–507 10.1002/ijc.20767 [DOI] [PubMed] [Google Scholar]
- 12.Zhou XL, Djureinovic T, Werelius B, Lindmark G, Sun XF, Lindblom A (2005) Germline mutations in the MYH gene in Swedish familial and sporadic colorectal cancer. Genet Test 9:147–151 10.1089/gte.2005.9.147 [DOI] [PubMed] [Google Scholar]
- 13.Martin DO, Austin H (2000) An exact method for meta-analysis of case-control and follow-up studies. Epidemiology 11:255–260 10.1097/00001648-200005000-00005 [DOI] [PubMed] [Google Scholar]