Abstract
Systemic lupus erythematosus is a prototypic autoimmune disease. Apart from rare monogenic deficiencies of complement factors, where lupuslike disease may occur in association with other autoimmune diseases or high susceptibility to bacterial infections, its etiology is multifactorial in nature. Cutaneous findings are a hallmark of the disease and manifest either alone or in association with internal-organ disease. We describe a novel genodermatosis characterized by painful bluish-red inflammatory papular or nodular lesions in acral locations such as fingers, toes, nose, cheeks, and ears. The lesions sometimes appear plaquelike and tend to ulcerate. Manifestation usually begins in early childhood and is precipitated by cold and wet exposure. Apart from arthralgias, there is no evidence for internal-organ disease or an increased susceptibility to infection. Histological findings include a deep inflammatory infiltrate with perivascular distribution and granular deposits of immunoglobulins and complement along the basement membrane. Some affected individuals show antinuclear antibodies or immune complex formation, whereas cryoglobulins or cold agglutinins are absent. Thus, the findings are consistent with chilblain lupus, a rare form of cutaneous lupus erythematosus. Investigation of a large German kindred with 18 affected members suggests a highly penetrant trait with autosomal dominant inheritance. By single-nucleotide-polymorphism–based genomewide linkage analysis, the locus was mapped to chromosome 3p. Haplotype analysis defined the locus to a 13.8-cM interval with a LOD score of 5.04. This is the first description of a monogenic form of cutaneous lupus erythematosus. Identification of the gene responsible for familial chilblain lupus may shed light on the pathogenesis of common forms of connective-tissue disease such as systemic lupus erythematosus.
Systemic lupus erythematosus is a complex autoimmune disease with a prevalence of 0.06% in the general population. Its etiology is multifactorial and is influenced by both genetic and environmental factors.1,2 Cutaneous findings are a hallmark of the disease and include butterfly rash, discoid lesions, oral ulcers, and alopecia.3 Moreover, 4 of the 11 diagnostic criteria for systemic lupus erythematosus comprise cutaneous findings.4 The formation of immune complexes consisting of autoantibodies against nuclear antigens is thought to be the principal cause of the inflammatory process leading to skin rashes, vasculitis, arthritis, and nephritis.4,5 To date, several susceptibility loci have been identified by both genomewide and association approaches.1,2 However, most of the genetic basis and the molecular pathogenesis of lupus erythematosus remains undefined.
Apart from autosomal recessively inherited deficiencies of complement factors, which play an important role in adaptive immunity, no monogenic form of lupus erythematosus has been identified so far. Thus, selective deficiency of C1q, C1r, C1s, C2, or C4a has been associated with multiple autoimmune diseases, including a lupuslike phenotype,6–9 whereas selective deficiency of C3 and C5 leads to high susceptibility to bacterial infections in addition to lupuslike phenotypes.10,11
In the present study, we describe a large, nonconsanguineous German family with 18 members over 5 generations affected with chilblain lupus, a rare cutaneous form of lupus erythematosus (fig. 1). Affected individuals presented with painful bluish-red papular or nodular lesions of the skin in acral locations—including the dorsal aspects of fingers and toes, heels, nose, cheeks, ears, and, in some cases, also knees—precipitated by cold and wet exposure at temperatures <10°C (fig. 2). Sometimes a plaquelike appearance was noted, and ulceration was commonly seen. Although deep ulceration led to necrotic destruction of the distal interphalangeal joint of the left fifth finger in the index patient at age 15 years, the lesions usually healed without scars, occasionally leaving atrophic skin and pigmentary changes. The onset of the skin lesions was in early childhood, and, in most patients, the lesions tended to improve during summer. Mucous membranes and nails were not affected, although subungual lesions were sometimes seen. There was no associated Raynaud phenomenon or photosensitivity. Apart from arthralgias affecting mainly large joints, such as knees and shoulders, there was no history of associated disease of any internal organ (including the CNS), immune deficiency, or malignancy. Serological data were available from seven affected individuals. There was no evidence for cryoglobulinemia, cryofibrinogenemia, hypergammaglobulinemia, irregular antibodies, cold agglutinins, viral or bacterial infection, rheumatic factor, or anticardiolipin antibodies (table 1). In two cases, antinuclear antibodies were found, although further differentiation did not show the presence of known nuclear autoantibodies (table 1). One affected child was found to have increased C3d-binding circulating immune complexes, and one affected woman showed decreased levels of C4 complement, indicating the formation of immune complexes (table 1).
Figure 1. .
Pedigree of the family with chilblain lupus. The arrow indicates the index patient. The asterisks (*) indicate family members included in the genomewide linkage analysis.
Figure 2. .
Cutaneous findings. A, Hands of proband (V1) at 6 years of age and his mother (IV2) at 30 years of age, showing multiple ulcerating nodular lesions over dorsal aspects of fingers and knuckles. B, Dorsal feet and left heel with purpuric appearance of individual IV2. C, Left ear of individual V11 at 3 years of age, with ulcerating lesion. D, Face and hands of individual V1 at 8 years of age. The erythematous plaquelike lesions over cheeks, nose, and chin resemble butterfly rash. E, Fingers showing fresh lesions, which appear as bluish-red, indurated, and tender papules.
Table 1. .
Phenotypic Data of Seven Affected Individuals
| Individual | Age at Onset (years) |
Distribution of Skin Lesions |
Additional Findings | Laboratory Findingsa | Histology Consistent with Lupusb |
| V1 | 1 | Fingers, toes, face, ears | Arthralgia (knees, shoulders), necrosis of distal left fifth finger | Unremarkable | Yes |
| V2 | 3 | Fingers, toes, heels, knees | None | Unremarkable | NA |
| IV2 | 2 | Fingers, toes, heels, knees | Arthralgia (knees, shoulders) | Decreased C4 complement | Yes |
| V12 | 3 | Fingers, toes, ears | None | C3d binding immune complexes | NA |
| V11 | 1 | Fingers, toes, face, ears | None | Unremarkable | NA |
| IV11 | 3 | Fingers, toes, heels | Arthralgia (knees) | ANA (1:300) speckled and homogenous pattern | Yes |
| III4 | 3 | Fingers, toes, ears | Arthralgia (knees, fingers, spine) | ANA (1:300) speckled pattern | NA |
Laboratory workup included complete blood count with differential, cryoglobulins, cryofibrinogen, cold agglutinins, autoantibodies (antinuclear antibodies [ANA], cardiolipin IgA/IgG/IgM, phosphatidylserin IgA/IgG/IgM, ssDNA, dsDNA, Ro/SSA, La/SSB, Sm, U1RNP, Scl-70, Jo), rheumatic factor, direct Coombs test, irregular antibodies, complement (C1q, C3c, C4), total hemolytic complement, circulating immune complexes (C1q and C3d binding), serum electrophoresis, signs of viral and bacterial infection (hepatitis A, B, and C, cytomegaly virus, borrelia burgdorferi, streptococci), liver transaminases, and urine analysis.
NA = not analyzed.
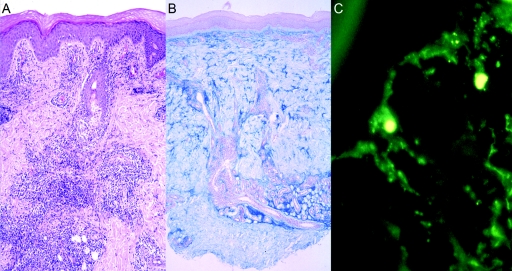
Three affected individuals underwent biopsy of skin lesions for histological analysis. Staining with hematoxylin-eosin, Alcian blue, and periodic acid Schiff (PAS) and direct immunofluorescence against IgG, IgM, IgA, and C3 were performed according to standard procedures. The histology showed characteristic findings of lupus erythematosus, whereas edematous changes or intraluminal fibrin—which are typically seen in true cold-induced perniosis or cryoglobulinemia, respectively—were absent (fig. 3). There was a lamellar orthohyperkeratosis within a regularly built epidermis. The junctional zone showed areas of hydropic degeneration of the stratum basale and occasional single-cell necrosis. A pronounced superficial and deep lymphocytic and histiocytic infiltration, with periadnexial and perivascular distribution along with interface dermatitis, could be seen. Throughout the stratum reticulare, there were increased mucin deposits (fig. 3). PAS staining revealed discrete broadening of the basement membrane without intraluminal fibrin deposits (data not shown). Direct immunofluorescence showed granular deposits of IgM and IgA immunoglobulins and C3 complement within the basement membrane zone (fig. 3). Thus, the findings in this family were consistent with the diagnosis of chilblain lupus, a rare cutaneous form of lupus erythematosus, which was first described by Hutchinson.12 The presence of histological and serological findings typically seen in lupus constitutes diagnostic criteria and differentiates chilblain lupus from true cold-induced perniosis or chilblains.13 The distinct morphology and distribution of the lesions, together with the absence of photosensitivity and autoantibodies against Ro/SSA and La/SSB, distinguish the phenotype of this family from subacute cutaneous lupus erythematosus. Chilblain lupus occurs predominantly in adult women and has only rarely been described in children.14,15 Apart from two affected brothers, no familial occurrence had been described at the time of this study.15 Progression to systemic lupus erythematosus has been reported in up to 18% of affected individuals, which is comparable to the progression rate of classic chronic cutaneous lupus erythematosus.14
Figure 3. .
Histology of lesional skin biopsy from proband V1. A, Hematoxylin-eosin staining (magnification 1:100) showing lamellar orthohyperkeratosis and regions of hydropic degeneration of the stratum basale as well as single-cell necrosis. Inflammatory infiltrates with perivascular and periadnexial distribution and interface dermatitis can be seen. B, Alcian blue staining (magnification 1:40) showing increased deposits of mucin throughout entire stratum reticulare. C, Direct immune fluorescence (magnification 1:100) showing broad granular deposits of C3 along basement membrane zone. A similar pattern was also seen with staining for IgM or IgA.
The vertical transmission of the disease in both males and females and the consistency of the phenotype suggested autosomal dominant inheritance with high penetrance. The phenotype of all family members was established through direct clinical examination or review of medical records. All participating individuals granted informed written consent before the study. To map the disease locus, whole-genome linkage analysis was performed on genomic DNA from peripheral blood leukocytes from 26 family members, including 14 affected individuals, by use of the Affymetrix GeneChip Human Mapping 10K Xba 142 2.0 Array. Genotyping of microsatellite markers was performed with fluorescently labeled primers on an ABI3100 Sequencer (Applied Biosystems). GRR and PedCheck were used to verify relationship and sex and to identify Mendelian errors.16,17 Nonparametric and parametric linkage analysis was done with Merlin, version 1.0.18 For parametric analysis, an autosomal dominant trait with a penetrance of 99%, without phenocopy, and a disease allele frequency of 0.001 were assumed. The disease locus was localized on chromosome 3p21–3p14, with a maximum LOD score of 5.04 (fig. 4). Nonparametric analysis confirmed this locus, with a Z-mean score of 6.2. A common haplotype was identified that was present in all affected and absent in all unaffected individuals. On the basis of critical recombination events in two affected individuals, the disease interval was defined to a 13.8-cM region delimited by markers rs704920 and d3s1300 (fig. 4). The mapped interval comprises 16.4 Mb and includes >100 known genes (Ensembl v38). By systematic mining of the Ensembl and GeneCards databases, the function and expression of all genes were investigated, and candidate genes were selected for sequencing. All coding exons, including flanking intronic regions, were sequenced in two affected individuals and two unrelated, healthy control individuals on an ABI3100 sequencer, according to standard procedures. Priority was given to genes that had been implicated in the pathogenesis of lupus erythematosus or other autoimmune diseases, such as the genes encoding toll-like receptor 9 (TLR9 [MIM *605474]),19,20 deoxyribonuclease I-like 3 (DNASE1L3 [MIM *602244]),5,21 ubiquitin-activating enzyme E1-like (UBE1L [MIM *191325]),22 protein kinase C delta (PRKCD [MIM *176977]),23,24 interleukin 17 receptors B and D (IL17RB [MIM *605458] and IL17RD [MIM *606807]),25 and the chemokine receptor cluster (CCR9 [MIM *604738], CXCR6 [MIM *605163], XCR1 [MIM *600552], CCR1 [MIM *601159], CCR2 [MIM *601267], CCR3 [MIM *601268], CCR5 [MIM *601373], and CCRL2 [MIM *608379]).26,27 We also investigated the collagen VII alpha-1 gene (COL7A1 [MIM *120120]), since it is highly expressed in the skin and known to cause autosomal dominant bullous epidermolysis dystrophica (MIM *131750), although blistering is not a feature of chilblain lupus. A complete list of genes analyzed for mutations so far is given in table 2. Several known and novel SNPs were identified, but no pathogenic mutation was observed within the regions analyzed. All novel coding SNPs were interrogated for segregation with the trait and their allele frequencies estimated by genotyping 100 unrelated healthy controls (data not shown).
Figure 4. .
Graphic summary of linkage analysis. A, Parametric linkage results identifying a single locus on chromosome 3p. B, Relative position of markers and refined physical interval for chilblain lupus, on the basis of haplotype analysis and genotyping of additional microsatellite markers. Key recombinant markers are marked in bold. AGS1 = Aicardi-Goutières syndrome, type 1.
Table 2. .
List of Genes Screened for Mutations by Comparative Sequencing
| Gene Name | Gene Symbol | Accession Numbera |
| Toll-like receptor 9 | TLR9 | MIM *605474 |
| Deoxyribonuclease I-like 3 | DNASE1L3 | MIM *602244 |
| Ubiquitin-activating enzyme E1-like | UBE1L | MIM *191325 |
| Protein kinase C delta | PRKCD | MIM *176977 |
| Interleukin 17 receptor B | IL17RB | MIM *605458 |
| Interleukin 17 receptor D | IL17RD | MIM *606807 |
| Chemokine, CC motif, receptor 9 | CCR9 | MIM *604738 |
| Chemokine, CXC motif, receptor 6 | CXCR6 | MIM *605163 |
| Chemokine, C motif, receptor 1 | XCR1 | MIM *600552 |
| Chemokine, CC motif, receptor 1 | CCR1 | MIM *601159 |
| Chemokine, CC motif, receptor 2 | CCR2 | MIM *601267 |
| Chemokine, CC motif, receptor 5 | CCR3 | MIM *601268 |
| Chemokine, CC motif, receptor 5 | CCR5 | MIM *601373 |
| Chemokine, CC motif, receptor-like protein 2 | CCRL2 | MIM *608379 |
| Collagen VII alpha-1 | COL7A1 | MIM *120120 |
| Chemokine-binding protein 2 | CCBP2 | MIM *602848 |
| Cytokine-inducible SH2-containing protein | CISH | MIM *602441 |
| C-type lectin domain family 3, member B | CLEC3B | NM_003278 |
| Decapping enzyme 1 | DCP1A | MIM *607010 |
| Family with sequence similarity 3, member 3 | FAM3D | MIM *608619 |
| Hyaluronidase 2 | HYAL2 | MIM *603551 |
| Interferon-related developmental regulator 2 | IFRD2 | MIM *602725 |
| Inositol hexaphosphate kinase 1 | IHPK1 | MIM *606991 |
| Inositol hexaphosphate kinase 2 | IHPK2 | MIM *606992 |
| Leucine-rich repeat-containing protein 2 | LRRC2 | MIM *607180 |
| Leucine zipper transcription factor-like 1 | LZTFL1 | MIM *606568 |
| Macrophage stimulating 1 | MST1 | MIM *142408 |
| Macrophage stimulating 1 receptor | MST1R | MIM *600168 |
| Natural killer tumor recognition sequence | NKTR | MIM *161565 |
| Protein phosphatase, 1M | PP2CE | MIM *608979 |
| Ring finger protein 123 | RNF123 | NM_022064 |
| Stabilin 1 | STAB1 | MIM *608560 |
| TRAF-interacting protein | TRIP | MIM *605958 |
| Ubiquitin-specific protease 4 | USP4 | MIM *603486 |
For genes not listed in the OMIM database, a representative RefSeq accession number is listed.
Interestingly, the locus mapped for chilblain lupus overlaps with a locus for Aicardi-Goutières syndrome 1 (AGS1 [MIM *225750]), a progressive encephalopathy, which mimicks congenital viral infection.28 Aicardi-Goutières syndrome is identical to pseudo-TORCH syndrome (MIM *251290) and Cree encephalitis (MIM *608505)29 and has been shown to be genetically heterogeneous. Thus, in addition to the locus on chromosome 3p, there is evidence for a second locus on chromosome 13q.30 Children affected with Aicardi-Goutières syndrome suffer from progressive microcephaly and severe cerebral dysfunction associated with calcification of basal ganglia, chronic lymphocytosis, and elevated interferon alpha in the spinal fluid.28,31 Most patients die in early childhood. Some patients have chilblainlike lesions that resemble those found in the family studied here, although unaffected parents appear not to show any cutaneous findings.31,32 Moreover, Aicardi-Goutières syndrome has been suggested to be a form of systemic lupus erythematosus, because of the findings of hypocomplementemia and antinuclear autoantibodies in addition to lupuslike skin lesions in some patients.33–36 Therefore, it is possible that chilblain lupus and Aicardi-Goutières syndrome are allelic phenotypes representing different spectrums of the same disease, although the exact molecular mechanisms and the different modes of inheritance remain to be clarified.
Systemic lupus erythematosus is a complex polygenic disease and is considered a prototypic autoimmune disease. The observation that the locus identified in this study does not localize within any hitherto mapped susceptibility locus for systemic lupus erythematosus does not preclude it from playing a major role,1,2 since previous studies of complex diseases have demonstrated that genomewide and association approaches may lead to completely different results. Thus, further investigation of the potential significance of this locus for common forms of lupus erythematosus is warranted.
The clinical spectrum of systemic lupus erythematosus is very broad, encompassing exclusive internal-organ disease, exclusive cutaneous findings, and both internal and cutaneous findings. It is likely that different genetic causes determine at which end of the phenotypic spectrum a susceptible individual will manifest the disease. In contrast with other monogenic diseases that have lupuslike features, the phenotype presented here displays only features seen in sporadic cutaneous lupus erythematosus, suggesting that the underlying genetic cause also contributes to genetic susceptibility to common forms of cutaneous lupus erythematosus. With all observations taken together, we have described a novel genodermatosis termed “familial chilblain lupus” and have mapped its genetic locus to chromosome 3p. Identification of the responsible gene may shed light on the pathogenesis of common forms of connective-tissue disease, such as systemic lupus erythematosus.
Acknowledgments
The authors thank the members of the family for their participation in this study. We are grateful for the excellent technical assistance of Kerstin Engel and Lydia Senenko. This work was supported in part by a Marie Curie Development Host Fellowship (HPMD-CT-2000-00034, to M.L.K.); grants by the Medizinische Fakultät, TU Dresden (MeDDrive 2004 and 2005, to M.L. and M.L.K.); and a grant-in-aid from the National Genome Research Network of the German Ministry of Science and Education (BMBF) (NGFN2, to N.H.).
Web Resources
The URLs for data presented herein are as follows:
- ENSEMBL genome browser, http://www.ensembl.org/homo_sapiens/index.html
- GeneCards, http://www.genecards.org/index.shtml
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=OMIM
References
- 1.Alarcon-Riquelme ME (2005) The genetics of systemic lupus erythematosus. J Autoimmun Suppl 25:46–48 10.1016/j.jaut.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 2.Cunninghame Graham DS, Vyse TJ (2004) The candidate gene approach: have murine models informed the study of human SLE? Clin Exp Immunol 137:1–7 10.1111/j.1365-2249.2004.02525.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sontheimer RD (1997) The lexicon of cutaneous lupus erythematosus—a review and personal perspective on the nomenclature and classification of the cutaneous manifestations of lupus erythematosus. Lupus 6:84–95 [DOI] [PubMed] [Google Scholar]
- 4.Mills JA (1994) Systemic lupus erythematosus. N Engl J Med 330:1871–1879 10.1056/NEJM199406303302608 [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ (2000) Lupus, DNase and defective disposal of cellular debris. Nat Genet 25:135–136 10.1038/75963 [DOI] [PubMed] [Google Scholar]
- 6.Lee SL, Wallace SL, Barone R, Blum L, Chase PH (1978) Familial deficiency of two subunits of the first component of complement: C1r and C1s associated with a lupus erythematosus–like disease. Arthritis Rheum 21:958–967 [DOI] [PubMed] [Google Scholar]
- 7.Dragon-Durey MA, Quartier P, Fremeaux-Bacchi V, Blouin J, de Barace C, Prieur AM, Weiss L, Fridman WH (2001) Molecular basis of a selective C1s deficiency associated with early onset multiple autoimmune diseases. J Immunol 166:7612–7616 [DOI] [PubMed] [Google Scholar]
- 8.Hannema AJ, Kluin-Nelemans JC, Hack CE, Eerenberg-Belmer AJ, Mallee C, van Helden HP (1984) SLE like syndrome and functional deficiency of C1q in members of a large family. Clin Exp Immunol 55:106–114 [PMC free article] [PubMed] [Google Scholar]
- 9.Provost TT, Arnett FC, Reichlin M (1983) Homozygous C2 deficiency, lupus erythematosus, and anti-Ro (SSA) antibodies. Arthritis Rheum 26:1279–1282 [DOI] [PubMed] [Google Scholar]
- 10.Alper CA, Bloch KJ, Rosen FS (1973) Increased susceptibility to infection in a patient with type II essential hypercatabolism of C3. N Engl J Med 288:601–606 [DOI] [PubMed] [Google Scholar]
- 11.Snyderman R, Durack DT, McCarty GA, Ward FE, Meadows L (1979) Deficiency of the fifth component of complement in human subjects: clinical, genetic and immunologic studies in a large kindred. Am J Med 67:638–645 10.1016/0002-9343(79)90247-X [DOI] [PubMed] [Google Scholar]
- 12.Hutchinson J (1888) Harveian lectures on lupus, lecture III: on the various forms of lupus vulgaris and erythematosus. BMJ 1:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franceschini F, Calzavara-Pinton P, Quinzanini M, Cavazzana I, Bettoni L, Zane C, Facchetti F, Airo P, McCauliffe DP, Cattaneo R (1999) Chilblain lupus erythematosus is associated with antibodies to SSA/Ro. Lupus 8:215–219 10.1191/096120399678847632 [DOI] [PubMed] [Google Scholar]
- 14.Millard LG, Rowell NR (1978) Chilblain lupus erythematosus (Hutchinson): a clinical and laboratory study of 17 patients. Br J Dermatol 98:497–506 10.1111/j.1365-2133.1978.tb01935.x [DOI] [PubMed] [Google Scholar]
- 15.Weston WL, Morelli JG (2000) Childhood pernio and cryoproteins. Pediatr Dermatol 17:97–99 10.1046/j.1525-1470.2000.01722.x [DOI] [PubMed] [Google Scholar]
- 16.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2001) GRR: graphical representation of relationship errors. Bioinformatics 17:742–743 10.1093/bioinformatics/17.8.742 [DOI] [PubMed] [Google Scholar]
- 17.O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abecasis GR, Cherny SS, Cookson WO, Cardon LR (2002) Merlin—rapid analysis of dense genetic maps using sparse gene flow trees. Nat Genet 30:97–101 10.1038/ng786 [DOI] [PubMed] [Google Scholar]
- 19.Ng MW, Lau CS, Chan TM, Wong WH, Lau YL (2005) Polymorphisms of the toll-like receptor 9 (TLR9) gene with systemic lupus erythematosus in Chinese. Rheumatology (Oxford) 44:1456–1457 [DOI] [PubMed] [Google Scholar]
- 20.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, Duramad O, Coffman RL (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202:1131–1139 10.1084/jem.20050914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilber A, O’Connor TP, Lu ML, Karimi A, Schneider MC (2003) Dnase1l3 deficiency in lupus-prone MRL and NZB/W F1 mice. Clin Exp Immunol 134:46–52 10.1046/j.1365-2249.2003.02267.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, Roberts IS, Copley RR, Bell JI, Cornall RJ, Goodnow CC (2005) A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 435:452–458 10.1038/nature03555 [DOI] [PubMed] [Google Scholar]
- 23.Mecklenbrauker I, Saijo K, Zheng NY, Leitges M, Tarakhovsky A (2002) Protein kinase Cδ controls self-antigen-induced B-cell tolerance. Nature 416:860–865 10.1038/416860a [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI (2002) Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature 416:865–869 10.1038/416865a [DOI] [PubMed] [Google Scholar]
- 25.Kurasawa K, Hirose K, Sano H, Endo H, Shinkai H, Nawata Y, Takabayashi K, Iwamoto I (2000) Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum 43:2455–2463 [DOI] [PubMed] [Google Scholar]
- 26.Amoura Z, Combadiere C, Faure S, Parizot C, Miyara M, Raphael D, Ghillani P, Debre P, Piette JC, Gorochov G (2003) Roles of CCR2 and CXCR3 in the T cell–mediated response occurring during lupus flares. Arthritis Rheum 48:3487–3496 10.1002/art.11350 [DOI] [PubMed] [Google Scholar]
- 27.Eriksson C, Eneslatt K, Ivanoff J, Rantapaa-Dahlqvist S, Sundqvist KG (2003) Abnormal expression of chemokine receptors on T-cells from patients with systemic lupus erythematosus. Lupus 12:766–774 10.1191/0961203303lu467oa [DOI] [PubMed] [Google Scholar]
- 28.Aicardi J, Goutieres F (1984) A progressive familial encephalopathy in infancy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis. Ann Neurol 15:49–54 10.1002/ana.410150109 [DOI] [PubMed] [Google Scholar]
- 29.Crow YJ, Black DN, Ali M, Bond J, Jackson AP, Lefson M, Michaud J, Roberts E, Stephenson JB, Woods CG, Lebon P (2003) Cree encephalitis is allelic with Aicardi-Goutieres syndrome: implications for the pathogenesis of disorders of interferon alpha metabolism. J Med Genet 40:183–187 10.1136/jmg.40.3.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali M, Highet LJ, Lacombe D, Goizet C, King MD, Tacke U, van der Knaap MS, Lagae L, Rittey C, Brunner HG, van Bokhoven H, Hamel B, Oade YA, Sanchis A, Desguerre I, Cau D, Mathieu N, Moutard ML, Lebon P, Kumar D, Jackson AP, Crow YJ (2006) A second locus for Aicardi-Goutieres syndrome at chromosome 13q14–21. J Med Genet 43:444–450 10.1136/jmg.2005.031880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goutieres F, Aicardi J, Barth PG, Lebon P (1998) Aicardi-Goutieres syndrome: an update and results of interferon-α studies. Ann Neurol 44:900–907 10.1002/ana.410440608 [DOI] [PubMed] [Google Scholar]
- 32.Tolmie JL, Shillito P, Hughes-Benzie R, Stephenson JB (1995) The Aicardi-Goutieres syndrome (familial, early onset encephalopathy with calcifications of the basal ganglia and chronic cerebrospinal fluid lymphocytosis). J Med Genet 32:881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aicardi J, Goutières F (2000) Systemic lupus erythematosus or Aicardi-Goutières syndrome? Neuropediatrics 31:444–450 [DOI] [PubMed] [Google Scholar]
- 34.De Laet C, Goyens P, Christophe C, Ferster A, Mascart F, Dan B (2005) Phenotypic overlap between infantile systemic lupus erythematosus and Aicardi-Goutieres syndrome. Neuropediatrics 36:399–402 10.1055/s-2005-873058 [DOI] [PubMed] [Google Scholar]
- 35.Dale RC, Tang SP, Heckmatt JZ, Tatnall FM (2000) Familial systemic lupus erythematosus and congenital infection-like syndrome. Neuropediatrics 31:155–158 10.1055/s-2000-7492 [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen M, Skullerud K, Bakke SJ, Lebon P, Jahnsen FL (2005) Cerebral thrombotic microangiopathy and antiphospholipid antibodies in Aicardi-Goutieres syndrome—report of two sisters. Neuropediatrics 36:40–44 10.1055/s-2004-830532 [DOI] [PubMed] [Google Scholar]