Abstract
Pulmonary alveolar microlithiasis (PAM) is a rare disease characterized by the deposition of calcium phosphate microliths throughout the lungs. We first identified a PAM locus by homozygosity mapping to 4p15, then identified, by a candidate-gene approach, the gene responsible for the disease as SLC34A2 (the type IIb sodium-phosphate cotransporter gene), which is involved in phosphate homeostasis in several organs. We identified six homozygous exonic mutations in the seven unrelated patients with PAM we studied. Three of the mutations were frameshifts, one was a chain termination, one was an amino acid substitution, and one was a deletion spanning the minimal promoter and the first exon. Absence of functional protein product of the gene is compatible with calcium phosphate deposition in alveolar airspaces. We show that impaired activity of the phosphate transporter is presumably responsible for the microliths and that PAM is a recessive monogenic disease with full penetrance. Testicular microlithiasis (TM) is a disease that is more common than PAM. It is often associated with cancer and infertility. Since the gene we identified is also expressed in testis, we searched for mutations in subjects with TM. In 2 of the 15 subjects with TM we studied, we identified two rare variants, one synonymous and the other noncoding, that are possibly associated with the condition.
Pulmonary alveolar microlithiasis (PAM [MIM 265100]) is a rare disease characterized by the deposition of calcium phosphate microliths throughout the lungs. Most patients are asymptomatic for several years or even for decades, and, generally, the diagnosis is incidental to clinical investigations unrelated to PAM. Cases with early onset or rapid progression are rare. A “sandstorm-appearing” chest roentgenogram is a typical diagnostic finding. The onset of this potentially lethal disease varies from the neonatal period to old age, and the disease follows a long-term, progressive course, resulting in a slow deterioration of lung functions. A variety of environmental factors have been suggested as the etiology, and about one-third of the reported cases are familial (reviewed by Ucan et al.,1 Castellana et al.,2 and Mariotta et al.3). A report on six related, affected individuals was the best support for autosomal recessive inheritance of the disease.4 Testicular microlithiasis (TM), in contrast, is not rare; it has a prevalence of 0.6%–9% in the population.5 It was found to be associated with the majority of primary testicular malignancies6 and with ∼1% of male idiopathic infertility cases.7 We identified the gene responsible for PAM by positional cloning and subsequent candidate-gene approach. Here, we describe (1) the localization of the gene in the family with six affected individuals mentioned above and (2) the screening of SLC34A2 (the type IIb sodium-phosphate cotransporter gene) for mutations in patients with PAM and subjects with TM.
Material and Methods
Subjects
A large consanguineous family4 was used for linkage analysis. A total of 7 unrelated patients with PAM and 15 men with diffuse bilateral TM were included in the SLC34A2 mutation screening. Informed written consent was obtained from all subjects or their parents. The study was approved by the Committee on Research with Human Participants at Boğaziçi University.
Linkage Analysis
A genome scan of three brothers with PAM in family 1 (individuals 501, 503, and 504 in the pedigree shown in fig. 1) was performed using the CHLC/Weber Human screening set version 8a. The set contained 156 polymorphic microsatellite markers that spanned the human autosomes with an average density of 1 per 25 cM. The positions of the markers were retrieved from the STS map of GenBank (NCBI Map Viewer and UCSC Genome Browser). Those loci exhibiting shared homozygosity were further analyzed with more densely spaced markers in the family members available for study. Marker alleles were resolved on 8% denaturing polyacrylamide gels and were visualized by staining with silver nitrate.8 Linkage analysis was performed under the assumption of autosomal recessive inheritance, full penetrance, a disease gene frequency of 1 in 100,000, consanguinity, equal recombination frequencies in both sexes, and equal frequencies of marker alleles. PedCheck version 1.1 was used to detect any Mendelian or genotyping errors in the linkage data.9 SimWalk2 version 2.91 was used for the calculation of multipoint LOD scores and for the construction of haplotypes, with allowance for the minimum number of recombination events.10
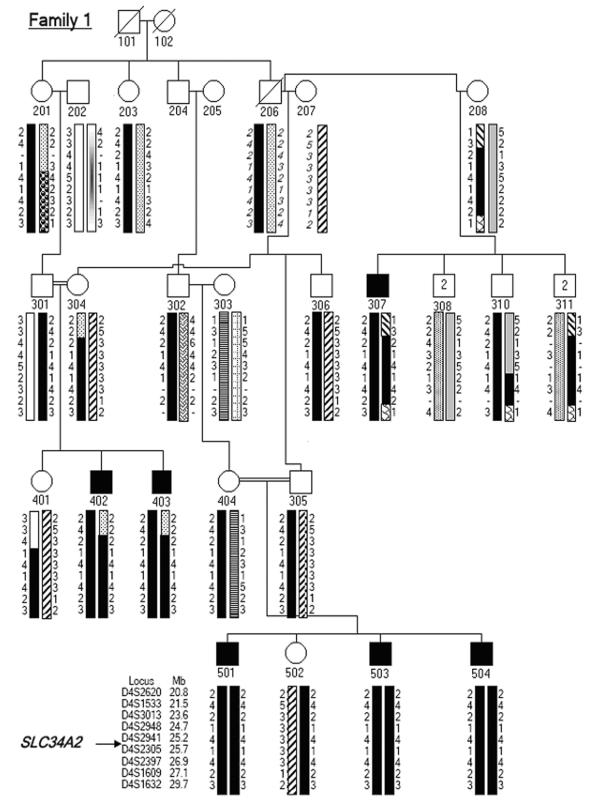
Figure 1. .
Partial pedigree diagram and haplotype analysis at 4p15.31-p15.1 for family 1. Haplotypes are shown by differently shaded bars. Deduced alleles are shown in italics.
Mutation Analysis
All 12 coding exons of SLC34A2 and the flanking intronic sequences were amplified with intronic primers (table 1) designed using Primer3 software. Exon 13 was amplified as three overlapping fragments: 13a, 13b, and 13c. Each fragment was amplified in a 25-μl volume containing 125 ng of genomic DNA, PCR buffer with 2 mM MgCl2, deoxynucleoside triphosphates (125 mM of each), Taq DNA polymerase (0.8 U/reaction), and 0.4 mM of the primer pair under the following cycle conditions: an initial denaturation step at 95°C for 5 min; followed by 35 cycles for 30 s at 94°C, 30 s of annealing at the appropriate temperature (given in table 1), and 2 min of elongation at 72°C; and a final extension step for 10 min at 72°C. The noncoding exon 1 was amplified for the family’s patients and parents only. Because of the GC-rich nature of the region, a GC-Rich PCR System (Roche) containing 0.5-M GC-rich resolution buffer and GC-rich enzyme mix (2 U/reaction) were used. The 462-bp product was amplified using the 522-bp product as a template (table 1). PCR amplification for the 522-bp product was performed using the protocol stated by the manufacturer, whereas the cycling conditions for the 462-bp product were as follows: an initial denaturation step at 95°C for 5 min; followed by 35 cycles for 30 s at 94°C, 30 s of annealing at 55°C for 5 cycles and 52°C for the remaining 30 cycles, and 50 s of elongation at 72°C; and a final extension step for 10 min at 72°C.
Table 1. .
Primers for the Amplification of SLC34A2 Exons
| Primer Sequence(5′ → 3′) |
||||
| Exon | Forward | Reverse | Length (bp) |
Annealing Temperature (°C) |
| 1: | ||||
| Reaction 1 | GTTCTTGAGGAGAAGGCAGG | CCAGCCAAGTTCCTTGAAGT | 522 | 57.0 (10 cycles); 54.0 (25 cycles) |
| Reaction 2 | GTTCTTGAGGAGAAGGCAGG | TCCTTGAAAGAGCGAGGAAG | 462 | 55.0 (5 cycles); 52.0 (30 cycles) |
| 2a | AGGGGAACCACAGAGGAAAT | CTTTTATCAGGGGCAGTGGG | 359 | 59.0 |
| 3a | AGCCCAACCCCGATAAGTA | ACCACAGGAAGTTTCCCTCC | 300 | 61.5 |
| 4 | TTGCCAAACTTCTCAGGGTT | CCTAAAACACTCCAGGAGGC | 319 | 61.5 |
| 5 | GCCTTGGATGGAGACTTCTG | AAACACATTGTAGCTGGGGC | 383 | 61.5 |
| 6 | GGTAACTTTAGCCTGCCTCC | CGTGGCCAAGAACAATAGAG | 213 | 60.0 |
| 7 | ACTAATCTGGCTGTCGGGGT | GGGATGTTGTGGGTAGGAAG | 284 | 63.5 |
| 8 | CCCTGGGTTTGTGTCCTAAA | GATTTTTCAGGGACTCCCAA | 211 | 60.0 |
| 9 | AGTGTTGTGGGCATTTGTCA | CGGATAAATAGGTCACCCCC | 219 | 58.0 |
| 10 | CATGCCCTCCTGACAAGATT | GTGAGGCCTACAAGTGAGGG | 236 | 59.0 |
| 11 | GAGGCCATGACATCTCTTCC | GGATGACAGGAAATGGCAGTG | 204 | 61.0 |
| 12 | ACTGCCATTTCCTGTCATCC | AGTTTGCAAGACCATGGGTG | 246 | 55.0 |
| 13a | TCCAACCTCTTGTGTTGCAG | AGTCGGAGGCACAGTACCAG | 283 | 61.5 |
| 13b | GTTCCCGTCGTCTTCATCAT | CTCCTCCAAGTCCTCGCA | 273 | 61.5 |
| 13c | CTGTGTGACTGCCCCAAGT | ATCTCCTCGAGGTGGTGAAA | 277 | 57.0 |
These primer pairs were designed to produce overlapping fragments that contained exons 2 and 3.
The amplified fragments were subjected to SSCP, and any fragment exhibiting an aberrant pattern was further investigated with sequence analysis. SSCP analysis was performed on 8% polyacrylamide gels with crosslinking ratios of 2%, both with glycerol (10%) and without glycerol. Sequencing was performed with an ABI 310 analyzer (Applied Biosystems) at the Department of Molecular Biology and Genetics, Boğaziçi University.
Results
Linkage Analysis
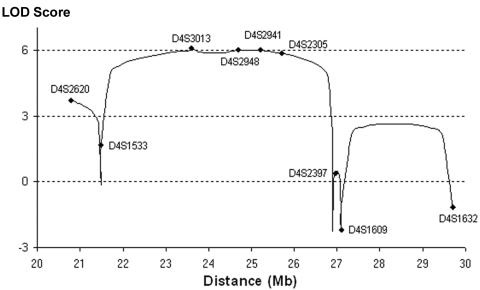
The genome scan of the three affected brothers in the large consanguineous family and the subsequent genotyping at homozygous loci with additional markers in all family members available for study pointed to a single candidate locus at chromosome 4p15. We narrowed the gene locus to a 4.2-Mbp region at 4p15.31-15.2, flanked by markers D4S1533 and D4S2305. The haplotype data are given in figure 1. LOD scores were calculated to assess the significance of the results. The multipoint LOD score peaked to 6.0 between D4S3013 and D4S2305 (fig. 2).
Figure 2. .
Multipoint linkage analysis at 4p15.31-p15.1 for family 1. The microsatellite markers used in this study are plotted on the graph.
Mutation Analysis
The gene locus contained 16 genes, according to National Center for Biotechnology Information (NCBI) build 35.1. SLC34A2 stood out as the likely disease gene, since it was a phosphate transporter (reviewed by Murer et al.11) expressed strongly in lung.12,13 We analyzed all 12 coding exons in the patients with PAM by SSCP and performed subsequent DNA sequence analysis for samples displaying aberrant patterns. A total of five homozygous mutations in the six unrelated patients were identified (table 2), but no mutation was detected in the family. All mutations were predicted to result in loss of function of the protein product of the gene. Mutations c.114delA and c.1328delT resulted in the shift of the translational reading frame and the truncation of the protein 7 and 5 codons downstream, respectively, whereas c.1342delG led to immediate truncation. c.226→T (p.Q76X) created a premature termination codon. The remaining mutation was c.316G→C, which substituted an arginine for glycine—a basic residue for a polar, uncharged one. The glycine residue is conserved across species (chimpanzee, mouse, dog, and chicken). While analyzing the normal controls, we identified the novel variant c.989C→T (p.T330M) in a single chromosome in an unaffected female. Threonine is replaced with methionine (substituting a polar residue with a nonpolar one), most likely inactivating the protein. The residue is conserved in chimpanzee and mouse but is substituted with serine in dog and chicken.
Table 2. .
Novel SLC34A2 Sequence Variants Identified in Patients with PAM, Subjects with TM, and a Normal Control
| Phenotype and Subject(s) |
Mutation | Location | Effect on Translation | Predicted Consequence on Protein |
| PAM: | ||||
| 1 | c.[−6773_−6588del]+[−6773_−6588del] | Promoter exon 1 | … | Not synthesized |
| 3 and 5 | c.[114delA]+[114delA] | Exon 3 | Frameshift | Truncation |
| 7 | c.[226C→T]+ [226C→T] | Exon 3 | p.Q76X | Truncation |
| 6 | c.[316G→C]+[316G→C] | Exon 4 | p.G106R | Substitution |
| 2 | c.[1328delT]+[1328delT] | Exon 11 | Frameshift | Truncation |
| 4 | c.[1342delG]+[1342delG] | Exon 12 | p.V448X | Truncation |
| TM: | ||||
| 4 | c.[T552C]+[-] | Exon 6 | p.I184I | Aberrant splicing? |
| 6 | c.[*27G→T]+[-] | 3′ UTR | Unknown | Unknown |
| Normal: | ||||
| 30 | c.[989C→T]+[-] | Exon 9 | p.T330M | Substitution |
Having failed to identify the putative mutation in the family despite sequence analysis of the coding exons, we analyzed exon 1 and the flanking sequences by direct sequence analysis of a seminested PCR product. A deletion of 186 nt was identified. The sequences flanked by copies of hexamer GGCAGG, together with one of the copies, had been lost. The deletion spanned the 40-bp noncoding exon and the minimal promoter.14
Moderate expression of SLC34A2 in testis12,13 prompted us to search for mutations in men with diffuse bilateral TM. We identified two rare variants in the heterozygous state in 2 of the 15 subjects with TM studied (table 2). The first variant was c.552T→C, a synonymous T→C conversion in exon 5. The nucleotide residue is conserved in chimpanzee but, intriguingly, is a C in mouse, dog, and chicken. The second variant was noncoding: c.*27G→T, a G→T transversion 27 nt downstream of the termination codon. The residue is conserved in chimpanzee; however, the sequences downstream of termination are not conserved at all among other species. Although the second variant was not found in any other individual studied, the first was carried by two individuals in the normal group.
None of the mutations or rare variants we identified had been reported previously. All identified variants that were not predicted to truncate the protein product (i.e., c.316G→C, c.989C→T, c.552T→C, and c.*27G→T) were screened in 7 unrelated patients with PAM, 15 subjects with TM, and a control group of 105–123 individuals, to achieve at least 80% power to distinguish a normal sequence variant.15 This population control group comprised anonymized, unrelated individuals randomly chosen from our Turkish DNA collection.
Discussion
We localized the gene responsible for PAM in a large family and identified homozygous mutations in SLC34A2 in all patients studied. Six mutations presumably affected the protein product, whereas the remaining mutation abolished gene expression. That mutation was a 186-bp deletion that possibly resulted from an ancestral unequal crossover at two copies of a hexamer. There are four copies of the hexamer within a 424-bp region around the exon, all with the same orientation, but no function has been proposed for it.
In contrast with the predicted severe effects of the identified mutations on the protein product of the gene or on the expression of the gene, the patients with PAM had mild clinical phenotypes, with the exception of the smokers (table 3). The lack of a genotype-phenotype correlation was supported also by the variation in age at onset among the affected members of the large family. The general clinical course of PAM seems to be that microliths begin forming early in childhood, but clinical symptoms arise much later, and lung deterioration is very slow in nonsmokers.
Table 3. .
Clinical Features of Patients with PAM
| Age (years) at |
|||||
| Family and Patient |
Sex | Appearance of Symptoms | Last Visit | Clinical Course | Comment(s) |
| Family 1: | |||||
| 307, or 1 | M | 25 | 34 | Severe | Heavy smoker |
| 403 | M | 7 | 17 | Moderate | Smoker; progressive roentgenographic changes |
| 402 | M | 3 | 13 | Slow | … |
| 501 | M | … | 17 | Asymptomatic | Received diagnosis at age 11 years4 |
| 503 | M | … | 15 | Asymptomatic | Received diagnosis at age 9 years4 |
| 504 | M | … | 11 | Asymptomatic | Received diagnosis at age 5 years4 |
| Family 2: | |||||
| 2 | M | 21 | 24a | Slow | Died of lung infection |
| Family 3: | |||||
| 3 | F | … | 38 | Asymptomatic | PAM diagnosed incidentally |
| Family 4: | |||||
| 4 | M | 26 | 39 | Slow | … |
| Family 5: | |||||
| 5 | F | 9 | 17 | Improving | Receiving treatment with disodium ethidronate16 |
| Family 6: | |||||
| 6 | F | 5 | 22 | Stable | Receiving treatment with disodium ethidronate16 |
| Family 7: | |||||
| 7 | F | 29 | 35 | Slow | … |
Deceased.
SLC34A2 has 13 exons, the first one noncoding, and encodes a 2,280-nt mRNA and a 690-aa protein. It is a member of the solute carrier family SLC34A that plays a major role in the homeostasis of inorganic phosphate. The gene is expressed most strongly in fetal and adult lung; therefore, it has been suggested that the gene has an important physiological function in lung. It was shown to be expressed in lung only in alveolar type II cells, which are responsible for surfactant production.17,18 This finding led to the proposal that the function of the gene protein was to uptake liberated phosphate from the alveolar fluid for surfactant production, the major components of which are phospholipids. This hypothesis is in line with the observation that the microliths are located in alveolar airspaces.19 In addition, calcifications along interlobular septa, bronchovascular bundles, and pleura were observed.19 The finding that mutations in the gene are responsible for the disease in all our patients with PAM suggests that phosphate uptake in lung is performed mainly by this gene’s protein product.
In light of our findings, it is certain that PAM is a recessively inherited disease and is not caused by environmental factors. It has full penetrance, since none of the unaffected members of the large family was homozygous for the disease haplotype. Genetic heterogeneity is not likely for this disease, since we identified mutations in all seven unrelated patients studied. The highest incidence of the disease has been reported in Turkey.1 We did not find a common mutation among our patients. Only the mutations in patients 3 and 5 were the same, and those patients originated from different parts of the country. Thus, it is more likely that the mutation is recurrent rather than identical by descent. We propose that the high incidence of the disease in Turkey is due to the high proportion of consanguineous marriages. Indeed, all patients carried homozygous mutations, in accordance with identity by descent.
Moderate expression of SLC34A2 in testis12,13 prompted us to search for mutations in men with diffuse bilateral TM, to investigate any role of the gene in the etiology of the condition. The two rare variants we identified could not be assigned as mutations as readily as could those found in patients with PAM. Further studies are needed to determine whether they have any effect on the expression of the gene or on its protein product. Both variants were associated with infertility, but only c.*27G→T was associated with a tumor. Variant c.552T→C created a hexamer that has been proposed to be an exonic splicing enhancer that plays an important role in constitutive or alternative splicing.20,21 Alternative splicing would decrease the number of full transcripts in the cell and, subsequently, decrease the activity of the gene. The other TM variant (c.*27G→T) was noncoding, located downstream of the translational termination codon. The residue is conserved in chimpanzee; however, the sequences downstream of termination have not been conserved at all among other species. We investigated whether the variant had an effect on the conformation of the mRNA, using the program RNA2 (GeneBee Molecular Biology Server), but we did not obtain a strongly supportive result. Therefore, we are unable to propose any possible effect of the variant on mRNA stability. In addition, the fact that testis has a lower temperature than the body temperature raises the question of whether the altered forms of the mRNA and the protein would have less effective conformations at the lower temperature. Whether the variants contribute in any way to susceptibility to TM, a common condition, needs to be investigated. We also mention that none of our seven male patients with PAM had positive findings when investigated for TM.
So far, calcium ions have been blamed for the pathogenesis. Now, it is clear that microlith formation is the result of phosphate-chelating calcium in the extracellular fluid. Microliths from both patients with PAM and subjects with TM had been found to be composed of calcium and phosphate.22,23 The rare variants carried by two of our subjects with TM indicated that SLC34A2 could be responsible for the condition, at least in those subjects. It should be noted that the efficiency of SSCP is not 100%; thus, probable variants in other subjects with TM might have escaped detection. Also, variants could possibly be located in those regions of the gene we did not analyze. Thus, >2/15 of the subjects could possibly be carrying variants. The prevalence of TM in the Turkish population is reported as 2.4% in 17–42-year-old healthy men, which is within the range reported for other populations.24
This is the first report of a pathological role of SLC34A2. Although defects in the gene lead to calcium phosphate deposits, defects in the other members of the gene family—namely, SLC34A1 and SLC34A3—result in hypophosphatemia, because those genes are responsible for phosphate reabsorption in kidney.25,26 Interestingly, SLC34A2 is also expressed in kidney. We were able to investigate phosphate uptake in one of our patients (patient 5). The observed value of 92% maximal renal tubular reabsorption was within the normal range. Thus, all our observations together indicate that SLC34A2 does not play a role in renal reabsorption as important as the role of its paralogs. Also, serum phosphate levels were normal in all 9 of our 12 patients with PAM, and none of the 10 patients investigated had calcifications in the kidneys.
The identification of the gene responsible for calcium phosphate deposition in lung has broad implications, because the same gene might be responsible also for calcifications in several other tissues. This hypothesis is based on two observations. First, SLC34A2 is expressed also in kidney, pancreas, prostate, ovary, small intestine, mammary gland, liver, and placenta,12,13 and idiopatic isolated calcifications of some of these organs are well documented. Second, calcium deposits were reported in additional organs in some patients with PAM: pleura and kidneys,27 seminal vesicles,28 urethra,29 and gall bladder.29 The possible role of the gene in calcification in various tissues as well as in diseases with clinical manifestations resembling PAM and TM can now be investigated. Particularly interesting would be prostate microlithiasis and mammary calcifications, since malignancies are associated with them. Microcalcifications associated with malignant breast lesions are more commonly calcium phosphate30 than calcium oxalate. The former was reported to enhance mitogenesis in both mammary epithelial cells and breast cancer cell lines.31
The identification of the gene responsible for PAM will facilitate genetic diagnosis of isolated cases. Also, early diagnosis in asymptomatic individuals in affected families would be most conveniently performed by a genetic test. Several therapeutic approaches have been applied with no knowledge of the molecular basis of PAM. Now, there is hope for the development of gene therapy. Since the gene encodes an integral membrane protein, as does the cystic fibrosis transmembrane regulator gene, strategies developed for gene therapy for cystic fibrosis might benefit patients with PAM in the future.
Acknowledgments
We thank the individuals for their participation, Dr. O. Sanli for providing the TM samples, and Dr. E. S. Ucan for his support. This work was supported by the Turkish State Planning Organization, the Turkish Academy of Sciences (to A.T.), a fellowship of the Scientific and Technological Research Council of Turkey (to S.A.U.), and Boğaziçi University Research Fund.
Web Resources
Accession numbers and URLs for data presented herein are as follows:
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for sequences from human [accession numbers NM_006424.1 and NP_006415.1], chimpanzee [accession numbers XM_526805.1 and XP_526805.1], mouse [accession numbers NM_011402.2 and NP_035532.2], dog [accession numbers XM_545968.2 and XP_545968.2], chicken [accession numbers NM_204474.1 and NP_989805.1], and Caenorhabditis elegans [accession numbers NM_076180.3 and NP_508581.2])
- GeneBee Molecular Biology Server, http://www.genebee.msu.su/genebee.html (for RNA secondary-structure prediction program)
- NCBI Map Viewer, http://www.ncbi.nlm.nih.gov/mapview/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for PAM) [PubMed]
- UCSC Genome Browser, http://genome.ucsc.edu/
References
- 1.Ucan ES, Keyf AI, Aydilek R, Yalcin Z, Sebit S, Kudu M, Ok U (1993) Pulmonary alveolar microlithiasis: review of Turkish reports. Thorax 48:171–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Castellana G, Gentile M, Castellana R, Fiorente P, Lamorgese V (2002) Pulmonary alveolar microlithiasis: clinical features, evolution of the phenotype, and review of the literature. Am J Med Genet 111:220–224 10.1002/ajmg.10530 [DOI] [PubMed] [Google Scholar]
- 3.Mariotta S, Ricci A, Papale M, De Clementi F, Sposato B, Guidi L, Mannino F (2004) Pulmonary alveolar microlithiasis: report on 576 cases published in the literature. Sarcoidosis Vasc Diffuse Lung Dis 21:173–181 [PubMed] [Google Scholar]
- 4.Senyigit A, Yaramis A, Gurkan F, Kirbas G, Buyukbayram H, Nazaroglu H, Alp MN, Topcu F (2001) Pulmonary alveolar microlithiasis: a rare familial inheritance with report of six cases in a family. Contribution of six new cases to the number of case reports in Turkey. Respiration 68:204–209 10.1159/000050494 [DOI] [PubMed] [Google Scholar]
- 5.Kim B, Winter TC, Ryu JA (2003) Testicular microlithiasis: clinical significance and review of the literature. Eur Radiol 13:2567–2576 10.1007/s00330-003-2014-5 [DOI] [PubMed] [Google Scholar]
- 6.Middleton WD, Teefey SA, Santillan CS (2002) Testicular microlithiasis: prospective analysis of prevalence and associated tumor. Radiology 224:425–428 [DOI] [PubMed] [Google Scholar]
- 7.Miller FNAC, Sidhu PS (2002) Does testicular microlithiasis matter? A review. Clin Radiol 57:883–890 10.1053/crad.2002.1005 [DOI] [PubMed] [Google Scholar]
- 8.Kavaslar GN, Onengut S, Derman O, Kaya A, Tolun A (2000) The novel genetic disorder microhydranencephaly maps to chromosome 16p13.3-12.1. Am J Hum Genet 66:1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Connell JR, Weeks DE (1998) PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet 63:259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sobel E, Lange K (1996) Descent graphs in pedigree analysis: applications to haplotyping, location scores, and marker-sharing statistics. Am J Hum Genet 58:1323–1337 [PMC free article] [PubMed] [Google Scholar]
- 11.Murer H, Forster I, Biber J (2004) The sodium phosphate cotransporter family SLC34. Pfugers Arch Eur J Physiol 447:763–767 10.1007/s00424-003-1072-5 [DOI] [PubMed] [Google Scholar]
- 12.Feild JA, Zhang L, Brun KA, Brooks DP, Edwards RM (1999) Cloning and functional characterization of a sodium-dependent phosphate transporter expressed in human lung and small intestine. Biochem Biophys Res Commun 258:578–582 10.1006/bbrc.1999.0666 [DOI] [PubMed] [Google Scholar]
- 13.Xu H, Bai L, Collins JF, Ghishan FK (1999) Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium-phosphate (Na+-Pi) transporter (SLC34A2). Genomics 62:281–284 10.1006/geno.1999.6009 [DOI] [PubMed] [Google Scholar]
- 14.Xu H, Uno JK, Inouye M, Collins JF, Ghishan FK (2005) NF1 transcriptional factor(s) is required for basal promoter activation of the human intestinal NaPi-IIb cotransporter gene. Am J Physiol Gastrointest Liver Physiol 288:G175–G181 10.1152/ajpgi.00396.2004 [DOI] [PubMed] [Google Scholar]
- 15.Collins JS, Schwartz CE (2002) Detecting polymorphisms and mutations in candidate genes. Am J Hum Genet 71:1251–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozcelik U, Gulsun M, Gocmen A, Ariyurek M, Kiper N, Anadol D, Cobanoglu N (2002) Treatment and follow-up of pulmonary alveolar microlithiasis with disodium editronate: radiological demonstration. Pediatr Radiol 32:380–383 10.1007/s00247-001-0651-x [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto M, Wang DY, Kamo T, Zhu Y, Tsujiuchi T, Konishi Y, Tanaka M, Sugimura H (2000) Isolation and localization of type IIb Na/Pi cotransporter in the developing rat lung. Am J Pathol 157:21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traebert M, Hattenhauer O, Murer H, Kaissling B, Biber J (1999) Expression of type II Na-Pi cotransporter in alveolar type II cells. Am J Physiol 277:L868–L873 [DOI] [PubMed] [Google Scholar]
- 19.Sumikawa H, Johkoh T, Tomiyama N, Hamada S, Koyama M, Tsubamoto M, Murai S, et al (2005) Pulmonary alveolar microlithiasis: CT and pathologic findings in 10 patients. Monaldi Arch Chest Dis 63:59–64 [DOI] [PubMed] [Google Scholar]
- 20.Fairbrother WG, Yeh RF, Sharp PA, Burge CB (2002) Predictive identification of exonic splicing enhancers in human genes. Science 297:1007–1013 10.1126/science.1073774 [DOI] [PubMed] [Google Scholar]
- 21.Yeo G, Hoon S, Venkatesh B, Burge CB (2004) Variation in sequence and organization of splicing regulatory elements in vertebrate genes. Proc Natl Acad Sci 101:15700–15705 10.1073/pnas.0404901101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pracyk JB, Simonson SG, Young SL, Ghio AJ, Roggli VL, Piantadosi CA (1996) Composition of lung lavage in pulmonary alveolar microlithiasis. Respiration 63:254–260 [DOI] [PubMed] [Google Scholar]
- 23.De Jong BW, De Gouveia Brazao CA, Stoop H, Wolffenbuttel KP, Oosterhuis JW, Puppels GJ, Weber RF, Looijenga LH, Kok DJ (2004) Raman spectroscopic analysis identifies testicular microlithiasis as intratubular hydroxyapatite. J Urol 171:92–96 10.1097/01.ju.0000101948.98175.94 [DOI] [PubMed] [Google Scholar]
- 24.Serter S, Gümüs B, Ünlü M, Tunçyürek Ö, Tarhan S, Ayyildiz V, Pabuscu Y (2006) Prevalence of testicular microlithiasis in an asymptomatic population. Scand J Urol Nephrol 40:212–214 10.1080/00365590600589641 [DOI] [PubMed] [Google Scholar]
- 25.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, Friedlander G (2002) Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med 347:983–991 10.1056/NEJMoa020028 [DOI] [PubMed] [Google Scholar]
- 26.Lorenz-Depiereux B, Benet-Pages A, Eckstein G, Tenenbaum-Rakover Y, Wagenstaller J, Tiosano D, Gershoni-Baruch R, Albers N, Lichtner P, Schnabel D, Hochberg Z, Strom TM (2005) Hereditary hypophosphatemic rickets with hypercalciuria is caused by mutations in the sodium-phosphate cotransporter gene SLC34A3. Am J Hum Genet 78:193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pant K, Shah A, Mathur RK, Chhabra SK, Jain SK (1990) Pulmonary alveolar microlithiasis with pleural calcification and nephrolithiasis. Chest 98:245–246 [DOI] [PubMed] [Google Scholar]
- 28.Arslan A, Yalin T, Akan H, Belet U (1996) Pulmonary alveolar microlithiasis associated with calcifications in the seminal vesicles. J Belge Radiol 79:118–119 [PubMed] [Google Scholar]
- 29.Piesiak P, Kasibowska-Kuzniar K, Jankowska R (2001) Pulmonary alveolar microlithiasis in a patient with urolithiasis and cholelithiasis. Pneumonol Alergol Pol 69:285–289 [PubMed] [Google Scholar]
- 30.Frappart L, Boudeulle M, Boumendil J, Lin HC, Martinon I, Palayer C, Mallet-Guy Y, et al (1984) Structure and composition of microcalcifications in benign and malignant lesions of the breast: study by light microscopy, transmission and scanning electron microscopy, microprobe analysis, and X-ray diffraction. Hum Pathol 15:880–889 [DOI] [PubMed] [Google Scholar]
- 31.Morgan MP, Cooke MM, Christopherson PA, Westfall PR, McCarthy GM (2001) Calcium hydroxyapatite promotes mitogenesis and matrix metalloproteinase expression in human breast cancer cell lines. Mol Carcinog 32:111–117 10.1002/mc.1070 [DOI] [PubMed] [Google Scholar]