Abstract
During early pregnancy, interleukin-1 (IL-1) is mainly produced and secreted by maternal decidua. Yet, its biological function on placental cells is not well defined. In this study, we employed JAR choriocarcinoma cell line as a model of human placental trophoblast to study the effect of IL-1. Treatment with recombinant human IL-1β resulted in significant inhibition of JAR proliferation (P < .05) paralleled with increased cytotoxicity. The inhibitory effect was blocked by both IL-1 receptor antagonist (IL-1Ra) and antihuman IL-1β monoclonal antibody. Analyzing the mode of action, IL-1β was found to induce cell cycle arrest in the G0/G1 phase and triggered apoptotic cell death. These findings demonstrated that IL-1 regulates human trophoblast growth by induction of cell cycle delay and cell death.
INTRODUCTION
IL-1 is a pleiotropic proinflammatory cytokine produced by both activated lymphoid and nonlymphoid cells. There are two known forms of IL-1, a membrane-bound IL-1α and a secretory form IL-1β [1], both of which exert similar effects. Binding to their receptor complex, IL-1 leads to increased activation of several transcription factors especially NF-κB. This results in a wide spectrum of biological effects, such as local inflammation and endocrine effect [2]. During pregnancy, IL-1 is mainly produced by maternal decidua [3]. IL-1α and IL-1β production has been localized to macrophages, glandular epithelium, and stromal cells in the endometrium [4]. Several studies have suggested a potential role of IL-1 during pregnancy. IL-1 may function in the regulation of blastocyst implantation [5] and in stimulating the production of endometrial leukemia inhibitory factor (LIF) production [6]. IL-1 upregulation is thought to modulate decidualization in an autocrine/paracrine manner [7]. In mice, IL-1α, IL-1β, and IL-1R mRNA are all expressed in the uterus during the preimplantation period [8]. In addition, treatment with IL-1Ra can block embryonic implantation [9]. These studies support a potential functional role of IL-1 in decidualization and implantation.
IL-1 may promote placental trophoblast invasion by stimulating metalloproteinase (MMP-9) release by human cytotrophoblast [10]. It is also associated with trophoblast differentiation by increasing hCG production by isolated first-trimester villous trophoblast [11] and human choriocarcinoma cell lines [12]. IL-1 also regulates the production of other placental cytokines including M-CSF and IL-6 [13, 14]. Furthurmore, it was recently demonstrated that maternal decidual IL-1 could stimulate proliferation of human first trimester extravillous trophoblast cell lines [15] by induction of other growth factors especially IL-6 and LIF [16, 17]. Increased cell proliferation and survival in the extravillous trophoblast cell lines is mediated by the induction of the phosphatidylinositol-3 kinase (PI3K) and mitogen-activated protein kinase (MAPK) signal transduction pathways [18]. However, the effect of IL-1 on villous trophoblast is not well defined. In this study, the effect of IL-1 and its mode of action on regulation of human placental villous trophoblast proliferation was investigated.
MATERIALS AND METHODS
Cytokine and cell line
Recombinant human IL-1β and antihuman IL-1β monoclonal antibody were purchased from Peprotech Inc. (Rocky Hill, NJ). IL-1Ra was purchased from Serotec (Kidlington, Oxford). Insulin-transferrin-selenium growth supplement (ITS) was purchased from Life Technology (Gaithersburg, MD). Actinomycin D was purchased from Amersham Bioscience (Uppsala, Sweden). Choriocarcinoma cell line (JAR) is directly derived from a trophoblastic tumor of placenta. It produces estrogen, progesterone, gonadotrophin, and lactogen in culture. This cell line expresses IL-1 receptor. The cells were maintained in RPMI 1640 (Life Technologies, Gaithersburg, MD) with 10% FBS and antibiotics (100 unit/mL of penicillin G, 100 μg/mL of streptomycin sulfate, and 0.25 μg/mL of amphotericin B). The JAR cell line was purchased from ATCC (Manassas, VA) and cultured at 37°C in 5% CO2.
MTT proliferation assay
Unless stated otherwise, JAR cells (10,000 cells/well) were cultured in collagen I (50 μg/mL) coated 96-well culture plates, in a total volume of 200 μL serum-free RPMI 1640 supplemented with insulin-transferrin-selenium (ITS) solution. Cells were cultured in the presence of increasing concentrations of recombinant human IL-1β (0−100 ng/mL). Both treatment and control groups were performed in 6−8 replicate wells. The number of viable cells was then determined after 72-hour incubation, by adding 1 mg/mL of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) and incubating for a further 4 hours. Live cells assimilated MTT, resulting in the accumulation of formazan crystals. These were then solubilized with acid isopropanol (90% isopropyl alcohol, 0.004 N HCL) for 1 hour. The optical density of this solution measured at 595 nm is directly related to the live cell numbers. These experiments were repeated at least three times to ensure the reproducibility. Inhibition of the IL-1 effect was tested by using antihuman IL-1β monoclonal antibody and IL-1Ra.
Lactate dehydrogenase (LDH) assay
LDH release from trophoblasts was used to detect cytotoxicity and was measured at the end of each proliferation experiment. Briefly, culture plates were centrifuged at 1500 rpm for 15 minutes at room temperature to ensure accumulation of cells at the bottom of the wells. Cell-free culture media (100 μL) was collected and then incubated with 100 μL of the reaction mixture Cytotoxicity Detection Kit (Boehringer Mannheim, Indianapolis, IN) for 30 minutes at room temperature in the dark. 1 N HCl (50 μL) was added into each well to stop the enzymatic reaction. The optical density of the solution was then measured by using an ELISA plate reader with a 490 nm filter. Percent cytotoxicity to the control was then determined.
Cell synchronization and staining for flow cytometry
JAR cells were cultured in 4 mL of RPMI 1640 containing 10% FBS in 6-well culture plate (100 × 20 mm) until 70−80% confluence was achieved. The culture medium was then replaced with serum-free fresh medium and incubated with 0.1 mM hydroxyurea for 24 hours. Synchronized JAR cells were then cultured in 4 mL of fresh RPMI 1640 in the presence of 5% FBS with or without IL-1β (50 ng/mL) for 24, 32, and 48 hours. Cells were then trypsinized, centrifuged, and resuspended into PBS and stained with propidium iodide solution. The percent cell in each cell cycle phase was determined in each sample (30 000 counts) using FACSCalibur and ModFit LT software.
DNA laddering
JAR cells were cultured in 4 mL of RPMI 1640 containing 10% FBS in 6-well culture plate until 70−80% confluence was achieved. Culture media were replaced with fresh ITS-containing media with or without IL-1β (50 ng/mL) and incubated for 24, 48, and 72 hours. Cells were then washed with cold PBS and lysed with lysis solution. DNA sample was then extracted from the solution using a phenol-chloroform-isoamyl alcohol extraction protocol. The DNA concentration was determined using a spectrophotometer (OD260). DNA (2 μg) samples, along with positive controls, were then subjected to agarose gel (1.5%) electrophoresis. DNA ladder was analyzed by ethidium bromide staining and visualized using UV transilluminator.
In situ detection of apoptotic cell death
JAR cells were cultured in 4 mL of RPMI 1640 containing 10% FBS in 6-well culture plate containing a glass slide until 70−80% confluence was achieved. Culture medium was replaced with fresh medium containing ITS with or without IL-1β (50 ng/mL) and incubated for 72 hours. Apoptosis staining was carried out using an Apoptosis Detection Kit (R&D systems, Minneapolis, MN) in culture plate as follows. Glass slides were washed with ice cold PBS then fixed using freshly prepared paraformaldehyde solution 3.7% for 10 minutes at room temperature. Slides were then rinsed with PBS and incubated in permeabilization solution for 30 minutes at room temperature. Slides were then rinsed with washing buffer and incubated with TdT reaction mixture for 60 minutes at 37°C. The labeling reaction was stopped and then incubated with streptavidin-FITC for 10 minutes at room temperature. Slides were then washed with PBS and visualized using fluorescent microscopy. Similar experiments were conducted without glass slide and apoptotic cells were determined using flow cytometry.
Statistics
Data are presented as mean ± standard error of the mean (SEM). Differences in data between two groups in cell cycle studies were analyzed using the Student t test. In cell proliferation and cytotoxicity experiments, Dunnett's method of One-Way ANOVA was used to assess differences among control groups and treatment groups.
RESULTS
IL-1 inhibition of human placental trophoblast proliferation
The overall purpose of this project was to investigate the effect of IL-1β and its mode of action on JAR cell proliferation. The proliferative response of JAR cells to IL-1 is shown in Figure 1(a). IL-1β, as low as 0.01 ng/mL, inhibited JAR trophoblast proliferation compared to the control groups (*P < .05). At highest dose of IL-1β used in this study, proliferation was diminished by 46%. In a related study, the cytotoxic effect of IL-1β on JAR cells was determined by measuring the release of cytosolic LDH. The LDH concentration in conditioned media was proportional to cell death, and IL-1β treatment in these cells led to the increase of LDH release. Percent cytotoxicity to the control (Figure 1(b)) was increased in dose-dependent fashion, indicating that IL-1β promoted cytotoxicity. To confirm the inhibitory effect of IL-1 on JAR cell proliferation, blocking of proliferation inhibition was studied by using IL-1Ra and antihuman IL-1β monoclonal antibody. IL-1Ra (50 and 100 ng/mL) were preincubated with JAR cells for 1 hour at 37°C, prior to the addition of IL-1β (to final concentration of 50 ng/mL). Neutralizing activity of antihuman IL-1β monoclonal antibody was studied by incubating the antibody with IL-1β for at least one hour prior to the addition to the cell culture. The MTT assay was then performed after 72-hour incubation. Figure 1(c) showed that IL-1β (50 ng/mL) inhibited JAR cells by 55% (A, *P < .05) compared to the control groups (H). Antihuman IL-1 antibody at 5 and 10 μg/mL blocked IL-1β activity by 34% and 28%, respectively (B, C, **P < .05). IL-1Ra at a concentration of 50 and 100 ng/mL blocked IL-1β activity by 83% (D, E, ***P < .05). These findings confirmed that IL-1 exerts its inhibitory action via specific receptor.
Figure 1.
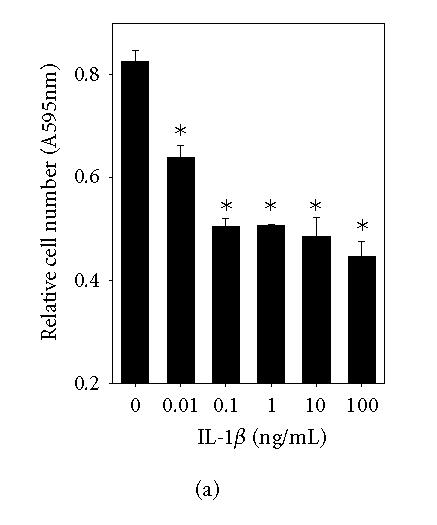
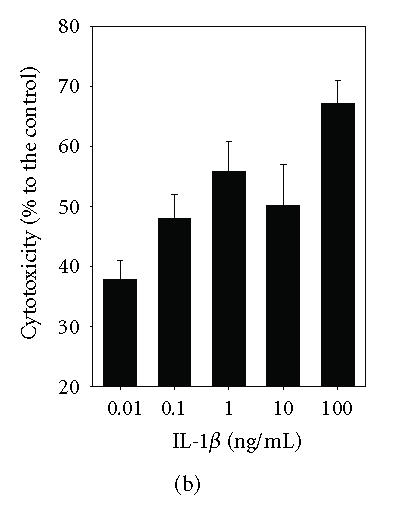
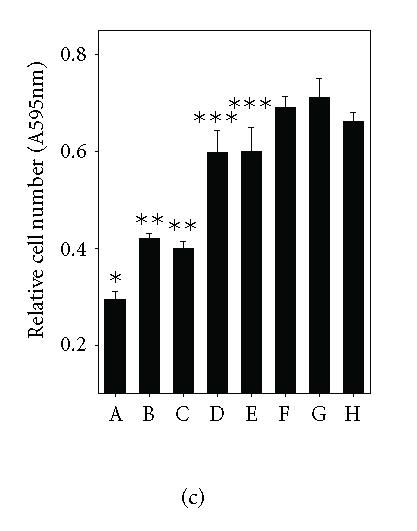
IL-1 inhibition of JAR proliferation. (a) JAR cells were cultured in the presence of increasing concentrations of IL-1 for 72 hours. Relative cell numbers were measured by MTT assay. (b) LDH release assay was performed for cytotoxicity of IL-1. (c) JAR trophoblasts were cultured for 72 hours with either IL-1 (50 ng/mL) alone (A), IL-1 with anti- IL-1 antibody (5 μg/mL) (B), IL-1 with anti- IL-1 antibody (10 μg/mL) (C), IL-1 with IL-1Ra (50 ng/mL) (D), IL-1 with IL-1Ra (100 ng/mL) (E), IL-1Ra (100 ng/mL) (F), anti- IL-1 antibody (10 μg/mL) (G), or untreated control (H). The MTT assay was then performed. The asterisks (*, **, ***P < .05) represent significant differences between treatment and control groups as analyzed by One-Way ANOVA.
Involvement of IL-1 and cell cycle progression
To determine the effect of IL-1 on control of JAR cell cycle progression, the DNA content was measured by flow cytometry, and the percentage of cells in each stage was analyzed and compared. As shown in Table 1, the number of cells in each phase was comparable in both control and treatment groups at 24 hours after cell cycle restimulation. At 32 hours, the percentage of cells in the G0/G1 phase was increased (*, P < .0001), while the percentage of cells in S phase was decreased in treatment groups compared to the control group (FBS alone). These findings indicated that IL-1 delayed cell cycle progression stimulated with FBS, resulting in accumulation of cells in G0/G1 phase. This could also indicate that IL-1 may inhibit DNA synthesis since the number of cells in the S phase was lowered. Interestingly, changes in the percentage of cells in the G2/M phase between the test and control group were not observed. Within the treatment groups, the number of cells deposited in the G0/G1 phase at 48 hours was not different from the 32-hour treatment indicating that the IL-1 effect on delaying cell cycle lasted at least for 48 hours. In addition, treatment with IL-1 for more than 48 hours resulted in detached dead cells. This indicated that inhibition of proliferation is also accompanied by the induction of cell death.
Table 1.
IL-1 modulation of cell cycle progression. Synchronized JAR cells were cultured with or without IL-1 at concentration of 50 ng/mL for 24, 32, and 48 hours. The cells were harvested and stained with propidium iodide. Percent cells (mean ± SEM) in each cycle phase were then analyzed in cells collected using flow cytometer. The asterisk (*, P < .0001) represents significant difference between antimalarial drug treatment and control groups as analyzed by T-test.
IL-1 induction of apoptosis
Since the increase of cytotoxicity and cell death resulting from IL-1 treatment of JAR cells, we therefore hypothesized that this could be attributed to the induction of apoptosis. To test this hypothesis, DNA fragmentation, as a marker of apoptosis, in JAR cells treated with IL-1 was determined using both DNA laddering technique and enzymatic in situ labeling of apoptotic cells.
In DNA ladder analysis (Figure 2), fragmented DNA was observed in cells treated with IL-1β after both 48- and 72-hour incubation as well as in cells that had been treated with actinomycin D (positive control, 1 μg/mL) for 4 hours. In contrast, there was no obvious DNA ladder in the untreated control group. In addition, when cells were incubated with IL-1β and IL-1Ra (50 ng/mL) for 72 hours, the DNA ladders were markedly decreased. This indicated that IL-1β induction of apoptotic cell death can be prevented by IL-1Ra. Further analysis to confirm the induction of apoptosis using enzymatic in situ labeling (Figure 3) revealed that IL-1β (50 ng/mL) (C) treatment for 72 hours could lead to apoptosis cell death as visualized by fluorescent microscopy, whereas there was little spontaneous cell death observed in the untreated control group (B). In addition, this result was consistent with similar experiments analyzed by using flow cytometry (D).
Figure 2.

IL-1 induction of DNA fragmentation. JAR cells were cultured in serum-free RPMI 1640 with or without IL-1 (50 ng/mL) for 24, 48, and 72 hours. Inhibition of DNA fragmentation was also performed using IL-1Ra. DNA samples were extracted and fractionated by agarose gel (1.5%) electrophoresis and DNA ladders were visualized after gel staining.
Figure 3.
IL-1 induction of apoptosis. JAR cells were cultured in serum-free RPMI 1640 with or without IL-1 and incubated for 72 hours. In situ cell death detection was then performed and apoptosis was visualized by fluorescent microscopy. Apoptotic cells showed a green fluorescent nucleus. (a) Nuclease treated (positive control); (b) untreated control; (c) JAR treated with IL-1 (50 ng/mL). Similar experiments were conducted without glass slide and apoptotic cells were determined using flow cytometry (d).
DISCUSSION
Maternal immune response during pregnancy is thought to be detrimental to the survival of the fetus, but in fact it may be beneficial. The success of pregnancy relies on adequate growth of the placenta. The mechanism of regulation of placental growth is thought to be hormone dependent, and perhaps also under the influence of maternal inflammatory cytokines. In 1984, Wegmann proposed the “immunotrophism” hypothesis stating that maternal immune response to the fetus has a beneficial role in regulating placental growth and development [19], whereas some cytokines may play a role in the downregulation of placental trophoblast development. These cytokines could be categorized as immunodystrophism cytokines. IL-1, a mediator of inflammation at maternal-fetal interface, is produced mainly by decidua with a tissue level of 175 pg/mg total protein and secretory rate at 190 pg/ml/24 hours [15]. In the present study, this proinflammatory cytokine was investigated for its role in the inhibition of trophoblast prolilferation. The findings in this study provided a new insight into the interaction between the maternal imune response and the biology of trophoblast cells. In a previous study using invasive extravillous trophoblast cell lines, maternal decidual IL-1 acted as a growth factor for these cells via the activation of PI3K and MAPK pathways [18]. This was a new evidence that placental growth is also, in part, controlled by the maternal cytokine [15].
In contrast to invasive extravillous trophoblast cell lines, in JAR choriocarcinoma cell line, which has the characteristics of early placental trophoblast residing on the floating placental villi, IL-1 was found to have the opposite effect. Recombinant human IL-1β inhibited JAR proliferation and this effect was blocked by using IL-1-Ra or antihuman IL-1β monoclonal antibody, indicating a specific biological activity mediated through the receptor complex. Furhermore, the inhibition of proliferation by IL-1 was shown to be mediated by the induction of cell cycle arrest followed by the induction of apoptotic cell death. Consistenly, this biological activity of IL-1 was also demonstrated in other studies. For example, IL-1 was elevated during labour and preterm premature rupture of the membrane and was found to induce apoptosis of placental membrane [20]. In a mouse model of preterm delivery, trophoblastic apoptosis was observed accompanied with the increase serum level of TNF-α and IL-1α [21]. In other studies, the antiproliferative effect of IL-1 on human melanoma is mediated by G0/G1 arrest [22]. More specifically, the IL-1 growth arrest of melanoma is induced by hypophosphorylation of the retinoblastoma susceptibility gene product RB [23], but it is independent of p53 and p21/WAF1 function [24].
Collectively, the data presented in this study indicate that IL-1 regulates human placental trophoblast growth by delaying cell cycle transition followed by induction of apoptosis. This property may render IL-1 as an immunodistrophism cytokine. In addition, this could also raise an interesting issue concerning choriocarcinoma, which is often an outcome of hydatidiform mole, whether this complex interplay between maternal IL-1 and placental trophoblast is similar to the interaction of IL-1 with hydatidiform mole in vivo.
ACKNOWLEDGMENTS
We thank all technical assistance from the Department of Biochemistry, the Department of Anatomy, and the Scientific Equipment Centre at Prince of Songkla Univerisity. This work was supported by a Grant from BIOTEC (BT-B-06-MM-18-4504) and Prince of Songkla University Grant, Thailand.
References
- 1.Dinarello CA. Interleukin-1 and its biologically related cytokines. Advances in Immunology. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- 3.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstetrics & Gynecology. 1989;73(1):31–34. [PubMed] [Google Scholar]
- 4.Simon C, Piquette GN, Frances A, Polan ML. Localization of interleukin-1 type I receptor and interleukin-1 beta in human endometrium throughout the menstrual cycle. The Journal of Clinical Endocrinology & Metabolism. 1993;77(2):549–555. doi: 10.1210/jcem.77.2.8345061. [DOI] [PubMed] [Google Scholar]
- 5.Kauma SW. Cytokines in implantation. Journal of Reproduction and Fertility. Supplement. 2000;55:31–42. [PubMed] [Google Scholar]
- 6.Sawai K, Matsuzaki N, Okada T, et al. Human decidual cell biosynthesis of leukemia inhibitory factor: regulation by decidual cytokines and steroid hormones. Biology of Reproduction. 1997;56(5):1274–1280. doi: 10.1095/biolreprod56.5.1274. [DOI] [PubMed] [Google Scholar]
- 7.Frank GR, Brar AK, Jikihara H, Cedars MI, Handwerger S. Interleukin-1 beta and the endometrium: an inhibitor of stromal cell differentiation and possible autoregulator of decidualization in humans. Biology of Reproduction. 1995;52(1):184–191. doi: 10.1095/biolreprod52.1.184. [DOI] [PubMed] [Google Scholar]
- 8.Takacs P, Kauma S. The expression of interleukin-1α, interleukin-1β, and interleukin-1 receptor type I mRNA during preimplantation mouse development. Journal of Reproductive Immunology. 1996;32(1):27–35. doi: 10.1016/s0165-0378(96)00987-4. [DOI] [PubMed] [Google Scholar]
- 9.Simon C, Frances A, Piquette GN, et al. Embryonic implantation in mice is blocked by interleukin-1 receptor antagonist [see comments] Endocrinology. 1994;134(2):521–528. doi: 10.1210/endo.134.2.8299552. [DOI] [PubMed] [Google Scholar]
- 10.Librach CL, Feigenbaum SL, Bass KE, et al. Interleukin-1 beta regulates human cytotrophoblast metalloproteinase activity and invasion in vitro. The Journal of Biological Chemistry. 1994;269(25):17125–17131. [PubMed] [Google Scholar]
- 11.Yagel S, Lala PK, Powell WA, Casper RF. Interleukin-1 stimulates human chorionic gonadotropin secretion by first trimester human trophoblast. The Journal of Clinical Endocrinology & Metabolism. 1989;68(5):992–995. doi: 10.1210/jcem-68-5-992. [DOI] [PubMed] [Google Scholar]
- 12.Seki H, Zosmer A, Elder MG, Sullivan MHF. The regulation of progesterone and hCG production from placental cells by interleukin-1β. Biochimica et Biophysica Acta. 1997;1336(2):342–348. doi: 10.1016/s0304-4165(97)00042-1. [DOI] [PubMed] [Google Scholar]
- 13.Kauma SW. Interleukin-1 beta stimulates colony-stimulating factor-1 production in human term placenta. The Journal of Clinical Endocrinology & Metabolism. 1993;76(3):701–703. doi: 10.1210/jcem.76.3.8445029. [DOI] [PubMed] [Google Scholar]
- 14.Kauma SW, Turner TT, Harty JR. Interleukin-1 beta stimulates interleukin-6 production in placental villous core mesenchymal cells. Endocrinology. 1994;134(1):457–460. doi: 10.1210/endo.134.1.8275959. [DOI] [PubMed] [Google Scholar]
- 15.Nilkaeo A, Green KL, Kauma SW. Maternal regulation of trophoblast proliferation by decidual interleukin-1 (IL-1) Fertility and Sterility. 2000;74(3 suppl 1):S8. [Google Scholar]
- 16.Nilkaeo A, Green K, Kauma S. Interleukin-1 (IL-1) stimulation of trophoblast proliferation is mediated by the induction of interleukin-6 (IL-6) production. Journal of the Society for Gynecologic Investigation. 2000;7(1 suppl):162A. [Google Scholar]
- 17.Nilkaeo A, Takacs P, Kauma S. Leukemia inhibitory factor (LIF) stimulates trophoblast proliferation and is upregulated in trophoblast by IL-1. Journal of the Society for Gynecologic Investigation. 2000;7(1 suppl):210A. [Google Scholar]
- 18.Nilkaeo A, Kauma S. Activation of phosphoinositol-3 kinase (PI3K) and mitogen activated protein kinase (MAPK) signal transduction pathway are necessary for iterleukin-1β (IL-1β) dependent trophoblast proliferation. Journal of the Society for Gynecologic Investigation. 2001;8(1 suppl):82A. [Google Scholar]
- 19.Wegmann TG. Maternal T cells promote placental growth and prevent spontaneous abortion. Immunology Letters. 1988;17(4):297–302. doi: 10.1016/0165-2478(88)90001-6. [DOI] [PubMed] [Google Scholar]
- 20.Fortunato SJ, Menon R. IL-1β is a better inducer of apoptosis in human fetal membranes than IL-6. Placenta. 2003;24(10):922–928. doi: 10.1016/s0143-4004(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 21.Kakinuma C, Kuwayama C, Kaga N, Futamura Y, Katsuki Y, Shibutani Y. Trophoblastic apoptosis in mice with preterm delivery and its suppression by urinary trypsin inhibitor. Obstetrics & Gynecology. 1997;90(1):117–124. doi: 10.1016/S0029-7844(97)00176-2. [DOI] [PubMed] [Google Scholar]
- 22.Morinaga Y, Hayashi H, Takeuchi A, Onozaki K. Antiproliferative effect of interleukin 1 (IL-1) on tumor cells: G0-G1 arrest of a human melanoma cell line by IL-1. Biochemical and Biophysical Research Communications. 1990;173(1):186–192. doi: 10.1016/s0006-291x(05)81039-3. [DOI] [PubMed] [Google Scholar]
- 23.Muthukkumar S, Sells SF, Crist SA, Rangnekar VM. Interleukin-1 induces growth arrest by hypophosphorylation of the retinoblastoma susceptibility gene product RB. The Journal of Biological Chemistry. 1996;271(10):5733–5740. doi: 10.1074/jbc.271.10.5733. [DOI] [PubMed] [Google Scholar]
- 24.Nalca A, Rangnekar VM. The G1-phase growth-arresting action of interleukin-1 is independent of p53 and p21/WAF1 function. The Journal of Biological Chemistry. 1998;273(46):30517–30523. doi: 10.1074/jbc.273.46.30517. [DOI] [PubMed] [Google Scholar]




