Abstract
To determine whether there was a specific inflammatory process in severe asthmatics, the phenotypic characteristics of induced sputum immune cells were analysed among patients with severe asthma. Twenty-two induced sputa (10 severe asthmatics) were studied. Flow cytometric analysis was performed using immune cells of the sputum and monoclonal antibodies to CD3, CD4, CD8, CD56, CD25, and TCRγδ. The number of NKT (CD3+CD56+) cells was significantly higher in the sputum of severe asthmatics compared with mild asthmatic and healthy control groups (P < .05). CD8+CD56+ cells were the predominant subtype of the increased NKT cells in severe asthmatics. CD3+CD56+Vα24+, TCRγδ+ CD56+, and CD4+CD25+ T cells were significantly increased in severe asthmatic patients. These results suggest that the immunopathogenesis of severe asthmatics vary between severe and mild asthmatics, and that CD8+CD56+ NKT cells may play an important role in the immunopathogenesis of severe asthma.
INTRODUCTION
Asthma is recognized by the presence of reversible bronchoconstriction, airway hyperresponsiveness, and airway inflammation [1]. These clinical features result from a chronic inflammation of the airways, caused by a migration of leucocytes and an increase of inflammatory mediators in the bronchial wall [2]. This pathological reaction in asthma is thought to arise from a complex interaction between genes and the environment. Different immunopathogenic mechanisms and immune cells may be involved in the development of asthma [3], natural killer T (NKT) cells constitute one of the regulatory cells not well defined in asthma.
Natural killer T (NKT) cells are heterogeneous T-cell populations that are characterised by the coexpression of TCRs and various NK receptors, including CD16, CD56, CD161, CD94, CD158a, and CD158b [4]. NKT cells have been considered to regulate autoimmunity and adaptive immune responses [4–6], even though the pleiotropic nature of NKT cells creates some controversy regarding their functional role at different inflammatory sites [7–10]. Disturbances in the numbers and functions of the NKT cells have been implicated in several organ-specific animal models of autoimmunity as well as in humans [11, 12]. The proportion of NKT cells among the induced sputum cells in the severe asthmatic patients has not yet been studied.
Therefore, in this study we asked whether there are phenotypic differences in the sputum immune cell types, including the NKT cells, in patients with severe asthma and in patients with mild asthma. The subtypes of CD56+ T cells, including γδ T cells, Vα24+ T cells, and CD8+CD56+ T cells, were analysed.
PATIENTS AND METHODS
Patients
Induced sputum samples were collected from 22 successive patients with asthma (10 patients in severe asthma) (Table 1). All were outpatients in steady state, regulatory followed by an asthma specialist. The severity of the disease was classified according to GINA recommendations [13]. The samples were obtained on the day of the fixed visit. A precise history of the patient was previously obtained with functional respiratory tests. The following patients were excluded: acute exacerbation of asthma, concomitant respiratory infection, other pulmonary diseases, and smokers.
Table 1.
Cellular characteristics of induced sputum. Differential sputum cell counts are expressed as percentage of nonsquamous cells.
| Healthy controls | Severe asthmatics | Mild asthmatics | |
| Number/Sex (F) | 10 (F) | 10 (F) | 12 (F) |
| Age | 28.7 ± 6.2 | 48.6 ± 3.5 | 55.2 ± 3.2 |
| FEV1 (% predicted) | 93.2 ± 4.8 | 58.7 ± 6.2† | 87.3 ± 2.5 |
| Total cell count (106/ml) | 1.65 ± 0.7 | 3.1 ± 0.5† | 1.9 ± 0.32 |
| Macrophages (%) | 47.6 ± 5.2 | 33.6 ± 8 | 48.9 ± 4.5 |
| Neutrophils (%) | 30 ± 4.5 | 29.2 ± 4.9 | 25.7 ± 6.1 |
| Eosinophils (%) | 0.7 ± 0.2 | 5.7 ± 3.6† | 1.2 ± 0.7 |
| Lymphocytes (%) | 1.7 ± 1.2 | 6.5 ± 2† | 2.7 ± 1.8 |
| Squamous cells (%) | 4.5 ± 2 | 4.8 ± 1.9 | 4.50 ± 1.8 |
| Viability (%) | 84 ± 4 | 82 ± 6 | 87 ± 5 |
† significant differences (p < .05) compared to mild asthmatic patients and healthy controls. Successful sputum induction was achieved in 9/10 severe asthmatics and 8/12 mild asthmatics.
Ten induced sputum from healthy subjects—females with a mean age of 28.7 years (range 18–39 years), who had normal pulmonary radiographs and showed no clinical signs of respiratory diseases—acted as controls. Informed consent was obtained from all the patients. The study was approved by our Ethics Committee.
Sputum induction and processing
Before sputum induction all patients inhaled salbutamol (200 μg) via a metered dose inhaler. Baseline FEV1 was measured and this was repeated following salbutamol and after each 5-minute inhalation of nebulised hypertonic saline (3.5%). The procedure was stopped if the FEV1 fell by > 10% following saline or by > 20% at any time during the induction procedure. Solid sputum material was separated from saliva before processing as we have recently reported [14]. Briefly, selected sputum was weighed and 0.1% DTT (Sigma-Aldrich, Poole, UK) in phosphate-buffered saline (PBS) was added at a ratio of 4 ml to 1 g sputum. The sputum was incubated with DTT at room temperature for 15 minutes on a rolling mixer. The same volume (4 ml to 1 g sputum) of PBS was added to the sputum and then filtered through 48 μm nylon gauze. The filtrate was centrifuged at 400 g (Sorvall RT6000D, Kendro, Bishop's Stortford, UK) for 10 minutes at 4°C to pellet cells. The cells were resuspended in PBS containing 0.1% bovine serum albumin (BSA). The viability of the sample was determined by trypan blue exclusion staining (Sigma-Aldrich) in a Neubauer hemocytometer (Merck Eurolabs, Lutterworth, UK). Cytospins (Shandon Scientific, Sewickley, Pa, USA) of sputum cells that were used for determination of differential cell counts were fixed with methanol and stained with May-Grunwald-Giemsa stain (Merck-Eurolabs).
Flow cytometric analysis
Two-colour staining of the sputum cells was performed using monoclonal antibodies (mAbs) in two combinations: tube A contained mAbs to CD4 (helper T cell)/CD8 (cytotoxic T cell), and tube B contained mAbs to CD3 (pan T cell)/CD56 (natural killer cell) (all were products of BD Biosciences). The isotype-matched control mAbs were used. 50 μl aliquots of the mononuclear cells from the sputum cells were added to the polypropylene round-bottom test tube, and the directly conjugated mAbs (conjugated fluorescein isothiocyanate (FITC), phycoerythrin (PE), Cy-Chrome (Cy), or peridinin chlorophyll-a protein (PerCP)) were added at predetermined optimal dilution. After a 30-minute incubation, aliquots were washed twice in FACS buffer (phosphate-buffered saline/0.2% bovine serum albumin). The acquisition was performed using a FACSCalibur flow cytometer (Becton Dickinson), and analysed using the CellQuest software program (BD Biosciences). A gate for the live lymphocyte population was defined by the forward and side scatter characteristics, and the data was displayed on a log scale of increasing fluorescence intensity. In addition, the subtype analysis of CD3+CD56+ cells was done in the sputum of patients with severe asthma and mild asthma. The subtype analysis was also done to 10 healthy controls for the comparison. CD3+CD56+ T cells belong to a highly heterogeneous subset that includes chronically stimulated conventional CD8+ T cells, T cells, and so called CD1d-restricted NKT cells, the hallmark of which is the recurrent expression of Vα24Vβ11 T-cell receptors [15]. For the further detailed phenotypic analysis of CD3+CD56+ T cells, three- or four-colour staining, using CD3, CD56, CD25, and additional markers such as pan TCRγδ mAb, pan TCRβ mAb, CD4, and CD8 coreceptors and Vα24Vβ11 (all were products of BD Biosciences) was done.
Statistics
The differences in the phenotypic characteristics of the mononuclear cell populations among the patients with severe asthma, mild asthma, and healthy controls were statistically compared using Anova test. For significant Anova values, the groups were compared by a Tukey's post hoc test for the multiple comparisons with an unequal cell size. A P value < .05 was considered significant. In addition, the Wilcoxon matched-pair signed-rank test was used to demonstrate the differences between the values obtained from the sputum cells of severe and mild asthmatics.
RESULTS
Cell types in induced sputum of severe and mild asthmatics
Subject sputum cell counts characteristics were as summarised in Table 1. Baseline FEV1 expressed as percentage predicted differed significantly between severe asthmatics and mild asthmatics (P < .01). There were significant differences in the total cell count of the sputum cells between patients with severe asthma compared to patients with mild asthma and healthy controls (P < .05). Significant differences were observed in the percentages of eosinophils and lymphocytes between severe asthmatics and mild asthmatics (P < .01).
The sputum from the asthmatic patients was analysed for the percentages of B cells (CD19), CD4+, and CD8+ T-cell subsets among the lymphocytes. The phenotypes of the immune cells showed significant differences between the sputum of severe and mild asthmatic patients (Table 2). A slight but not significant reduction of CD19+ cells was observed in severe and mild asthmatics comparatively to healthy controls. Decreased percentages of CD8+ T-cells were observed in severe asthmatic. The percentage of CD4+ cells in the sputum of severe asthmatic patients was elevated resulting in a higher CD4+/CD8+ T-cell ratio compared to the sputum of mild asthmatics and healthy controls. There was a positive correlation between sputum CD4/CD8 ratio and the sputum percentage of eosinophils in severe asthmatic patients (r = 0.536, P < .05). Patients with severe asthma showed a higher TCRγδ+ cell percentage than mild asthmatics and healthy controls. Severe asthmatic patients expressed high level of CD4+CD25+ cells, when compared to mild asthmatics.
Table 2.
Percentages of lymphocyte subsets and CD4/CD8 ratio in the induced sputum of healthy control, mild asthmatic, and severe asthmatic patients.
| Healthy controls | Severe asthmatics | Mild asthmatics | |
| CD3+ | 91.42 ± 8.35 | 93.12 ± 6.11 | 94.21 ± 7.12 |
| CD4+ | 65.5 ± 18.66 | 72.65 ± 10.22† | 68.6 ± 9.33 |
| CD8+ | 25.6 ± 17.2 | 18.6 ± 9.8† | 23.6 ± 8.7 |
| CD4+/CD8+ | 1.97 ± 0.7 | 3.58 ± 0.37†† | 2.57 ± 0.29† |
| CD19+ | 6.33 ± 1.82 | 5.32 ± 1.07 | 4.30 ± 0.98 |
| TCR γδ+ | 2.63 ± 1.72 | 9.63 ± 0.28†† | 1.97 ± 0.63 |
| CD4+CD25+ | 5.25 ± 1.45 | 9.44 ± 0.63†† | 3.23 ± 0.30 |
† significantly different from healthy controls. †† significantly different from healthy controls and mild asthmatic patients.
Prevalence of CD3+CD56+ T cells in severe asthmatic patients
The proportions of CD3+CD56+ cells in the sputum of patients with severe asthma were highly expressed when compared to patients with mild asthma and healthy controls (Table 3). No significant differences were observed in the CD3−CD56+ cells between mild and severe asthmatics.
Table 3.
Percentages of subpopulations of NKT (CD3+CD56+) cells and NK (CD3−CD56+) cells in the induced sputum of patients with severe asthma, mild asthma, and healthy controls.
| Healthy controls | Severe asthma | Mild asthma | |
| CD3+CD56+ | 2.14 ± 1.72 | 13.52 ± 4.13†† | 2.86 ± 1.39 |
| CD4+CD56+ | 0.30 ± 0.20 | 0.58 ± 0.32 | 0.29 ± 0.07 |
| CD8+CD56+ | 2.05 ± 0.29 | 7.43 ± 1.05† | 2.83 ± 1.45 |
| CD3−CD56+ (NK cells) | 3.25 ± 0.29 | 5.58 ± 4.72 | 3.83 ± 1.45 |
| CD56+ αβ TCR | 0.93 ± 0.18 | 6.12 ± 1.29† | 0.92 ± 0.87 |
| CD56+ γδ TCR | 0.97 ± 0.25 | 6.50 ± 3.74† | 0.89 ± 0.45 |
| CD56+Vα24+ T cells | 0.09 ± 0.02 | 0.82 ± 0.07†† | 0.07 ± 0.05 |
†† denotes statistically significant differences of the value when compared with that of patients with mild asthma and healthy controls (P < .05).
The CD3+CD56+/CD3−CD56+ cellular ratio was significantly increased in severe asthmatics compared to patients with mild asthma and healthy controls, because of the more dominance of the NKT cells (P < .05), (Figure 1).
Figure 1.
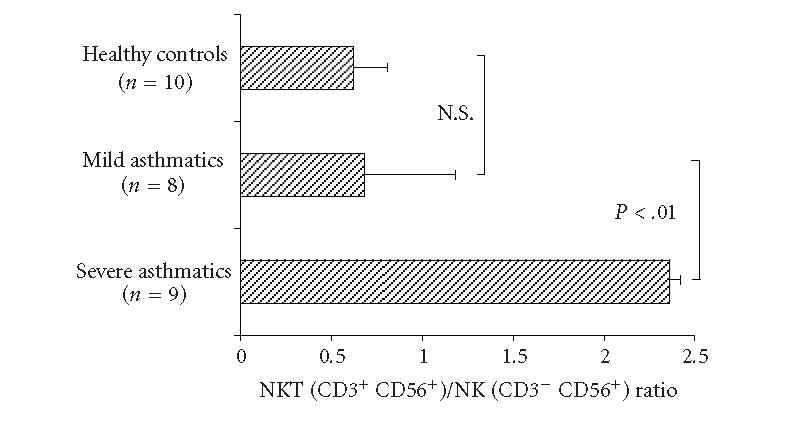
Ratio of NKT (CD3+CD56+) cells and NK (CD3−CD56+) cells in the induced sputum of patients with severe asthma, mild asthma, and healthy controls.
Phenotypic analysis of CD3+CD56+ T cells in patients with severe asthmatic
The subsets of CD3+CD56+ cells in patients with severe asthma and mild asthma were analysed in the sputum; we also analysed these cells in healthy controls (Table 3). The subsets of CD3+CD56+ cells, especially CD8+CD56+ cells and CD56+ γδ T cells, were significantly increased in patients with severe asthmatics compared to healthy controls and mild asthmatics. Vα24+ T cells among CD3+CD56+ cells were significantly increased in severe asthmatics. Vα24+ T cells did not show any statistically significant difference among the mild asthmatic group and healthy controls.
DISCUSSION
This study shows that sputum immune cells in severe asthmatic patients expressed different CD3+CD56+, CD8+CD56+, CD56+ γδ CD56+ Vα24+ T subpopulations, and NKT/NK cell ratio than patients with mild asthma. The values of CD8+CD56+ were similar as we have recently reported [14]. No differences were observed between healthy controls and mild asthmatics. These findings may be attributed to the fact that the aetiologies of the severe and mild asthma were different. CD4+ T cells were more predominant than CD8+ T cells in the sputum of mild and severe asthmatics. The CD4+ T cells in the sputum of severe asthmatic patients show increased CD25 expression, comparatively to mild asthmatics. CD25 characterise both activated cells and regulatory T cells (Treg), the most specific marker for Treg cells is Foxp3, a recently identified transcription factor gene that is specifically expressed in CD4+CD25+ Treg cells. The sputum eosinophils count was higher in severe asthmatics compared to mild asthmatics. Our study also confirms that inducted sputum is a safe method to investigate airway inflammation in asthma, as it has been reported by Jatakanon A et al [16].
Severe asthmatics exhibited an increase in CD4/CD8 cell ratio in comparison with mild asthmatic patients. The significantly higher CD4/CD8 ratio confirms the enhancement of CD4+ cells response in severe asthma. Increased CD4/CD8 ratios have been already suggested from severe asthmatics peripheral blood, bronchoalveolar lavage lymphocytes (BAL), and lung biopsy studies [17, 18]. In the same line of our report, the recent study of Fabbri et al [19], severe asthmatics expressed a higher CD4/CD8 T-cell ratio, infiltrating the airway mucosa. On the contrary, Leckie et al [20] failed to find any difference in the number of sputum CD4+ and CD8+ cell populations of patients with asthma or healthy subjects. CD4+ cells of type 2 have also been found increased in blood, biopsies, and BAL of asthmatic patients [17, 21]. CD4+ cells expressed regulatory cytokines, like IL-5 that promotes eosinophil differentiation and activation, as well as IL-4 and IL-13, which induce IgE production and eosinophil recruitment via vascular cell adhesion molecule-1 expression on the vascular endothelium [22]. Increased eosinophil cells were observed in our severe asthmatic patients. Eosinophils play a major role in the onset and maintenance of bronchial inflammation and tissue injury in asthma [23, 24]. The sputum CD4/CD8 ratio in our severe asthmatic group correlate to the number of eosinophils, but did not correlate to IgE levels. Possibly, the higher CD4 cell numbers observed in our study among severe asthmatics also reflect a persisting release of Th2 cytokines from the above cells, which both support higher eosinophil levels, despite steroid treatment. In asthma, Th2 cytokines (IL-13) is elevated in BAL, bronchial biopsies, and sputum [25], and several cell types have been described as important sources of IL-13, including T-lymphocytes [26, 27]. The role of eosinophils in severe asthmatics is not clear. Wenzel et al [28] have demonstrated that there are two subtypes of severe asthma, depending on the absence or presence of eosinophils in endobronchial biopsies. Sputum analysis of severe asthmatics has provided evidence for persistent eosinophilic inflammation [17, 19]. Similarly, our asthmatic group had significantly higher eosinophil percentages. Gibson et al [29] have already reported heterogeneity regarding sputum eosinophil numbers in severe asthmatics.
In this study, CD3+CD56+ cell population was much higher in the sputum of severe asthmatic patients than patients with mild asthma and healthy controls. These results indicate that CD3+CD56+ cells may play a key role only in the pathogenesis severe asthma. Ohkawa et al [30] suggested that human CD56+ T cells play an important role in the Th1 responses. In severe asthmatics, CD3+CD56+-cytokines production is under studies. Activated T cells with NK cell markers (NKT cells) in our severe asthmatics may be harmful to the lung. TCRγδ T cells with or without CD56 were increased in sputum of severe asthmatic patients compared to mild asthma. TCRγδ cells are predominantly CD4−CD8− T lymphocytes, appear early in thymic ontogeny, consist 1% to 10% of the peripheral blood T cells, and are dispersed in lymphatic and extralymphatic tissues. TCRγδ cells work as first-line defending cells of the innate immune response and are thought to influence the nature of the adaptive immune response [31]. T cells expressing NK receptors are believed to play key roles in patrolling, surveying, and destroying cells with an abnormal phenotype [32]. T cells expressing the γδ-receptor have been reported to be essential for Th2-mediated inflammation in patients with acute exacerbation of asthma [33]. In IL-12 rich microenvironments, CD56 expression was induced from T cells, which exerted potent cytotoxicity [34].
The predominance of CD8+CD56+ cells might be related to the chronic and massive tissue inflammation observed in severe asthmatics. In certain autoimmune disease, tissue destruction is dominated by CD8+ T cells in the chronic stage [35]. The importance of these cells in the development of branchial inflammation in severe asthma is further supported by the fact that CD8+CD56+ cells is more prominent in the sputum of severe asthmatics rather than in the sputum of mild asthmatics. Pittet et al [36] suggested that the CD8+ cytotoxic T lymphocyte (CTL) effector function correlates better with CD56 surface expression. Based on the tight association between cytolytic effector function and CD56 expression, they proposed that CD56 represents a useful marker to identify and monitor effector CD8+ T cells in different clinical situations. These immune cells with cytolytic effector potential may contribute to the more chronic and destructive nature that is mediated by CD4+ T cells. Functional study such as cytotoxic functional assay will be necessary in order to determine the exact pathogenic role of CD8+CD56+ cells in severe asthmatics.
In summary, the CD4+ T cells were prominent in the sputum of the patients with severe asthma. Despite decreased CD8+ T cells observed in severe asthmatics, the number of CD8+CD56+ cells was the predominant subtype, subscribing an increase of NKT cells in severe asthmatics than the other groups. These results suggest that the immunopathogenesis of severe asthma is different than mild asthma, and that CD8+CD56+ NKT cells may play an important role in the immunopathogenesis of severe asthma. The identification and analysis of the T lymphocytes from the sputum can assist in understanding the role of T cells in the pathogenesis of the different groups of asthma. The increased infiltration of the sputum by NK/NKT cells favors recovery? This information may help to characterise the pathogenesis of severe asthma.
ACKNOWLEDGMENT
This study was supported by the grant from “Secrétariat d'Etat à la Recherche Scientifique of Tunisia.”
References
- 1.Sheffer AL. Guidelines for the diagnosis and management of asthma. National Heart, Lung and Blood Institute. National Asthma Education Program. Expert Panel Report. Journal of Allergy and Clinical Immunology. 1991;88(3 pt 2):425–534. [PubMed] [Google Scholar]
- 2.Bittleman DB, Casale TB. Allergic models and cytokines. American Journal of Respiratory and Critical Care Medicine. 1994;150(5 pt 2):S72–S76. doi: 10.1164/ajrccm/150.5_Pt_2.S72. [DOI] [PubMed] [Google Scholar]
- 3.Wills-Karp M, Karp CL. Chitin checking—novel insights into asthma. The New England Journal of Medicine. 2004;351(14):1455–1457. doi: 10.1056/NEJMcibr041294. [DOI] [PubMed] [Google Scholar]
- 4.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunology Today. 2000;21(11):573–583. doi: 10.1016/s0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 5.van der Vliet HJ, von Blomberg BM, Nishi N, et al. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clinical Immunology. 2001;100(2):144–148. doi: 10.1006/clim.2001.5060. [DOI] [PubMed] [Google Scholar]
- 6.Wilson SB, Kent SC, Patton KT, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes. Nature. 1998;391(6663):177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 7.Shi F-D, Ljunggren HG, Sarvetnick N. Innate immunity and autoimmunity: from self-protection to self-destruction. Trends in Immunology. 2001;22(2):97–101. doi: 10.1016/s1471-4906(00)01821-4. [DOI] [PubMed] [Google Scholar]
- 8.Mempel M, Ronet C, Suarez F, et al. Natural killer T cells restricted by the monomorphic MHC class 1b CD1d1 molecules behave like inflammatory cells. The Journal of Immunology. 2002;168(1):365–371. doi: 10.4049/jimmunol.168.1.365. [DOI] [PubMed] [Google Scholar]
- 9.Smyth MJ, Godfrey DI. NKT cells and tumor immunity—a double-edged sword. Nature Immunology. 2000;1(6):459–460. doi: 10.1038/82698. [DOI] [PubMed] [Google Scholar]
- 10.Cui J, Watanabe N, Kawano T, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. The Journal of Experimental Medicine. 1999;190(6):783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mieza MA, Itoh T, Cui JQ, et al. Selective reduction of V α14+ NK T cells associated with disease development in autoimmune-prone mice. The Journal of Immunology. 1996;156(10):4035–4040. [PubMed] [Google Scholar]
- 12.Kukreja A, Costi G, Marker J, et al. NKT cell defects in NOD mice suggest therapeutic opportunities. Journal of Autoimmunity. 2002;19(3):117–128. doi: 10.1006/jaut.2002.0609. [DOI] [PubMed] [Google Scholar]
- 13.NHBLI/WHO. Global initiative for asthma. Global strategy for asthma management and prevention. Bethesda, Md: National Institutes of Health; 1995. NHBLI/WHO Workshop Report 95-3659. [Google Scholar]
- 14.Hamzaoui A, Chaouch N, Graïri H, Ammar J, Hamzaoui K. Inflammatory process of CD8+ CD28− T cells in induced sputum from asthmatic patients. Mediators of Inflammation. 2005;(3):160–166. doi: 10.1155/MI.2005.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musha N, Yoshida Y, Sugahara S, et al. Expansion of CD56+ NK T and γδ T cells from cord blood of human neonates. Clinical and Experimental Immunology. 1998;113(2):220–228. doi: 10.1046/j.1365-2249.1998.00645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic inflammation in severe persistent asthma. American Journal of Respiratory and Critical Care Medicine. 1999;160(5 pt 1):1532–1539. doi: 10.1164/ajrccm.160.5.9806170. [DOI] [PubMed] [Google Scholar]
- 17.Colavita AM, Reinach AJ, Peters SP. Contributing factors to the pathobiology of asthma. The Th1/Th2 paradigm. Clinics in Chest Medicine. 2000;21(2):263–277, viii. doi: 10.1016/s0272-5231(05)70265-3. [DOI] [PubMed] [Google Scholar]
- 18.Tsoumakidou M, Tzanakis N, Kyriakou D, Chrysofakis G, Siafakas NM. Inflammatory cell profiles and T-lymphocyte subsets in chronic obstructive pulmonary disease and severe persistent asthma. Clinical & Experimental Allergy. 2004;34(2):234–240. doi: 10.1111/j.1365-2222.2004.01858.x. [DOI] [PubMed] [Google Scholar]
- 19.Fabbri LM, Romagnoli M, Corbetta L, et al. Differences in airway inflammation in patients with fixed airflow obstruction due to asthma or chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2003;167(3):418–424. doi: 10.1164/rccm.200203-183OC. [DOI] [PubMed] [Google Scholar]
- 20.Leckie MJ, Jenkins GR, Khan J, et al. Sputum T lymphocytes in asthma, COPD and healthy subjects have the phenotype of activated intraepithelial T cells (CD69+ CD103+) Thorax. 2003;58(1):23–29. doi: 10.1136/thorax.58.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brightling CE, Symon FA, Birring SS, Bradding P, Pavord ID, Wardlaw AJ. TH2 cytokine expression in bronchoalveolar lavage fluid T lymphocytes and bronchial submucosa is a feature of asthma and eosinophilic bronchitis. Journal of Allergy and Clinical Immunology. 2002;110(6):899–905. doi: 10.1067/mai.2002.129698. [DOI] [PubMed] [Google Scholar]
- 22.Muro S, Minshall EM, Hamid QA. The pathology of chronic asthma. Clinics in Chest Medicine. 2000;21(2):225–244. doi: 10.1016/s0272-5231(05)70263-x. [DOI] [PubMed] [Google Scholar]
- 23.Druilhe A, Letuve S, Pretolani M. Eosinophil apoptosis in asthma [in French] Pathologie Biologie. 2000;48(6):566–573. [PubMed] [Google Scholar]
- 24.Leung DY, Martin RJ, Szefler SJ, et al. Dysregulation of interleukin 4, interleukin 5, and interferon gamma gene expression in steroid-resistant asthma. The Journal of Experimental Medicine. 1995;181(1):33–40. doi: 10.1084/jem.181.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komai-Koma M, McKay A, Thomson L, et al. Immuno-regulatory cytokines in asthma: IL-15 and IL-13 in induced sputum. Clinical & Experimental Allergy. 2001;31(9):1441–1448. doi: 10.1046/j.1365-2222.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 26.Kotsimbos TC, Ernst P, Hamid QA. Interleukin-13 and interleukin-4 are coexpressed in atopic asthma. Proceedings of the Association of American Physicians. 1996;108(5):368–373. [PubMed] [Google Scholar]
- 27.Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. The Journal of Immunology. 1995;155(5):2688–2694. [PubMed] [Google Scholar]
- 28.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. American Journal of Respiratory and Critical Care Medicine. 1999;160(3):1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 29.Gibson PG, Simpson JL, Saltos N. Heterogeneity of airway inflammation in persistent asthma: evidence of neutrophilic inflammation and increased sputum interleukin-8. Chest. 2001;119(5):1329–1336. doi: 10.1378/chest.119.5.1329. [DOI] [PubMed] [Google Scholar]
- 30.Ohkawa T, Seki S, Dobashi H, et al. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103(3):281–290. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrick DA, Schrenzel MD, Mulvania T, Hsieh B, Ferlin WG, Lepper H. Differential production of interferon-γ and interleukin-4 in response to Th1- and Th2-stimulating pathogens by γδ T cells in vivo. Nature. 1995;373(6511):255–257. doi: 10.1038/373255a0. [DOI] [PubMed] [Google Scholar]
- 32.Hayday AC. [gamma][delta] cells: a right time and a right place for a conserved third way of protection. Annual Review of Immunology. 2000;18:975–1026. doi: 10.1146/annurev.immunol.18.1.975. [DOI] [PubMed] [Google Scholar]
- 33.Hamzaoui A, Kahan A, Ayed K, Hamzaoui K. T cells expressing the γδ receptor are essential for Th2-mediated inflammation in patients with acute exacerbation of asthma. Mediators of Inflammation. 2002;11(2):113–119. doi: 10.1080/09629350220131971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satoh M, Seki S, Hashimoto W, et al. Cytotoxic gammadelta or alphabeta T cells with a natural killer cell marker, CD56, induced from human peripheral blood lymphocytes by a combination of IL-12 and IL-2. The Journal of Immunology. 1996;157(9):3886–3892. [PubMed] [Google Scholar]
- 35.Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. Journal of Investigative Dermatology. 1998;111(5):850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- 36.Pittet MJ, Speiser DE, Valmori D, Cerottini J-C, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. The Journal of Immunology. 2000;164(3):1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]


