Abstract
In inflammation, inducible nitric oxide synthase (iNOS) produces nitric oxide (NO), which modulates inflammatory processes. We investigated the effects of Janus kinase (JAK) inhibitors, AG-490 and WHI-P154, on iNOS expression and NO production in J774 murine macrophages stimulated with interferon-γ (IFN-γ). JAK inhibitors AG-490 and WHI-P154 decreased IFN-γ-induced nuclear levels of signal transducer and activator of transcription 1α (STAT1α). JAK inhibitors AG-490 and WHI-P154 decreased also iNOS protein and mRNA expression and NO production in a concentration-dependent manner. Neither of the JAK inhibitors affected the decay of iNOS mRNA when determined by actinomycin D assay. Our results suggest that the inhibition of JAK-STAT1-pathway by AG-490 or WHI-P154 leads to the attenuation of iNOS expression and NO production in IFN-γ-stimulated macrophages.
INTRODUCTION
Nitric oxide (NO) is a small gaseous signalling molecule that is synthesized from amino acid L-arginine in a reaction catalyzed by nitric oxide synthase (NOS). In mammalian cells, there are three isoforms of the enzyme: neuronal nNOS and endothelial eNOS are constitutively expressed and the third isoform, iNOS, is induced in response to proinflammatory cytokines and bacterial products in inflammatory and tissue cells [4, 8, 13]. Once iNOS is expressed, it produces high amounts of NO for prolonged periods. NO production through iNOS pathway is regulated mainly at the level of iNOS expression [8, 10]. In inflammation, NO modulates immune responses and inflammatory process [10, 16], and is associated with the pathophysiology of various inflammatory diseases such as asthma [18] and arthritis [23]. Compounds that inhibit iNOS expression or iNOS activity have a promise as antiinflammatory drugs based on their effects in various forms of experimentally-induced inflammation [22].
One of the central cytokines involved in the induction of iNOS expression and NO production in macrophages is interferon-γ (IFN-γ). IFN-γ regulates iNOS expression at transcriptional and post-transcriptional level [8, 10]. One of the intracellular signal transduction pathways that are activated by IFN-γ is Janus kinase (JAK)—signal transducer and activator of transcription (STAT) -pathway [17]. In the present study, we investigated the effects of two JAK inhibitors, AG-490 and WHI-P154, on the IFN-γ-induced iNOS expression and NO production in cultured macrophages. Both compounds inhibited iNOS expression and NO production in IFN-γ-treated macrophages along with their inhibitory effect on activation of STAT1.
MATERIALS AND METHODS
Materials
JAK inhibitors AG-490 (tyrphostin B42) and WHI-P154 (Calbiochem, La Jolla, Calif, USA), rabbit polyclonal mouse iNOS and STAT1α p91 antibodies and goat anti-rabbit HRP-conjugated polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, Calif, USA), rabbit polyclonal phospho-STAT1 (Tyr701) antibody (Cell Signaling Technology Inc, Beverly, Mass, USA) and recombinant mouse γ-interferon (R&D systems, Minneapolis, Minn, USA) were obtained as indicated. All other reagents were from Sigma Chemical Co (St Louis, Mo, USA).
Cell culture
J774 macrophages (ATCC, Manassas, Virginia, USA) were cultured at 37°C in 5% CO2 atmosphere in Dulbecco's modified Eagle's medium with Glutamax-I (Cambrex BioScience, Verviers, Belgium) containing 10% heat-inactivated fetal bovine serum (Cambrex BioScience), 100 U/mL penicillin, 100 μg/mL streptomycin, and 250 ng/mL amphotericin B (all from Gibco, Paisley, UK). Cells were seeded on 24-well plates for nitrite measurement and RT-PCR, on 6-well plates for Western blot and on 10 cm dishes for nuclear extract preparation, and were grown for 72 h to confluence before the commencement of the experiments.
Toxicity of the tested compounds was ruled out by measuring cell viability using Cell Proliferation Kit II (XTT) (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer's instructions.
Preparation of cell lysates
At indicated time points, cells were rapidly washed with ice-cold phosphate-buffered saline (PBS) containing 2 mM sodiumorthovanadate. For pSTAT1 Western blot, the cells were solubilized in cold lysis buffer (1% NP-40, 150 mM NaCl, 50 mM Tris pH 7.5, 1 mM EDTA, 1 mM phenylmethylsulfonylfluoride, 2 mM sodiumorthovanadate, 80 μM leupeptin, 1 μg/mL aprotinin, 1 mM NaF, 1 μg/mL pepstatin, 2 mM sodiumpyrophosphate, 0.25% sodiumdeoxycholate and 10 μM N-octyl-β-D-glucopyranoside). After incubation for 15 min on ice, lysates were centrifuged (13 500 g, 5 min). The protein content of the supernatants was measured by the Coomassie blue method.
For iNOS Western blot, the cells were resuspended in lysis buffer containing 1% Triton X, 50 mM NaCl, 10 mM Tris-base pH 7.4, 5 mM EDTA, 0.5 mM phenylmethylsulfonylfluoride, 1 mM sodiumorthovanadate, 40 μM leupeptin, 50 μg/mL aprotinin, 5 mM NaF, 2 mM sodiumpyrophosphate, 10 μM N-octyl-β-D-glucopyranoside. Otherwise the lysis was performed as described above.
Preparation of nuclear extracts
At indicated time points, the cells were rapidly washed with ice-cold PBS and solubilized in hypotonic buffer A (10 mM HEPES-KOH pH 7.9, 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiotreitol, 0.2 mM phenylmethylsulfonylfluoride, 10 μg/mL leupeptin, 25 μg/mL aprotinin, 0.1 mM EGTA, 1 mM sodiumorthovanadate, 1 mM NaF). After incubation for 10 min on ice, the cells were vortexed for 30 s and the nuclei separated by centrifugation at 4°C, 21 000 g for 10 s. The pellet was resuspended in buffer C (20 mM HEPES-KOH pH 7.9, 420 mM NaCl, 25% glycerol, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiotreitol, 0.2 mM phenylmethylsulfonylfluoride, 10 μg/mL leupeptin, 25 μg/mL aprotinin, 0.1 mM EGTA, 1 mM sodiumorthovanadate, 1 mM NaF) and incubated on ice for 20 min. Nuclei were vortexed for 30 s and nuclear extracts were obtained by centrifugation at 4°C, 21 000 g for 2 min. The protein content of the supernatant was measured by the Coomassie blue method. The samples were boiled in SDS sample buffer and stored at −20°C.
Western blotting
Protein (20 μg of lysates or nuclear extracts) was loaded on 8% SDS-polyacrylamide electrophoresis gel and was electrophoresed for 2 h at 120 V in buffer containing 25 mM Tris base, 250 mM glycine and 0.1% SDS. After electrophoresis, the proteins were electrically transferred to Hybond ECL™ nitrocellulose membrane (Amersham Biosciences UK, Ltd., Little Chalfont, Buckinghamshire, UK) in buffer containing 25 mM Tris, 192 mM glycine, 20% methanol, and 0.005% SDS. After transfer, the membrane was blocked in TBST (20 mM Tris base pH 7.6, 150 mM NaCl, 0.1% Tween-20) containing 5% skimmed milk for 1 h at room temperature. The membrane was incubated with anti-STAT1α or anti-iNOS in the blocking solution for 1 h at room temperature or with anti-pSTAT1 in TBST containing 5% bovine serum albumin at 4°C overnight. Thereafter the membrane was washed three times with TBST for 5 min, incubated with secondary antibody in the blocking solution for 50 min at room temperature, and washed three times with TBST for 5 min. Bound antibody was detected using Super Signal West Pico or Dura chemiluminescent substrate (Pierce, Rockford, Ill, USA) and FluorChem™ 8800 imaging system (Alpha Innotech Corporation, San Leandro, Calif, USA). The quantitation of the chemiluminescent signal was carried out with the use of FluorChem™ software version 3.1.
RNA extractions and quantitative PCR
Cell homogenization, RNA extraction, reverse transcription, and quantitative PCR were performed as described in [11]. Mouse iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primers and probes [6-FAM (6-carboxy-fluorescein) as 5′-reporter dye and TAMRA (6-carboxy-tetramethyl-rhodamine) as 3′ -quencher] were designed using Express Software (Applied Biosystems, Foster City, Calif, USA) and were 5′-CCTGGTACGGGCATTGCT-3′ (miNOS forward), 5′-GCTCATGCGGCCTCCTT-3′ (miNOS reverse), 5′-CAGCAGCGGCTCCATGACTCCC-3′ (miNOS probe), 5′-GCATGGCCTTCCGTGTTC-3′ (GAPDH forward), 5′-GATGTCATCATACTTGGCAGGTTT-3′ (GAPDH reverse), and 5′-TCGTGGATCTGACGTGCCGCC-3′ (the GAPDH probe). The primers were used at 300 nM and the probes at 150 nM concentrations. All primers and probes were purchased from Metabion Planegg-Martinsried, Germany. Thermal cycling conditions were: incubation at 50°C for 2 min, 95°C for 10 min, thereafter 40 cycles of denaturation at 92°C for 15 s, and annealing/extension at 60°C for 1 min. The relative mRNA levels were quantified and compared using the relative standard curve method as described in Applied Biosystems User Bulletin #2. Each sample was determined in duplicate.
Nitrite assays
After 24 h incubation, the culture medium was collected for the nitrite measurement, which was used as a measure of NO production. Culture medium (100 μL) was incubated with 100 μL of Griess reagent (0.1% napthalethylenediamine dihydrochloride, 1% sulfanilamine, 2.4% H3PO4) and the absorbance was measured at 540 nm. The concentration of nitrite was calculated with sodium nitrite as a standard [5].
Statistics
Results are expressed as mean ± standard error of mean (SEM). When indicated, statistical analysis was carried out by analysis of variances supported by Dunnett adjusted significance levels. Differences were considered significant at P < .05.
RESULTS
Activation of STAT1 by IFN-γ
Activation of the JAK-STAT signalling pathway in J774 mouse macrophages was studied by measuring STAT1 phosphorylation and nuclear translocation of STAT1α after IFN-γ-treatment. In cells treated with IFN-γ, tyrosine (Tyr701) phosphorylation of STAT1 was detected 15 min after addition of IFN-γ and it was further enhanced up to 60 minutes (Figure 1(a)). Phosphorylated STATs dimerize and diffuse into the nucleus to initiate transcription [6]. Therefore we investigated the nuclear translocation of STAT1α in IFN-γ-stimulated J774 macrophages. The presence of STAT1α in nuclear extracts was measured by Western blot. The level of STAT1α in the nucleus increased in a time-dependent manner after addition of IFN-γ into the culture. In nuclei, low levels of STAT1α were detected already 5 min after exposure to IFN-γ and it was increased up to 30 minutes (Figure 1(b)).
Figure 1.
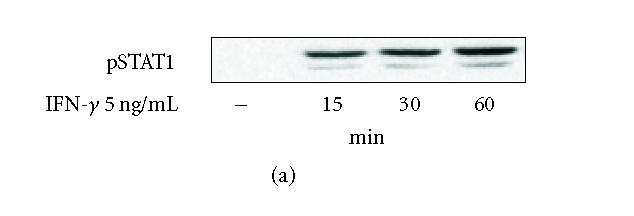
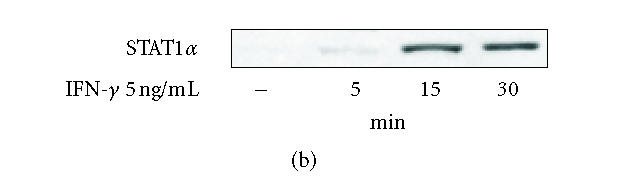
(a) Time-dependent activation (Tyr701 phosphorylation) of signal transducer and activator of transcription 1 (STAT1) by interferon-γ (IFN-γ) in J774 macrophages. Cells were treated with IFN-γ (5 ng/mL) for different times as indicated. Proteins were extracted with modified RIPA-buffer, and the protein contents were measured. Equal amounts of lysates (20 μg protein) were subjected to immunoblot analysis with antibody specific for STAT1 phosphorylated at the tyrosine residue 701. Similar results were obtained in three independent experiments. (b) Time-dependent nuclear translocation of STAT1α in IFN-γ-stimulated J774 macrophages. Cells were treated with IFN-γ (5 ng/mL) for different times as indicated. The nuclear proteins were extracted as described in materials and methods. The protein content of the samples was measured, and equal amounts of proteins (20 μg) were subjected to immunoblot analysis with antibody against STAT1α. Similar results were obtained in two independent experiments.
Effects of JAK inhibitors AG-490 and WHI-P154 on STAT1 activation
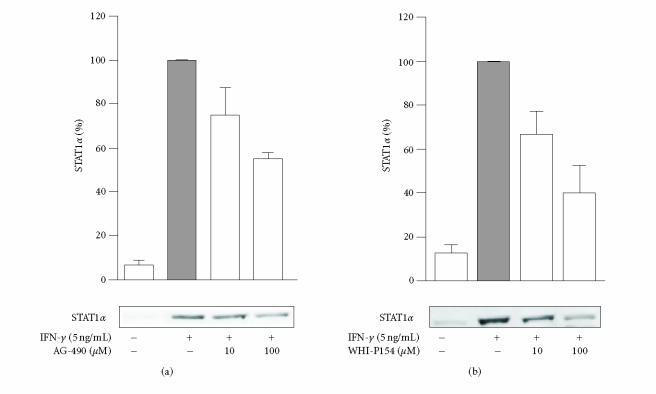
The action of JAK inhibitors AG-490 and WHI-P154 on STAT1 activation was studied by measuring their effects on nuclear translocation of STAT1α in IFN-γ-stimulated cells. Both AG-490 and WHI-P154 decreased the nuclear translocation of STAT1α in a concentration-dependent manner (Figure 2). WHI-P154 was somewhat more potent than AG-490, and at 10 μM drug concentration, WHI-P154 decreased the IFN-γ-induced nuclear translocation of STAT1α by approximately 50% when measured after 30 min incubation with IFN-γ.
Figure 2.
Effects of AG-490 and WHI-P154 on nuclear translocation of STAT1α in IFN-γ-stimulated J774 macrophages. The cells were pretreated with (a) AG-490 or (b) WHI-P154 for 30 minutes. Thereafter, the medium was replaced with fresh medium containing the combination of the inhibitor and IFN-γ (5 ng/mL). The cells were incubated for another 30 minutes, and the nuclear proteins were extracted as described in materials and methods. The protein content of the samples was measured and equal amounts (20 μg) were subjected to immunoblot analysis with antibody against STAT1α. The results are expressed as mean ± SEM (n = 2–3 for AG-490 and n = 4 for WHI-P154).
Effects of JAK inhibitors AG-490 and WHI-P154 on NO production in J774 macrophages
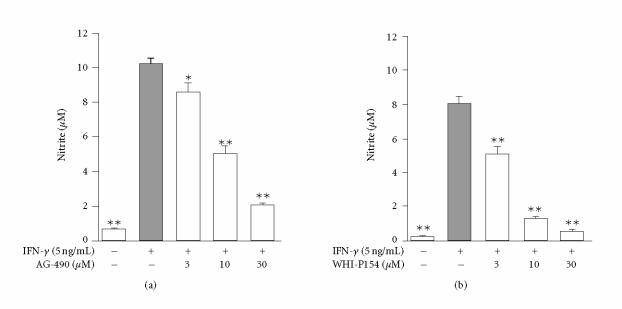
To investigate the effects of JAK inhibitors on NO production in J774 macrophages, the cells were treated with IFN-γ in the absence or in the presence of increasing concentrations (3, 10, and 30 μM) of JAK inhibitors AG-490 and WHI-P154, and NO production was detected as nitrite accumulation in the culture medium. IFN-γ induced NO production in J774 macrophages and it was inhibited in a concentration-dependent manner by AG-490 and WHI-P154 (Figure 3). WHI-P154 was somewhat more potent inhibitor of NO production than AG-490. Cytotoxicity as a contributing factor was ruled out by XTT test. When the compounds were added to cells 6 h after IFN-γ stimulation, no effect on NO production was seen. This suggests that the compounds do not inhibit iNOS activity but rather suppress iNOS expression.
Figure 3.
Effects of (a) AG-490 and (b) WHI-P154 on IFN-γ-induced nitric oxide (NO) generation in J774 macrophages. After 24-hour incubation with IFN-γ (5 ng/mL), the supernatants were collected and nitrite was measured in the culture medium as an indicator of NO production by Griess reaction. The values are mean ± SEM (n = 6), *P < .05, and **P < .01 when compared to cells treated with IFN-γ alone.
Effects of JAK inhibitors AG-490 and WHI-P154 on iNOS protein expression
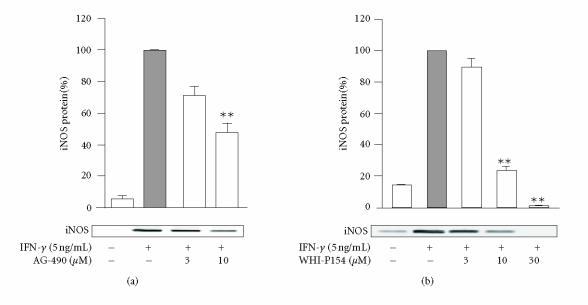
The effects of JAK inhibitors, AG-490 and WHI-P154, on iNOS protein expression were investigated by Western blot analysis. IFN-γ induced iNOS protein expression in J774 macrophages, and it was reduced in a concentration-dependent manner by AG-490 or WHI-P154 (Figure 4).
Figure 4.
Effects of (a) AG-490 and (b) WHI-P154 on iNOS protein expression in J774 macrophages. Cells were incubated with IFN-γ (5 ng/mL) in the presence or in the absence of the tested compound for 24 hours. Cells were lysed and the protein content of the lysates was measured, and equal amounts of proteins (20 μg) were subjected to immunoblot analysis with an antibody against iNOS. The results are shown as mean ± SEM (n = 6 for AG-490 and n = 4 for WHI-P154), **P < .01 when compared to cells treated with IFN-γ alone.
Effects of JAK inhibitors AG-490 and WHI-P154 on iNOS mRNA expression and decay
The effects of JAK inhibitors, AG-490 and WHI-P154, on iNOS mRNA expression in IFN-γ treated cells were measured by quantitative PCR. Both AG-490 (10 μM) and WHI-P154 (10 μM) reduced iNOS mRNA levels by 60% when measured after 4 h incubation (Figure 5(a)). To study whether the JAK inhibitors affect the rate of iNOS mRNA degradation, actinomycin D assay was applied. An inhibitor of transcription, actinomycin D (0.1 μg/mL), was added into the culture after 6 h incubation with IFN-γ or a combination of IFN-γ and the drugs tested. Cells were harvested at time points 0, 1, 2, 3, 4, and 6 h after the addition of actinomycin D. Neither AG-490 nor WHI-P154 affected the decay of iNOS mRNA (Figure). The results suggest that AG-490 and WHI-P154 suppress iNOS expression at the level of transcription rather than at the level of regulation of the stability of iNOS mRNA.
Figure 5.
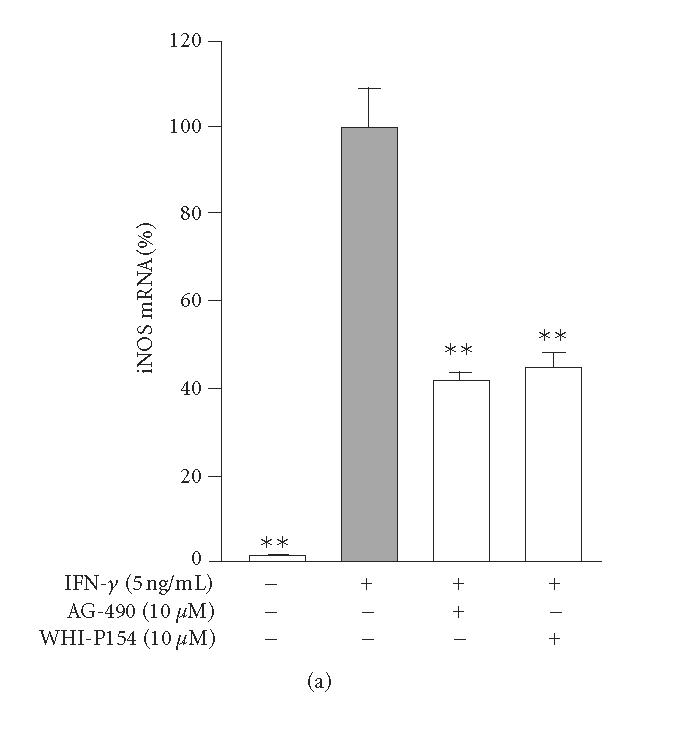
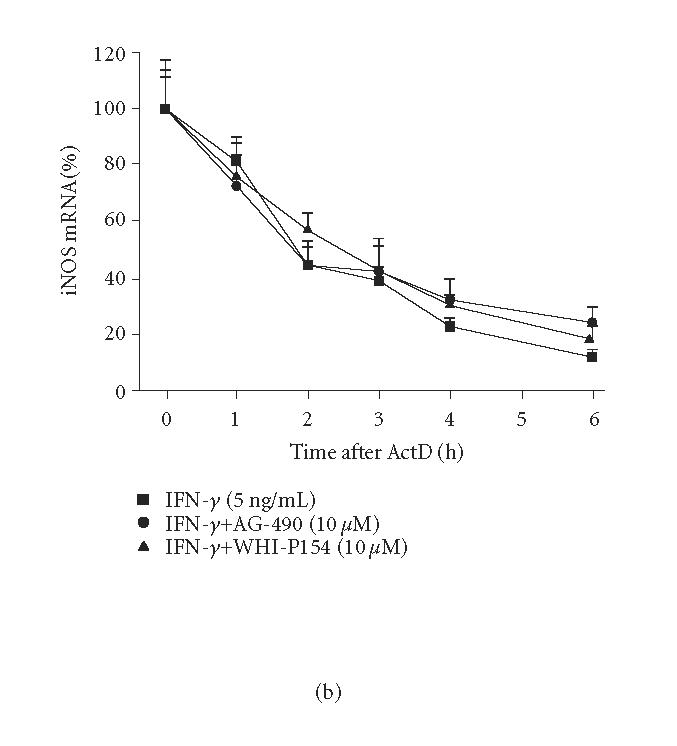
Effects of JAK inhibitors, AG-490 and WHI-P154, on IFN-γ-induced iNOS mRNA expression and degradation in J774 macrophages. (a) Cells were incubated with IFN-γ (5 ng/mL) in the absence or in the presence of AG-490 and WHI-P154 (10 μM). The cells were disrupted after 4-hour incubation and total RNA was collected. Isolated RNA was converted to cDNA. iNOS and GAPDH mRNAs were measured by quantitative PCR, and iNOS mRNA levels were normalized against GAPDH mRNA. The results are expressed as mean ± SEM, n = 3. **P < .01 when compared to cells treated with IFN-γ alone. (b) Cells were incubated as in (a) except that actinomycin D (actD) (0.1 μg/mL) was added after 6-hour incubation to stop transcription. Incubations were terminated at the indicated time points after addition of actD into the culture medium. Total RNA was isolated and converted to cDNA. iNOS and GAPDH mRNA were measured by quantitative PCR. iNOS mRNA levels were normalized against GAPDH mRNA. The results are expressed as mean ± SEM, n = 3.
DISCUSSION
In the present study, we tested the effects of two JAK inhibitors, AG-490 and WHI-P154, on the activation of JAK-STAT1-signalling pathway, iNOS expression, and NO production in IFN-γ-treated macrophages. JAK inhibitors AG-490 and WHI-P154 decreased IFN-γ-induced iNOS expression and NO production along with inhibition of STAT1 activation. To our knowledge, down-regulation of iNOS expression and NO production by JAK inhibitor WHI-P154 has not been reported previously. The inhibitors did not affect the decay of iNOS mRNA.
Typically, cytokine stimulation involves the ligation of two different receptor subunits, and this results in the formation of JAK heterodimers and their subsequent autophosphorylation. IFN-γ signalling preferentially leads to activation of STAT1 [6], which is phosphorylated on Tyr701 by JAK [12]. Phosphorylation of STAT1 induces STAT1 dimerization, nuclear translocation, and initiation of transcription of gamma activated site (GAS) -driven genes [7]. In our study, we followed STAT1 activation by detecting STAT1 (Tyr701) phosphorylation and by probing nuclear lysates for STAT1α at different time points after IFN-γ activation. The results show that STAT1 was activated in 15 minutes after IFN-γ-stimulation in J774 cells. Similar results have been reported recently when whole cell and nuclear lysates of J774 cells were immunoblotted for phosphorylated STAT1 [3].
STAT1 has been reported to act as a key transcription factor in IFN-γ-dependent mouse iNOS expression [1], whereas NF-κB, another important transcription factor in the induction of iNOS, is merely involved in lipopolysaccharide (LPS)-induced iNOS expression and has a minor role following IFN-γ stimulation [1, 24]. An IFN-γ-activated site (GAS) is necessary for full expression of iNOS in response to IFN-γ and LPS [2, 15]. In addition, macrophages derived from STAT1-deficient mice displayed severely impaired NO production in response to a combination of IFN-γ and LPS [15].
In the present study, stimulation of J774 macrophages by IFN-γ led to the phosphorylation and nuclear translocation of STAT1, which was inhibited by AG-490 and WHI-P154. On molar basis, WHI-P154 was somewhat more potent inhibitor than AG-490. Similarly to our results, AG-490 has previously been shown to prevent JAK2 phosphorylation and to decrease STAT1 phosphorylation in J774 cells [1] and to decrease activation of STAT1 pathway in B-cell chronic lymphocytic leukemia (B-CLL) cells [14]. WHI-P154 was designed to specifically inhibit JAK3, and it has been shown to inhibit IL-2-triggered JAK3-dependent STAT activation in 32Dc11-IL-2Rβ-cells [20]. WHI-P131 (another WHI-P154-related JAK inhibitor) has been shown to inhibit STAT1 activation in B-CLL cells, in platelets, and in mesenchymal stem cells [14, 19, 21]. Here we extend the earlier data by showing that WHI-P154 inhibits STAT1 activation also in IFN-γ-treated macrophages.
In the present study, IFN-γ induced iNOS expression and NO production in J774 macrophages, and it was inhibited by JAK inhibitors, AG-490 and WHI-P154, in a dose-dependent manner along with their inhibitory action on STAT1 activation. When the drugs were added to the culture 6 h after IFN-γ, no effect on NO production was detected suggesting that the compounds do not inhibit iNOS activity. The results confirm the earlier studies showing that AG-490 inhibits IFN-γ-induced iNOS expression in macrophages [1]. To our knowledge, down-regulation of iNOS expression and NO production by JAK inhibitor WHI-P154 has not been reported previously.
The regulation of iNOS expresion is controlled at the level of mRNA stability in addition to the transcriptional regulation [8, 10]. In murine macrophages, dexamethasone, and SP600125, an inhibitor of c-Jun N-terminal kinase (JNK), reduced LPS-induced iNOS expression by destabilizing the mRNA [9, 11]. In contrast, IFN-γ has been shown to retard iNOS mRNA degradation when compared to iNOS mRNA induced by LPS alone [9]. In the present study, the effects of AG-490 and WHI-P154 on iNOS mRNA decay were tested by actinomycin D assay. JAK inhibitors, AG-490 and WHI-P154 did not affect the rate of degradation of iNOS mRNA in cells treated with IFN-γ. This suggests that AG-490 and WHI-P154 inhibit iNOS expression at transcriptional level and they do not regulate mechanisms involved in the iNOS mRNA stabilization.
In conclusion, we have shown that JAK inhibitors, AG-490 and WHI-P154 down-regulate STAT1 activation, iNOS expression, and NO production in IFN-γ-treated macrophages. A better understanding of the mechanisms regulating iNOS expression and NO production in inflammation could facilitate the development of novel anti-inflammatory drugs acting through iNOS pathway.
ABBREVIATIONS
AG-490, α-cyano-(3,4-dihydroxy)-N-benzylcinnamide; B-CLL, B-cell chronic lymphocytic leukemia; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GAS, gamma activated site; IFN, interferon; IL, interleukin; iNOS, inducible nitric oxide synthase; JAK, Janus kinase; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide; NO, nitric oxide; STAT, signal transducer and activator of transcription; WHI-P154, 4-(3′-bromo-4′-hydroxylphenyl)-amino-6,7-dimethoxyquinazoline.
ACKNOWLEDGMENTS
We thank Mrs Niina Ikonen for the skillful technical assistance and Mrs Heli Määttä for secretarial help. This work was supported by The National Technology Agency (Tekes), Finnish Cultural Foundation, and the Medical Research Fund of Tampere University Hospital.
References
- 1.Blanchette J, Jaramillo M, Olivier M. Signalling events involved in interferon-gamma-inducible macrophage nitric oxide generation. Immunology. 2003;108(4):513–522. doi: 10.1046/j.1365-2567.2003.01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao J, Morrison DC, Parmely TJ, Russell SW, Murphy WJ. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. The Journal of Biological Chemistry. 1997;272(2):1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 3.Gatto L, Berlato C, Poli V, et al. Analysis of SOCS-3 promoter responses to interferon gamma. The Journal of Biological Chemistry. 2004;279(14):13746–13754. doi: 10.1074/jbc.M308999200. [DOI] [PubMed] [Google Scholar]
- 4.Geller DA, Billiar TR. Molecular biology of nitric oxide synthases. Cancer and Metastasis Reviews. 1998;17(1):7–23. doi: 10.1023/a:1005940202801. [DOI] [PubMed] [Google Scholar]
- 5.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 6.Ivashkiv LB, Hu X. Signaling by STATs. Arthritis Research & Therapy. 2004;6(4):159–168. doi: 10.1186/ar1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1-2):1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 8.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. European Journal of Pharmacology. 2004;500(1–3):255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Korhonen R, Lahti A, Hamalainen M, Kankaanranta H, Moilanen E. Dexamethasone inhibits inducible nitric-oxide synthase expression and nitric oxide production by destabilizing mRNA in lipopolysaccharide-treated macrophages. Molecular Pharmacology. 2002;62(3):698–704. doi: 10.1124/mol.62.3.698. [DOI] [PubMed] [Google Scholar]
- 10.Korhonen R, Lahti A, Kankaanranta H, Moilanen E. Nitric oxide production and signaling in inflammation. Current Drug Targets. Inflammation and Allergy. 2005;4(4):471–479. doi: 10.2174/1568010054526359. [DOI] [PubMed] [Google Scholar]
- 11.Lahti A, Jalonen U, Kankaanranta H, Moilanen E, editors. c-Jun NH2-terminal kinase inhibitor anthra(1,9-cd)pyrazol-6(2H)-one reduces inducible nitric-oxide synthase expression by destabilizing mRNA in activated macrophages. Molecular Pharmacology. 2003;64(2):308–315. doi: 10.1124/mol.64.2.308. [DOI] [PubMed] [Google Scholar]
- 12.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annual Review of Immunology. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 13.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annual Review of Immunology. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Lostao L, Briones J, Forne I, et al. Role of the STAT1 pathway in apoptosis induced by fludarabine and JAK kinase inhibitors in B-cell chronic lymphocytic leukemia. Leukemia & Lymphoma. 2005;46(3):435–442. doi: 10.1080/10428190400018398. [DOI] [PubMed] [Google Scholar]
- 15.Meraz MA, White JM, Sheehan KC, et al. Targeted disruption of the Stat1 gene in mice reveals unexpected physiologic specificity in the JAK-STAT signaling pathway. Cell. 1996;84(3):431–442. doi: 10.1016/s0092-8674(00)81288-x. [DOI] [PubMed] [Google Scholar]
- 16.Moilanen E, Whittle BJ, Moncada S. Nitric oxide as a factor of inflammation. In: Gallin JI, Snyderman R, editors. Inflammation: Basic Principles and Clinical Correlates. Philadelphia, Pa: Lippincott Williams & Wilkins; 1999. pp. 787–801. [Google Scholar]
- 17.Platanias LC. Mechanisms of type-I- and type-II-inter-feron-mediated signalling. Nature Reviews. Immunology. 2005;5(5):375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 18.Sanders SP. Nitric oxide in asthma. Pathogenic, therapeutic, or diagnostic? American Journal of Respiratory Cell and Molecular Biology. 1999;21(2):147–149. doi: 10.1165/ajrcmb.21.2.f158. [DOI] [PubMed] [Google Scholar]
- 19.Song HY, Jeon ES, Jung JS, Kim JH. Oncostatin M induces proliferation of human adipose tissue-derived mesenchymal stem cells. The International Journal of Biochemistry & Cell Biology. 2005;37(11):2357–2365. doi: 10.1016/j.biocel.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Sudbeck EA, Liu XP, Narla RK, et al. Structure-based design of specific inhibitors of Janus kinase 3 as apoptosis-inducing antileukemic agents. Clinical Cancer Research. 1999;5(6):1569–1582. [PubMed] [Google Scholar]
- 21.Tibbles HE, Vassilev A, Wendorf H, et al. Role of a JAK3-dependent biochemical signaling pathway in platelet activation and aggregation. The Journal of Biological Chemistry. 2001;276(21):17815–17822. doi: 10.1074/jbc.M011405200. [DOI] [PubMed] [Google Scholar]
- 22.Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nature Reviews. Drug Discovery. 2002;1(12):939–950. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- 23.van't Hof RJ, Ralston SH. Nitric oxide and bone. Immunology. 2001;103(3):255–261. doi: 10.1046/j.1365-2567.2001.01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie QW, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. The Journal of Biological Chemistry. 1994;269(7):4705–4708. [PubMed] [Google Scholar]