Abstract
Protection against Mycobacterium tuberculosis is based on cell-mediated immunity, most importantly involving CD4+ and CD8+ T-cell subsets. The aim of this study was to evaluate CD4+ and CD8+ T-cell profiles and CD19+ and CD3−CD(16+56)+ populations in patients with pulmonary tuberculosis. CD4+ and CD8+ T cells, B-lymphocytes, and natural killer (NK) cells were evaluated in 75 active (APTB) and 25 inactive (IPTB) pulmonary tuberculosis cases and 20 healthy subjects (HCs). The results were compared at different stages of antituberculosis treatment in the APTB patients and also according to X-ray findings in the newly diagnosed APTB patients. The percentages of CD4+ T cells were significantly lower (P < .01) and those of CD3−CD(16 + 56)+ cells were significantly higher (P < .01) in APTB patients than in HCs. CD8+ T cells were significantly decreased (P < .05), and CD3−CD(16+56)+ cells were significantly increased (P < .01), in IPTB patients compared to HCs. The percentages of CD4+, CD8+, CD3−CD19+, and CD3−CD(16+56)+ cells showed no differences at different times of the antituberculosis regimen, and different stages of newly diagnosed APTB patients. APTB patients have a reduced percentage of circulating CD4+ T cells and an increased percentage of NK cells compared with healthy individuals. These cells could play important roles in the immune response to M tuberculosis infection.
INTRODUCTION
The host immune response to Mycobacterium tuberculosis, especially cell-mediated immunity, is particularly important in preventing clinically evident disease following infection. CD4+ T cells have an essential role in this response but are supported by other T-cell subsets such as CD8+ [18]. Antigen-presenting cells express the costimulatory molecules necessary for activating T cells. How T cells help them to perform this task remains poorly understood. Primarily, CD4+ T cells are involved in amplifying immune responses by secreting various cytokines, but some CD4+ T cells also serve as cytotoxic effector cells capable of lysing target cells directly [1].
Although decreased CD4+ T cell counts and changes in CD8+ T cell counts are pivotal immune abnormalities in HIV patients, TB may be a cause of non-HIV associated CD4+ lymphopenia [2]. Despite the pivotal importance of CD4+ and CD8+ lymphocytes in antimicrobial immunity, very few researchers have studied changes in the peripheral blood counts of these cells in TB cases, and these few have obtained different results.
The aim of this study was to compare the baseline and posttreatment levels of the CD4+ and CD8+ T-cell subsets, and the CD3−CD19+ B-lymphocytes, and CD3−CD(16 + 56)+ NK cell counts, with the radiological extent of the disease in APTB patients and also in IPTB patients.
MATERIALS AND METHODS
Patients and study design
This study was performed on randomly selected 75 APTB patients (age: mean ± SD = 35.76 ± 15.8; 39 women, 36 men) who had been admitted to the Firat Medical Center and Elazig Tuberculosis Dispensary. APTBs were diagnosed on the basis of presence of recent clinical symptoms of tuberculosis, a positive sputum smear test for acid-fast bacilli confirmed by a positive culture of M tuberculosis and characteristics chest radiograph. No extrapulmonary involvement was detected in the patients. The exclusion criteria were HIV positivity, diabetes mellitus, pregnancy, and immunological or autoimmune diseases. Also, APTB patients were divided into three treatment groups as follows [1].
According to antituberculosis treatment (ATT)
Group 1. 25 newly diagnosed APTB patients. None of the cases had a priori history of TB or any relevant clinical or radiological findings.
Group 2. 25 patients who had undergone 2-month quadruplet antituberculosis treatment (Rifampin, Pyrazinamide, Isoniazid, and Streptomycin or Ethambutol).
Group 3. 25 patients who had been treated with antituberculosis drugs for 6 months.
In addition, Group 1 patients indicated above were divided into three stages according to the extent and type of X-ray findings as follows [3].
According to the X-ray findings
Stage 1 (mild cases, n = 11). Involving a single lobe without visible cavities.
Stage 2 (moderate cases, n = 8). Unilateral involvement of two or more lobes with cavities, if present, reaching a total diameter no more than 4 cm.
Stage 3 (advanced cases, n = 6). Bilateral disease with massive involvement and multiple cavities.
Twenty five inactive (ages 40.23 ± 20.5, 9 women, 16 men) pulmonary TB (IPTB) cases who had been registered and treated in the Elazig Tuberculosis Dispensary were examined, and 20 healthy volunteer subjects (ages 38.20 ± 17.6, 9 women, 11 men) were included as a control group (HC). The IPTB patients had had a previous episode of TB, with positive cultures documented at the time of diagnosis. There were abnormal but stable radiographic findings, and no changes had been observed in previous six months. At least three sputum cultures for M tuberculosis had been negative in all these patients [4]. The healthy subjects had no abnormal X-ray findings or history of TB and no other underlying disease, and they were not currently taking any medication.
Smears were pretreated with N-acetylcysteine and 4% sodium hydroxide. The samples were cultured in Lowenstein-Jensen medium and were examined weekly until growth was detected. Cultures were reported as negative if there was no growth after 8 weeks. Cultures with visible growth were processed for biochemical identification of species by standard methods.
Tuberculin was identified in the patients by the cutaneous Mantoux test: 5 IU PPD (BB-NCIPD Ltd, Sophia, Bulgaria) was injected intradermally into the volar surface of the forearm. Specifically trained nurses read the PPD reactivity, 72 hours after application, by measuring the transverse axis of induration (not erythema) with a flexible ruler. The exact number of millimeters was recorded. A transverse induration of ≥ 10 mm was considered a positive response. All APTB and IPTB patients were positive for the PPD skin test. Healthy subjects had been vaccinated with BCG as part of the obligatory Turkey National Vaccination Program and all were tuberculin positive.
Before the study, informed consent was obtained from all participants.
Flow cytometric analysis of peripheral blood
Peripheral venous blood samples (5 mL) were collected into tubes with EDTA from all the individuals diagnosed with tuberculosis and controls and were subjected to flow cytometry within 2 hours. Absolute lymphocyte counts (ALCs) were analyzed using a Coulter Max-M (Beckman Coulter, Fullerton, Calif, USA). Lymphocyte subsets analyses were performed using a Coulter EPICS XL-MCL (Beckman Coulter, Fullerton, Calif, USA). The results were counted on the same equipment using an Expo-32 analysis programmer and Immunotech (Margeille, France). Monoclonal antibodies against the following markers were deployed according to the manufacturer's instructions: CD14/CD45, CD3/CD4, CD3/CD8, CD3/CD19, and CD3/CD(16+56), and an isotype control. A total of 10000 cells were counted, and the results were interpreted using isotypic controls after performing the voltage tunings required. Each lymphocyte subpopulation count was expressed as a percentage of the total of CD3+ lymphocytes. Criteria of quality included a greater than 95% total lymphocyte frequency in the analysis gate and a homogenous CD45+ population.
Statistical analysis
Results are expressed as arithmetic means ± standard deviations. Statistical analysis was performed using Kruskal-Wallis test for multiple-group comparisons; Mann-Whitney U test was performed to test any observed differences for significance. Also, correlation analysis was performed with Spearman's test. All statistical analysis were performed by using SPSS version 12.00. Statistical significance was interfered at a P value < 0.05.
RESULTS
There were no statistically significant differences in age or gender among the subject groups. The mean PPD response was statistically higher in APTB (15.35 ± 2.78, range 11–24 mm) and IPTB (13.92 ± 2.25, range 10–17 mm) patients than in HC (11.80 ± 1.64, range 10–16 mm), (p = 0.000 for APTB and p = 0.007 for IPTB compared with HCs). There were no correlations between PPD and the T lymphocyte subset counts or the percentages of CD3−CD19+ or CD3−CD(16 + 56)+ in APTB or IPTB patients or controls (P > .05 for all groups).
The percentages (mean% ± SD) of serum total CD3+ lymphocytes (TL%), ALC, the percentages of T-cell subsets, B cells, and NK cells are given in Table 1.
Table 1.
The mean percentages of total lymphocytes, B-lymphocytes, T-lymphocyte subsets, CD3−CD(16 + 56)+, and absolute lymphocyte counts in the study groups.
| APTB (n = 75) | IPTB (n = 25) | HC (n = 20) | Statistics | |
| Total lymphocytes (%) | 25.70 ± 10.20a | 37.79 ± 7.23b | 30.13 ± 7.64 | P < .001a, P < .01b |
| ALC (cell/mm3) | 1407.6 ± 206.5a | 2136.1 ± 488.1b | 1528.1 ± 243.1 | P < .001a, P < .001b |
| CD3+CD4+(%) | 36.52 ± 8.66b | 39.28 ± 8.17 | 43.36 ± 6.01 | P < .01b |
| CD3+CD8+(%) | 26.30 ± 8.40 | 22.13 ± 5.75b | 27.16 ± 7.01 | P < .05b |
| CD3−CD19+(%) | 12.51 ± 6.37 | 14.13 ± 4.10 | 11.86 ± 3.26 | NS |
| CD3−CD(16 + 56)+(%) | 15.86 ± 7.76b | 17.36 ± 7.48b | 11.03 ± 6.71 | P < .01b |
| CD4+/CD8+ ratio | 1.57 ± 0.75 | 1.88 ± 0.64 | 1.69 ± 0.46 | NS |
ALC: absolute lymphocyte counts, acompared to IPTB, bcompared to HC.
The percentages of total CD3+ lymphocytes and ALC were lower significantly in APTB than IPTB patients (P < .001 for both) and these values significantly higher in IPTB patients than in HC (P < .01 for TL%, P < 0.001 for ALC). APTB patients had lower CD3+CD4+ (P < 0.01) and higher CD3−CD(16 + 56)+ (P < 0.01) levels than HC but similar CD3+CD8+ subset proportions. There were no statistically significant differences among the groups in the percentages of CD3−CD19+. IPTB patients had higher CD3−CD(16 + 56)+ (P < .01) and lower CD3+CD8+ levels (P < .05) than HCs.
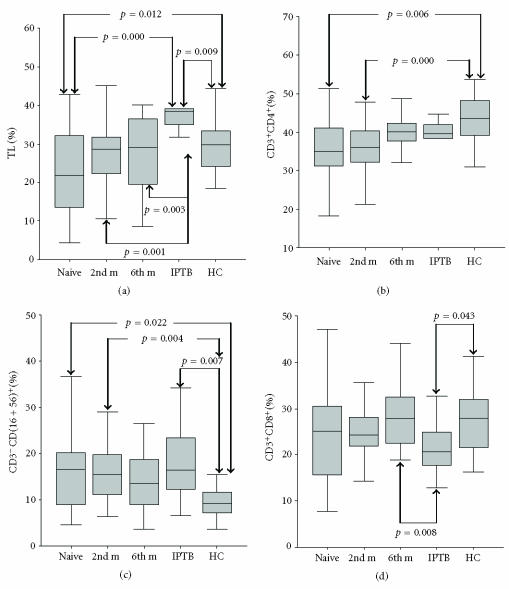
Group 1 APTB patients had lower TL% (p = 0.012) and ALC (p = 0.017) than HCs. Groups 1 and 2 APTB patients had lower CD3+CD4+ (p = 0.006 for Group 1, p = 0.000 for Group 2) and higher proportions of CD3−CD(16+56)+ (p = 0.022 for Group 1, p = 0.004 for Group 2) than HCs. However, there were no statistically significant differences in TL%, ALC, percentages of CD3+CD4+, or CD3−CD(16 + 56)+ among the three APTB groups, although there was a trend toward a higher level of TL%, ALC, and levels of CD3+CD4+ and lower levels of CD3−CD(16 + 56)+ in Groups 2 and 3. No statistically significant differences in these parameters were observed between APTB Group 3 and HCs (P > .05). The percentages of CD3+CD8+ and the CD3−CD19+ ratios in APTB Groups 1, 2, and 3 were similar to those in HCs (Figure 1).
Figure 1.
Percentages of total lymphocytes, CD3+CD4+, CD3−CD(16 + 56)+ and CD3+CD8+ lymphocytes in the study groups. (a) Group 1 APTB patients versus HC: p = 0.012; Group 1 APTB patients versus IPTB patients: p = 0.000; IPTB patients versus HC: p = 0.009, Group 2 APTB patients versus IPTB patients: p = 0.001; Group 3 APTB patients versus IPTB patients: p = 0.003, (b) Group 1 APTB patients versus HC: p = 0.006, Group 2 APTB patients versus HCs: p = 0.000, (c) Group 1 APTB patients versus HC: p = 0.022; IPTB patients versus HC: p = 0.007, Group 2 APTB patients versus HCs: p = 0.004, (d) IPTB patients versus HC: p = 0.043; Group 3 APTB patients versus IPTB patients: p = 0.008.
APTB Groups 1, 2, and 3 had fewer TL% (p = 0.000 for Group 1, p = 0.001 for Group 2, p = 0.003 for Group 3) and ALC (p = 0.000 for all) than ITBP. ITBP had more TL% (p = 0.009), ALC (p = 0.000), and CD3−CD(16 + 56)+ (p = 0.007) than HCs, and ITBP had lower CD3+CD8+ than HCs (p = 0.043) and Group 3 ATBP (p = 0.008) (Figure 1).
When the lymphocyte counts were compared to the X-ray findings, there were no significant differences among Stages 1 to 3 of Group 1 APTB patients in percentages of CD3+CD4+, CD3+CD8+, CD3−CD19+, or CD3−CD(16 + 56)+ (P > .05).
DISCUSSION
The major finding of this study was a decrease in CD3+CD4+ and increase in CD3−CD(16 + 56)+ in APTB, and in Group 1 of APTB, compared to HC. There were no significant differences in the percentages of CD3+CD4+ and CD3−CD(16 + 56)+ between HC and patients who had received six months of antituberculosis therapy. The percentages of total lymphocytes were lower in APTB patients than in IPTB patients or HCs. Similarly, the study by Montes Santiago et al [5] indicated a progressive lymphopenia, especially in patients with more extensive pulmonary TB. Another study suggested that the severity of TB disease is associated with greater depression of the total lymphocyte and CD4 cell counts [6].
T-lymphocyte subsets, particularly CD4+, play a crucial role in immunity against mycobacterium infections [7, 8]. In general, very few researchers have studied the changes in peripheral blood counts of T-cell subsets in TB cases, and even these few have obtained different results. In some studies, the percentages of CD4 cells were decreased while the percentages of CD8 cells were unchanged compared with controls [9–12]. However, another studies showed, especially in advanced or disseminated TB, that CD4+ T cells are reduced while CD8+ T cells are increased [6, 13]. In contrast, there are reports of unchanged proportions of CD4 cells in the peripheral blood of TB patients compared with controls. Shijubo et al [14] found only CD8+ T cells to be significantly decreased; the other T-cell subsets were normal. A study performed in our country showed no significant differences in CD4+ or CD8+ T cells between nonresistant TB patients and healthy controls [15]. In our study, the APTB patients had a significantly lower percentage of CD4+ T cells in the peripheral blood than HC. This finding accord with the results obtained from previous studies that suggest a decreased CD4+ lymphocyte percentage in the peripheral blood of TB patients [2, 9, 16]. In addition, in the present study, no significant differences were observed in the percentages of CD4+ T cell according to severity (extent and type of X-ray findings) of TB disease. This could be interpreted as indicating that CD4+ cells are critical at all stages of M tuberculosis infection.
We found that the percentages of CD3+CD8+ in APTB patients were similar to those in HCs the same as previous studies. [15, 17]. The role of CD8+ T cells in human immune responses to M tuberculosis, and their relationship to other T-cell subsets activated by M tuberculosis, is not well defined. For humans, it is not known at what stages of M tuberculosis infection CD8+ T cells are most critical [18].
In the present study, the percentages of CD3−CD19+ were similar in APTB patients and HCs. There are only a few studies explaining the role of B-lymphocytes in TB, and these have given different results. The role of B cells during M tuberculosis infection is not clear. Previous studies suggest little contribution from B cells to the control or exacerbation of M tuberculosis infections in mice during chronic phases of infection [19, 20]. Other studies have shown no difference in B-lymphocyte counts between pulmonary TB and control groups [5, 14]. In contrast to studies dealing with pulmonary TB patients and tuberculin skin-test-positive controls, Coraminas et al found a decrease in B cells. They interpreted this as indicating that B-cell counts are the best discriminating factor for infection, and that B-lymphocytes have an important role in immunity against tuberculosis [21].
The role of NK cells in the host defense against M tuberculosis remains unexplored. Some studies suggest that NK cells have a role in defense against mycobacterial infection especially in early resistance [22–25], and both the percentage of NK cells and the NK activity were increased in APTB patients [26, 27]. In our study, CD3−CD(16 + 56)+ was found to be increased in both APTB and IPTB patients compared to HCs. Thus, NK cells may have an important role in immunity against TB.
What are the effects of antituberculosis therapy on the decreased CD4+ and increased CD3−CD(16 + 56)+ cell counts in these patients? Although there was a trend toward higher levels of CD4+ and lower levels of CD3−CD(16 + 56)+ in APTB Groups 2 and 3, we found no significant differences among the APTB groups. When patients who had been treated for 6 months were compared with HCs, we observed no statistically significant differences in these cell populations. Previous studies have shown that the decreased CD4+ T-cell counts returned to normal levels after therapy [6, 13, 15, 28]. In patients with pulmonary TB, decreased CD4 and increased CD8 cell counts and near-normal levels of CD4/CD8 have been reported after successful chemotherapy [15]. Other studies showed no changes in the number of NK cells, but TB patients had a decreased NK activity, which increased after treatment [25, 29].
We found that APTB and IPTB patients differed only in TL% and ALC. Morikawa et al [30] demonstrated that APTB patients had higher NK activity than IPTB patients. A significantly greater percentage of BAL CD8+ cells have been reported in APTB compared with IPTB patients [31].
In the present study, we found no differences in the NK cells between patients treated for 6 months and HC, but the NK cell counts were higher in IPTB than HCs. Although no differences were observed between APTB and IPTB in CD8+T cells, IPTB had lower levels of CD8+T cells than HCs. Further studies will be needed to explain why lower levels of CD8+T cells were detected in IPTB.
How does the radiological extent of the disease influence the immune parameters? We found no differences among the different stages of APTB in any parameter. Other studies have also shown that lymphocyte subset numbers bear no relation to the radiographic scores [16, 17, 32]. Qin [27] found that NK activity increased as the TB lesion progressed, as judged from X-ray pictures; but the T-lymphocyte subsets were unrelated to the severity or extent of the lesion as judged radiologically.
CONCLUSIONS
Low percentages of CD4+ T cells and high percentages of NK cells in APTB, and high NK cells and low CD8+ T cells in IPTB, show that CD4+ and NK cells have an important role in the control of active disease and NK, and CD8+ T cells may have a role in the inactive disease. The low percentages of CD4+ T cells and high percentages of NK cells in APTB patients would be potentially reversible by antituberculosis treatment. In addition measuring of these parameters may be useful for monitoring the clinical effect of antituberculosis treatment. However, in this study, we studied different patients and different time periods of treatment. Thus, it can be more useful to evaluate the lymphocytes subsets on the same patients longitudinally.
References
- 1.Talreja J, Bhatnagar A, Jindal SK, Ganguly NK. Influence of Mycobacterium tuberculosis on differential activation of helper T-cells. Clinical and Experimental Immunology. 2003;131(2):292–298. doi: 10.1046/j.1365-2249.2003.02072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pilheu JA, De Salvo MC, Gonzalez J, Rey D, Elias MC, Ruppi MC. CD4+ T-lymphocytopenia in severe pulmonary tuberculosis without evidence of human immunodeficiency virus infection. The International Journal of Tuberculosis and Lung Disease. 1997;1(5):422–426. [PubMed] [Google Scholar]
- 3.Dlugovitzky D, Torres-Morales A, Rateni L, et al. Circulating profile of Th1 and Th2 cytokines in tuberculosis patients with different degrees of pulmonary involvement. FEMS Immunology and Medical Microbiology. 1997;18(3):203–207. doi: 10.1111/j.1574-695X.1997.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society . Diagnostic standard and classification of tuberculosis. The American Review of Respiratory Disease. 1990;142:725–735. doi: 10.1164/ajrccm/142.3.725. [DOI] [PubMed] [Google Scholar]
- 5.Montes Santiago J, Gambon Deza F, Pacheco Carracedo M, Cerda Mota T. Cellular immune response in tuberculosis: analysis of T-lymphocytes and their subsets, B-lymphocytes and natural cytotoxic cells in different tuberculosis states and body fluids. Revista Clinica Espanola. 1996;196(4):223–227. [PubMed] [Google Scholar]
- 6.Jones BE, Oo MM, Taikwel EK, et al. CD4 cell counts in human immunodeficiency virus-negative patients with tuberculosis. Clinical Infectious Diseases. 1997;24(5):988–991. doi: 10.1093/clinids/24.5.988. [DOI] [PubMed] [Google Scholar]
- 7.Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. The Journal of Infectious Diseases. 1993;167(6):1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 8.Yu CT, Wang CH, Huang TJ, Lin HC, Kuo HP. Relation of bronchoalveolar lavage T lymphocyte subpopulations to rate of regression of active pulmonary tuberculosis. Thorax. 1995;50(8):869–874. doi: 10.1136/thx.50.8.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho AC, Matteelli A, Airo P, et al. gamma/delta T lymphocytes in the peripheral blood of patients with tuberculosis with and without HIV co-infection. Thorax. 2002;57(4):357–360. doi: 10.1136/thorax.57.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Onwubalili JK, Edwards AJ, Palmer L. T4 lymphopenia in human tuberculosis. Tubercle. 1987;68(3):195–200. doi: 10.1016/0041-3879(87)90055-9. [DOI] [PubMed] [Google Scholar]
- 11.Ainslie GM, Solomon JA, Bateman ED. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992;47(7):513–518. doi: 10.1136/thx.47.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashtekar MD, Samuel AM, Kadival GV, Sakhalkar V, Rajadhyaksha S, Virdi SS. T lymphocytes in pulmonary tuberculosis. The Indian Journal of Medical Research. 1993;97:14–17. [PubMed] [Google Scholar]
- 13.Singhal M, Banavalikar JN, Sharma S, Saha K. Peripheral blood T lymphocyte subpopulations in patients with tuberculosis and the effect of chemotherapy. Tubercle. 1989;70(3):171–178. doi: 10.1016/0041-3879(89)90047-0. [DOI] [PubMed] [Google Scholar]
- 14.Shijubo N, Nakanishi F, Hirasawa M, et al. Phenotypic analysis in peripheral blood lymphocytes of patients with pulmonary tuberculosis [in Japanese] Kekkaku. 1992;67(9):581–585. [PubMed] [Google Scholar]
- 15.Yildiz P, Kadakal F, Tutuncu Y, et al. Natural killer cell activity in multidrug-resistant pulmonary tuberculosis. Respiration. 2001;68(6):590–594. doi: 10.1159/000050577. [DOI] [PubMed] [Google Scholar]
- 16.Turett GS, Telzak EE. Normalization of CD4+ T-lymphocyte depletion in patients without HIV infection treated for tuberculosis. Chest. 1994;105(5):1335–1337. doi: 10.1378/chest.105.5.1335. [DOI] [PubMed] [Google Scholar]
- 17.Uppal SS, Tewari SC, Verma S, Dhot PS. Comparison of CD4 and CD8 lymphocyte counts in HIV-negative pulmonary TB patients with those in normal blood donors and the effect of antitubercular treatment: hospital-based flow cytometric study. Cytometry. Part B, Clinical Cytometry. 2004;61(1):20–26. doi: 10.1002/cyto.b.20018. [DOI] [PubMed] [Google Scholar]
- 18.Boom WH, Canaday DH, Fulton SA, Gehring AJ, Rojas RE, Torres M. Human immunity to M. tuberculosis: T cell subsets and antigen processing. Tuberculosis (Edinburgh) 2003;83(1–3):98–106. doi: 10.1016/s1472-9792(02)00054-9. [DOI] [PubMed] [Google Scholar]
- 19.Vordermeier HM, Venkataprasad N, Harris DP, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clinical and Experimental Immunology. 1996;106(2):312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson CM, Cooper AM, Frank AA, Bonorino CB, Wysoki LJ, Orme IM. Mycobacterium tuberculosis aerogenic rechallenge infections in B cell-deficient mice. Tubercle and Lung Disease. 1997;78(5-6):257–261. doi: 10.1016/s0962-8479(97)90006-x. [DOI] [PubMed] [Google Scholar]
- 21.Corominas M, Cardona V, Gonzalez L, et al. B-lymphocytes and co-stimulatory molecules in Mycobacterium tuberculosis infection. The International Journal of Tuberculosis and Lung Disease. 2004;8(1):98–105. [PubMed] [Google Scholar]
- 22.Bermudez LE, Young LS. Natural killer cell-dependent mycobacteriostatic and mycobactericidal activity in human macrophages. Journal of Immunology. 1991;146(1):265–270. [PubMed] [Google Scholar]
- 23.Harshan KV, Gangadharam PR. In vivo depletion of natural killer cell activity leads to enhanced multiplication of Mycobacterium avium complex in mice. Infection and Immunity. 1991;59(8):2818–2821. doi: 10.1128/iai.59.8.2818-2821.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junqueira-Kipnis AP, Kipnis A, Jamieson A, et al. NK cells respond to pulmonary infection with Mycobacterium tuberculosis, but play a minimal role in protection. Journal of Immunology. 2003;171(11):6039–6045. doi: 10.4049/jimmunol.171.11.6039. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda T, Ellner JJ. CD4+ T cell and natural killer celldependent killing of Mycobacterium tuberculosis by human monocytes. American Journal of Respiratory and Critical Care Medicine. 1998;158(2):395–403. doi: 10.1164/ajrccm.158.2.9707102. [DOI] [PubMed] [Google Scholar]
- 26.Yoneda T, Kasai M, Ishibashi J, Nishikawa K, Tokunaga T, Mikami R. NK cell activity in pulmonary tuberculosis. British Journal of Diseases of the Chest. 1983;77(2):185–188. doi: 10.1016/0007-0971(83)90026-8. [DOI] [PubMed] [Google Scholar]
- 27.Qin J. NK cell activity and counts of T-lymphocyte subsets in pulmonary tuberculosis [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 1990;13(4):209–211. [PubMed] [Google Scholar]
- 28.Saenghirunvattana S. CD4 + T counts with a course of antituberculous therapy in healthy and HIV-infected patients. Journal of the Medical Association of Thailand. 1996;79(4):246–248. [PubMed] [Google Scholar]
- 29.Nirmala R, Narayanan PR, Mathew R, Maran M, Deivanayagam CN. Reduced NK activity in pulmonary tuberculosis patients with/without HIV infection: identifying the defective stage and studying the effect of interleukins on NK activity. Tuberculosis (Edinburgh) 2001;81(5-6):343–352. doi: 10.1054/tube.2001.0309. [DOI] [PubMed] [Google Scholar]
- 30.Morikawa F, Nakano A, Nakano H, Oseko F, Morikawa S. Enhanced natural killer cell activity in patients with pulmonary tuberculosis. Japanese Journal of Medicine. 1989;28(3):316–322. doi: 10.2169/internalmedicine1962.28.316. [DOI] [PubMed] [Google Scholar]
- 31.Taha RA, Kotsimbos TC, Song YL, Menzies D, Hamid Q. IFN-gamma and IL-12 are increased in active compared with inactive tuberculosis. American Journal of Respiratory and Critical Care Medicine. 1997;155(3):1135–1139. doi: 10.1164/ajrccm.155.3.9116999. [DOI] [PubMed] [Google Scholar]
- 32.Lyadova IV, Eruslanov EB, Khaidukov SV, et al. Comparative analysis of T lymphocytes recovered from the lungs of mice genetically susceptible, resistant, and hyperresistant to Mycobacterium tuberculosis-triggered disease. Journal of Immunology. 2000;165(10):5921–5931. doi: 10.4049/jimmunol.165.10.5921. [DOI] [PubMed] [Google Scholar]