Abstract
The aim of our study is to determine whether there is a relationship between familial Mediterranean fever (FMF) attacks and serum leptin levels. We enrolled 25 patients (22 males and 3 females) and 25 healthy controls (21 males and 4 females) with a mean age of 24.42 ± 1.22 (Mean ± SEM) years and 24.30 ± 1.19 years (Mean ± SEM), respectively. We investigated serum levels of leptin, interleukin-6 (IL-6), erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fibrinogen, and leukocyte counts before the attack and 8–12 hours after the attack started. The same parameters have been investigated in the control subjects. The mean serum leptin levels before the attacks were 6.45 ± 1.05 (Mean ± SEM) and during the attacks were 7.59 ± 1.3 (Mean ± SEM) in FMF group, respectively. There was a slight increase in serum leptin levels during the attacks but it was not statistically significant (P > .05). The mean serum leptin levels were 6.12 ± 2.81 in the control group which were not different from the mean serum leptin levels before and during the attack periods in the study group (P > .05). However, there were statistical differences in the serum levels of IL-6, ESR, CRP, fibrinogen, and leukocyte counts before and during the attack periods (P < .05). No correlation was found between serum leptin levels and IL-6, ESR, CRP, fibrinogen, and leukocyte counts (P > .05). Serum leptin levels do not increase during FMF attacks and therefore it is not useful for diagnostic purposes and follow-up during treatment.
INTRODUCTION
Familial Mediterranean fever (FMF) has been characterized by fever and recurrent aseptic inflammation of serosal spaces with a typical familial tendency [1]. A lot of metabolic and immunologic changes happen to occur in these patients during the attack period. Acute phase reactants, such as erythrocyte sedimentation rate (ESR), fibrinogen, and C-reactive protein (CRP) [2, 3], and pro-inflammatory cytokines, such as interleukin-6 (IL-6), have been found to be elevated during the attacks [4, 5].
The close relationship between IL-6 and FMF attacks is without any debate, that serum IL-6 shows meaningful increases during these attacks [1, 5, 6] and colchicum treatment turns IL-6 levels down to normal values [4].
Leptin is secreted from adipocytes, and after interaction with its receptors, it controls body weight and daily energy consumption [7, 8]. Increasingly, data show that leptin has some important role in the regulation of acute phase reaction [9]. Also it is such an immunoregulatory substance which belongs to class-1 cytokine family [10]. Indeed, leptin structurally looks like granulocyte colony stimulating factor which is a member of IL-6 cytokine family [11]. Adipocytes secrete leptin [12] and even IL-6 [13] in acute inflammatory conditions. During this acute inflammatory response, serum leptin levels have been shown to increase and possibly contribute to anorexia and cachexia that accompany chronic infections [14]. Interestingly, very low calorie diet in obese subjects leads to mutual decrease in blood IL-6 and leptin levels [15]. Moreover, in septic subjects, serum IL-6 and leptin levels correlate very well [14].
In this study, we plan to scrutinize whether or not leptin has any role in triggering and/or regulation of inflammatory response in FMF attacks. Accordingly, we investigated serum leptin levels before and during acute attacks of FMF.
MATERIALS AND METHODS
The study included 25 patients with FMF diagnosis according to Eliakim criteria [16]. The mean age was 24.42 ± 1.22 years (range: 20–52 years), and the gender distribution was 22 males and 3 females. Inclusion criteria were as follows: FMF attacks should have been started at least 1 year ago; absence of systemic diseases including chronic renal failure, diabetes mellitus, ischemic heart disease, malignancy, trauma, heavy exercise, and infectious and inflammatory disorders; and lack of any drug use including colchicine with potential effects on the biochemical and immunologic parameters during the last 4 weeks. The control group included 25 healthy people with a mean age 24.30 ± 1.19 (range: 21–43 years). The gender distribution was 21 males and 4 females.
Blood samples were taken before attack and 8–12 hours after the attack started in FMF cases. We obtained blood samples from the FMF patients and controls after 12 hours of fasting since serum IL-6 levels increase has been shown acutely stimulated by meals [17]. Moreover, concentration of circulating leptin is reduced in starvation [18, 19].
The total blood count was measured by STKS autoanalyzer (Hialeah, Finland). ESR was measured by Westergreen method on the same day of obtaining samples, and ESR within one hour was recorded. The rates of 0–15 mm/h for males and 0–20 mm/h for females were considered to be within normal ranges. CRP (Biosystems SA, Barcelona, Spain) levels were measured by turbidimetric method after assessing absorbance at BIS-310 photometer (Berlin, Germany), the levels equal to or below 6 mg/L were considered to be normal levels. Fibrinogen levels were measured by a photo-optical method using fibrinogen (Sigma Diagnostics) reagents in an Amelung AMAX 190 Plus device (Taufkirchen, Germany), and 200–400 mg/dL were considered to be normal values. Serum IL-6 values were measured by IMMUNOTECH enzyme immunoassay kit (Westbrook, Me) (Cat ≠ 1120, 96 tests), (normal < 8 pg/mL). Serum leptin analysis was performed by using Medizym Leptin ELISA kit (Medipan Diagnostica, GmbH, Selchow, Germany) in all patients and controls.
STATISTICS
The statistical analysis was made by using SPSS 11.0 (Chicago, Ill) in a personal computer. Groups were compared according to independent sample t test, and the results of serum leptin levels of presymptomatic and symptomatic periods were compared by using paired t test. Pearson correlation test was also used to make correlation analysis.
RESULTS
There was no statistical difference in respect to age distribution in between the controls and FMF study group. Out of 25 patients, 23 had fever and signs of aseptic peritonitis, 12 had pleural involvement, and 17 cases had joint inflammation. No case had dermatologic finding and/or amyloidal accumulation. Thirteen cases had positive family history. Five had previous history of appendectomy.
The mean serum leptin concentration in control group was between 1.81 and 9.64 ng/mL (Mean ± SEM: 4.282 ± 0.385). In FMF group, it was 6.4528 ± 1.05 (range: 0.32–20.55 ng/mL) and 7.5928 ± 1.3 (range: 0.2–20.40 ng/mL) before and during FMF attacks, respectively. The mean serum leptin levels in the study group before and during the attacks did not differ significantly from that of the control group (P > .05). Although serum leptin levels increased to some extent during the attacks, this was not significant statistically (P > .05) (Figure 1(a)). However, serum leukocyte counts, sedimentation rate, and C-reactive protein and IL-6 levels were found to increase significantly during the FMF attacks (P < .05) (Figure 1(b), Figure 2, Table 1). There was an apparent correlation between the body mass index and serum leptin levels (r = 0.433, P < .05) before attacks Figure 3. We could not find a correlation between serum leptin levels during attacks (r = 0.326, P > .05). BMI does not correlate with other parameters. There was no correlation comparing serum leptin levels with the leukocyte counts, sedimentation rate, fibrinogen, CRP levels and serum IL-6 (P > .05). IL-6 did not correlate with fibrinogen (P > .05) levels.
Figure 1.
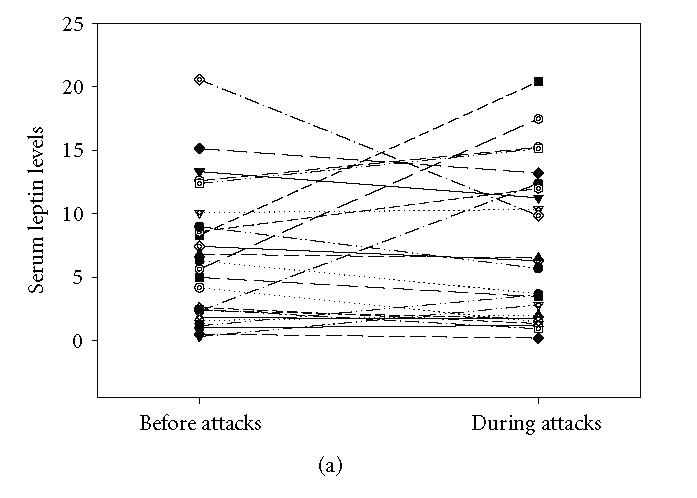
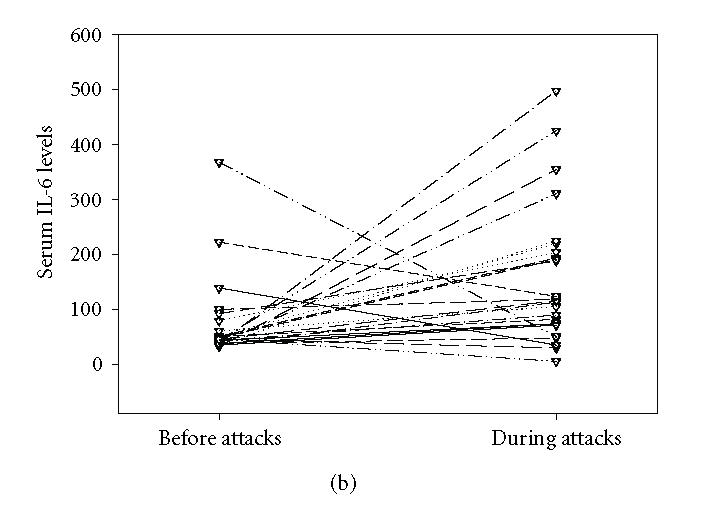
(a) Leptin levels before (Mean ± SEM: 6.4528 ± 1.05 ng/mL) and during (Mean ± SEM: 7.5928 ± 1.3 ng/mL) the attacks in FMF patients. Serum leptin levels did not increase significantly during the attacks (P > .05). (b) Serum IL-6 levels before (Mean ± SEM: 70.18 ± 14.8 pg/mL) and during (Mean ± SEM: 163.32 ± 25.25 pg/mL) attacks. IL-6 levels increased significantly during the attacks (P < .001).
Figure 2.
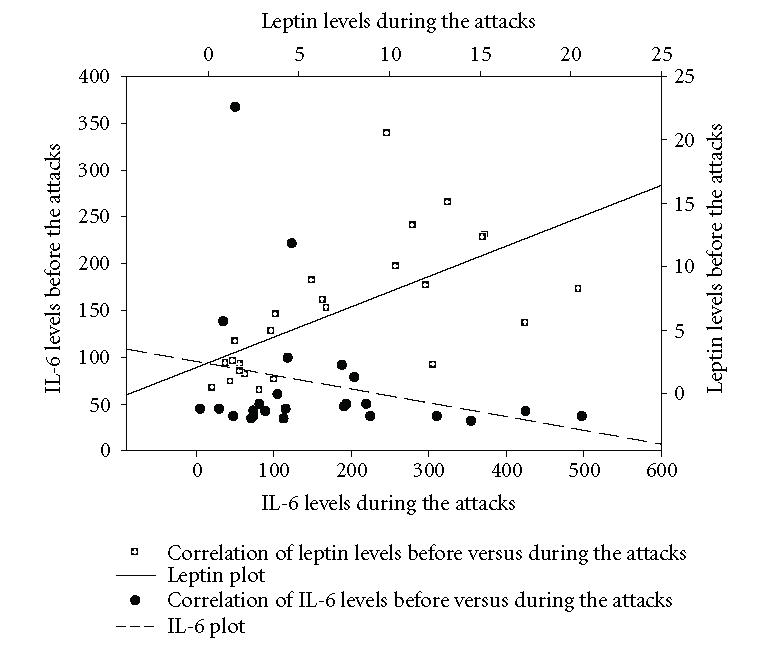
Correlation plot of leptin before and during FMF attacks together with IL-6 plot. Although serum leptin levels increased to some extent during the attacks, it was not statistical significant (P > .05). Serum IL-6 levels increased from 70.18 ± 14.8 (Mean ± SEM) to 163.32 ± 25.25 (Mean ± SEM) and they were statistically significant (P < .001).
Table 1.
Laboratory test before and during FMF attacks.
| Leukocyte (mm3) | Sedimentation (mm/h) | CRP (mg/L) | Fibrinogen (mg/L) | |
| Before the attacks: Mean ± SEM | 7244 ± 813.81 | 17.56 ± 2.23 | 18.28 ± 2.61 | 399.52 ± 16.38 |
| During the attacks: Mean ± SEM | 12220 ± 785 | 30.64 ± 2.86 | 26.88 ± 2.94 | 532.16 ± 24.6 |
| P value | < .01 | < .01 | < .01 | < .01 |
Figure 3.
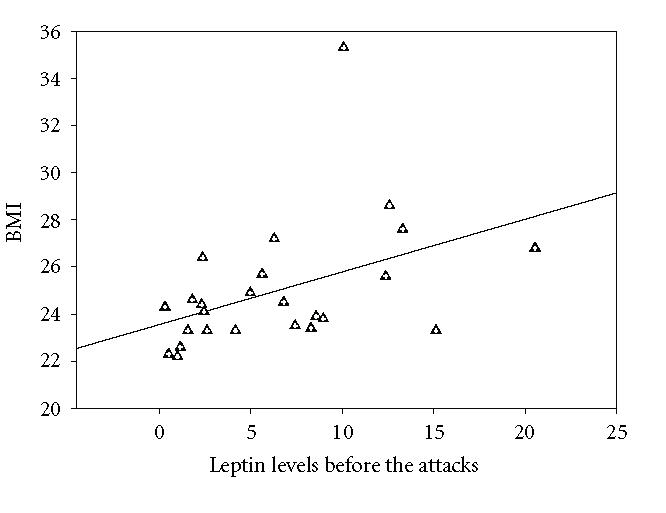
Correlation graph between BMI and preattack leptin levels in FMF patient (r = 0.433, P < .05).
DISCUSSION
Our aim was to define the role of leptin and find out if there is any relationship between serum leptin and IL-6 levels in a typical systemic inflammatory condition like FMF. Leptin has been already accepted as closely connected with the cytokine network. Though, we found that serum leptin levels increase during FMF attacks, this increase was statistically not significant (P > .05). However, serum leukocyte counts, fibrinogen, erythrocyte sedimentation rate, and C-reactive protein levels were shown to increase significantly as expected. Additionally, we could not find a correlation between serum IL-6 and leptin levels.
Acute inflammation can lead to elevated mouse serum leptin levels and interestingly serum leptin levels in mouse has a trend to increase even more after administration of IL-6 [20]. Leptin levels are acutely increased by many acute phase factors, such as TNF, IL-1, and IL-6, and during bacterial infection or lipopolysaccharide challenge [11]. Moreover, leptin itself was shown directly to induce the production of interleukin-6 and other acute phase reactants [21]. Leptin directly stimulates the production of interleukin-6 [22]. Additionally, leptin might stimulate acute phase protein production through the IL-6 dependent intracellular signaling pathway [23]. Moreover, there are some studies showing a correlation between leptin and IL-6. One of them indicated that there are clear correlations between the serum levels of IL-6 and leptin (r = 0.45, P < .05) TNF-alpha and leptin (r = 0.49, P < .05) in patients with intra-abdominal sepsis during postoperative period [14]. Similarly, in diabetic and nondiabetic obese persons, there were significant correlations between leptin and IL-6 and leptin and BMI (r = 0.841, P < .05). After weight loss, serum leptin and IL-6 concentrations decreased significantly in the same patient groups [15].
We measured fibrinogen to discriminate the attack and attack-free periods of the FMF patients [5, 24]. In the present study, we could not detect any correlation between fibrinogen and leptin and fibrinogen and IL-6 (P > .05).
On this background, it is very logical to consider leptin as a participant in the inflammatory process associated with FMF. However, our study could not indicate statistically significant increase in serum leptin levels during the acute attack periods. Furthermore, in the present study, serum leptin levels before FMF attacks were found to correlate well with the body mass indexes like some studies [25–27]. However, there were no statistically significant correlations between the body mass indexes and serum leptin levels during the attacks. A completely similar finding has been reported in septic patients in whom no correlation was found between these 2 parameters [14]. This may be related to some unknown complicated regulatory pathways for serum leptin levels during FMF attacks. Indeed, the activation of cytokine network in FMF is not based extensively on IL-6 and the interaction of the cytokine network is rather complex. Similarly, a relevant literature indicates no correlation between disease activity and serum leptin levels in cases with romatoid arthritis [27, 28].
As a conclusion, serum leptin levels do not increase statistically significantly in FMF attacks and therefore it is not useful for diagnostic purposes and/or follow-up during treatment.
ACKNOWLEDGMENT
We thank Dr Charles Chaya (Division of Gastroenterology, UTMB, Tex) for his excellent advice.
References
- 1.Gang N, Drenth JP, Langevitz P, et al. Activation of the cytokine network in familial Mediterranean fever. The Journal of Rheumatology. 1999;26(4):890–897. [PubMed] [Google Scholar]
- 2.Sohar E, Gafni J, Pras M, Heller H. Familial Mediterranean fever. A survey of 470 cases and review of the literature. The American Journal of Medicine. 1967;43(2):227–253. doi: 10.1016/0002-9343(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 3.Touitou I, Ben-Chetrit E, Notarnicola C, et al. Familial Mediterranean fever clinical and genetic features in Druzes and in Iraqi Jews: a preliminary study. The Journal of Rheumatology. 1998;25(5):916–919. [PubMed] [Google Scholar]
- 4.Kiraz S, Ertenli I, Arici M, et al. Effects of colchicine on inflammatory cytokines and selectins in familial Mediterranean fever. Clinical and Experimental Rheumatology. 1998;16(6):721–724. [PubMed] [Google Scholar]
- 5.Baykal Y, Saglam K, Yilmaz MI, Taslipinar A, Akinci SB, Inal A. Serum sIL-2r, IL-6, IL-10 and TNF-alpha level in familial Mediterranean fever patients. Clinical Rheumatology. 2003;22(2):99–101. doi: 10.1007/s10067-002-0682-1. [DOI] [PubMed] [Google Scholar]
- 6.Notarnicola C, Didelot MN, Seguret F, Demaille J, Touitou I. Enhanced cytokine mRNA levels in attack-free patients with familial Mediterranean fever. Genes & Immunity. 2002;3(1):43–45. doi: 10.1038/sj.gene.6363813. [DOI] [PubMed] [Google Scholar]
- 7.Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- 8.Halaas JL, Gajiwala KS, Maffei M, et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- 9.Barbier M, Cherbut C, Aubé AC, Blottière HM, Galmiche JP. Elevated plasma leptin concentrations in early stages of experimental intestinal inflammation in rats. Gut. 1998;43(6):783–790. doi: 10.1136/gut.43.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 11.Faggioni R, Feingold KR, Grunfeld C. Leptin regulation of the immune response and the immunodeficiency of malnutrition. The FASEB Journal. 2001;15(14):2565–2571. doi: 10.1096/fj.01-0431rev. [DOI] [PubMed] [Google Scholar]
- 12.Caro JF, Sinha MK, Kolaczynski JW, Zhang PL, Considine RV. Leptin: the tale of an obesity gene. Diabetes. 1996;45(11):1455–1462. doi: 10.2337/diab.45.11.1455. [DOI] [PubMed] [Google Scholar]
- 13.Mohamed-Ali V, Goodrick S, Rawesh A, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. The Journal of Clinical Endocrinology & Metabolism. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 14.Maruna P, Gürlich R, Fraško R, Haluzík M. Serum leptin levels in septic men correlate well with C-reactive protein (CRP) and TNF-alpha but not with BMI. Physiological Research. 2001;50(6):589–594. [PubMed] [Google Scholar]
- 15.Bastard J-P, Jardel C, Bruckert E, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. The Journal of Clinical Endocrinology & Metabolism. 2000;85(9):3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- 16.Eliakim M, Levy M, Ehrenfeld M. Recurrent Polyserositis: Familial Mediterranean Fever, Periodic Disease. New York, NY/Amsterdam, The Netherlands: Elsevier/North-Holland Biomedical Press; 1981. [Google Scholar]
- 17.Orban Z, Remaley AT, Sampson M, Trajanoski Z, Chrousos GP. The differential effect of food intake and β-adrenergic stimulation on adipose-derived hormones and cytokines in man. The Journal of Clinical Endocrinology & Metabolism. 1999;84(6):2126–2133. doi: 10.1210/jcem.84.6.5747. [DOI] [PubMed] [Google Scholar]
- 18.Ahima RS, Prabakaran D, Mantzoros C, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 19.Boden G, Chen X, Mozzoli M, Ryan I. Effect of fasting on serum leptin in normal human subjects. The Journal of Clinical Endocrinology & Metabolism. 1996;81(9):3419–3423. doi: 10.1210/jcem.81.9.8784108. [DOI] [PubMed] [Google Scholar]
- 20.Sarraf P, Frederich RC, Turner EM, et al. Multiple cytokines and acute inflammation raise mouse leptin levels: potential role in inflammatory anorexia. The Journal of Experimental Medicine. 1997;185(1):171–175. doi: 10.1084/jem.185.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulló M, García-Lorda P, Megias I, Salas-Salvadó J. Systemic inflammation, adipose tissue tumor necrosis factor, and leptin expression. Obesity Research. 2003;11(4):525–531. doi: 10.1038/oby.2003.74. [DOI] [PubMed] [Google Scholar]
- 22.Ble A, Windham BG, Bandinelli S, et al. Relation of plasma leptin to C-reactive protein in older adults (from the Invecchiare nel Chianti study) The American Journal of Cardiology. 2005;96(7):991–995. doi: 10.1016/j.amjcard.2005.05.058. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Kuropatwinski KK, White DW, et al. Leptin receptor action in hepatic cells. The Journal of Biological Chemistry. 1997;272(26):16216–16223. doi: 10.1074/jbc.272.26.16216. [DOI] [PubMed] [Google Scholar]
- 24.Korkmaz C , Özdogan H , Kasapçopur Ö, Yazici H. Acute phase response in familial Mediterranean fever. Annals of the Rheumatic Diseases. 2002;61(1):79–81. doi: 10.1136/ard.61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Crevel R, Karyadi E, Netea MG, et al. Decreased plasma leptin concentrations in tuberculosis patients are associated with wasting and inflammation. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):758–763. doi: 10.1210/jcem.87.2.8228. [DOI] [PubMed] [Google Scholar]
- 26.Frederich RC, Hamann A, Anderson S, Löllmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nature Medicine. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- 27.Nishiya K, Nishiyama M, Chang A, Shinto A, Hashimoto K. Serum leptin levels in patients with rheumatoid arthritis are correlated with body mass index [in Japanese] Rinsho Byori. 2002;50(5):524–527. [PubMed] [Google Scholar]
- 28.Anders H-J, Rihl M, Heufelder A, Loch O, Schattenkirchner M. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism. 1999;48(6):745–748. doi: 10.1016/s0026-0495(99)90174-9. [DOI] [PubMed] [Google Scholar]


