Abstract
Plague is a highly virulent disease believed to have killed millions during three historic human pandemics. Worldwide, it remains a threat to humans and is a potential agent of bioterrorism. Dissemination of Yersinia pestis, the etiological agent of plague, by blocked fleas has been the accepted paradigm for flea-borne transmission. However, this mechanism, which requires a lengthy extrinsic incubation period before a short infectious window often followed by death of the flea, cannot sufficiently explain the rapid rate of spread that typifies plague epidemics and epizootics. Inconsistencies between the expected rate of spread by blocked rat fleas and that observed during the Black Death has even caused speculation that plague was not the cause of this medieval pandemic. We used the primary vector to humans in North America, Oropsylla montana, which rarely becomes blocked, as a model for studying alternative flea-borne transmission mechanisms. Our data revealed that, in contrast to the classical blocked flea model, O. montana is immediately infectious, transmits efficiently for at least 4 d postinfection (early phase) and may remain infectious for a long time because the fleas do not suffer block-induced mortality. These factors match the criteria required to drive plague epizootics as defined by recently published mathematical models. The scenario of efficient early-phase transmission by unblocked fleas described in our study calls for a paradigm shift in concepts of how Y. pestis is transmitted during rapidly spreading epizootics and epidemics, including, perhaps, the Black Death.
Keywords: Oropsylla montana, transmission, vector efficiency, Black Death
Plague, caused by the Gram-negative bacterium Yersinia pestis, is a highly virulent disease believed to have killed millions during three historic human pandemics. It remains a threat worldwide and is a potential agent of bioterrorism. Although the disease is characterized by rapid spread within susceptible host populations, the mechanism by which this occurs remains unclear after a century of study (1–10). Dissemination of Y. pestis by blocked fleas has been the dominant paradigm for flea-borne transmission. However, this mechanism, which requires a lengthy extrinsic incubation period (i.e., length of time required from pathogen acquisition until a flea can transmit) before a short infectious window typically followed by death of the flea, cannot sufficiently explain the rapid rate of spread that typifies plague epidemics and epizootics. Lack of a flea-borne mechanism to explain the rapid spread of the second pandemic in medieval Europe (e.g., the Black Death) has even caused speculation that plague was not the cause of this event or that alternatives to flea-borne modes of transmission are necessary to explain the observed rapid spread (2, 8). Although respiratory droplet spread of Y. pestis among humans may have been a significant mode of rapid plague transmission during the Black Death, many local epidemics during this pandemic appear to have involved the bubonic form of the disease, which is primarily flea-borne (6).
Potential modes of flea-borne transmission of Y. pestis include mechanical transmission, in which bacteria are introduced into the skin via contaminated mouthparts or by infected flea feces; consumption of infected fleas; and regurgitation of bacteria by blocked or unblocked fleas (3, 4, 6, 11). In a seminal 1914 study, Bacot and Martin (9) demonstrated that plague bacilli multiply and form a block in the proventriculus of infected oriental rat fleas, Xenopsylla cheopis. This block prevents the ingested blood from reaching the midgut, causing the flea to starve. The resulting increase in the number of feeding attempts by blocked fleas, combined with regurgitation of ingested blood and infectious material from the blockage, makes them dangerous pathogen vectors. Indeed, comparative studies indicate that X. cheopis is virtually unrivaled as the most efficient flea vector of Y. pestis (3, 5, 6, 11–15). Consequently, transmission by blocked fleas has become the dominant paradigm for Y. pestis transmission by fleas and is the flea-borne transmission mode most commonly used in models of plague epizootics and epidemics, including the Black Death (2–4, 7, 16–18).
Despite the rarity of blockage formation in most species of fleas (3, 4, 11), including some presumed to be important vectors, few empirical studies have examined alternative mechanisms to transmission by blocked fleas or evaluated the efficiency of early-phase transmission before block formation. Although they are perceived as the most efficient vectors, blocked X. cheopis transmit the plague bacterium inefficiently (18). This is, in part, because of a long extrinsic incubation period (12–16 d) before blockage formation and subsequent transmission (11, 12). Extrinsic incubation periods are generally longer, and transmission efficiency rates are even lower in most other flea species (3, 11). Recent epidemiological models suggested that because blocked fleas die shortly after becoming infectious, they are not infectious long enough to drive epizootics unless the number of fleas per host is very high (7, 18). Inconsistencies between the rate of spread expected from transmission of Y. pestis by blocked fleas and those rates observed during epidemics or epizootics have also caused speculation on alternative reservoirs of Y. pestis. This includes transmission involving resistant rodent reservoirs and exposure via contact with contaminated and infectious soil, or consumption of infectious tissues from animal carcasses (2, 7, 19).
We used Oropsylla montana (formerly Diamanus montanus or Ceratophyllus acutus) (20) as a model flea for studying transmission of Y. pestis. This flea is considered the primary vector of Y. pestis to humans in North America and naturally infests highly plague-susceptible California ground squirrels (Spermophilus beechyii) and rock squirrels (Spermophilus variegatus), both of which commonly succumb to plague during epizootics (1, 3). We focused on the first 4 d postinfection (p.i.) (referred to as “early phase”) because a previous study using the CO96-3188 strain of Y. pestis and O. montana from the same colony evaluated vector efficiency from day 4 to 8 wk p.i. (12). The fact that block formation is rare in O. montana (11–13) strongly suggests that some other mode of transmission drives rapidly spreading epizootics in O. montana–Spermophilus rodent systems. Early-phase transmission of Y. pestis by fleas could be a key mode of transmission because it would greatly increase the rate of enzootic or epizootic spread (5, 11, 14).
In this report we show that early-phase transmission by O. montana is efficient in the absence of block formation. Mathematical modeling also demonstrated that early-phase transmission is sufficient to explain epizootic spread. Although our primary goal was to determine whether early-phase transmission can serve as the key mode of transmission during Spermophilus epizootics within human plague foci in North America, our results have implications for transmission in the absence of block formation for other flea species, including the so-called human flea (Pulex irritans), which has a low blockage rate and has been implicated on epidemiological grounds as a significant vector of human plague in modern times and possibly during the Black Death (2, 3, 21).
Results
Transmission of Y. pestis was observed for each of the five time points (3, 24, 48, 72, and 96 h p.i.). For time points from 24 to 96 h, transmission occurred in 83.3% (five of six) to 100% (six of six) of mice exposed to infected fleas (Table 1). Mean minimum transmission efficiency per time point ranged from 7.7% to 10.0%. Block formation, defined as the presence of fresh blood anterior but not posterior to the proventriculus, was not observed for any of the fleas (22). Although none of the five mice exposed to infected fleas 3 h p.i. became infected (Table 1), mice exposed to pools of 8 and 15 infected fleas at 3 h p.i. became infected in pilot experiments (see footnote to Table 1). Fleas were determined to have fed by observing fresh blood in the midgut, but this method was inadequate for the 3 h time point because the previous bloodmeal required >3 h but <24 h to be digested and lose its bright red color. Vector efficiency at 3 h p.i., therefore, was not quantified. It is likely that fleas were reluctant to take a bloodmeal on a naïve mouse as early as 3 h after feeding to repletion on the artificial feeders and, therefore, transmission was seldom observed at that time point.
Table 1.
Bacterial load and transmission efficiency for O. montanainfected in artificial feeders containing defibrinated rat blood infected with Y. pestis at a concentration of 1.495 to 4.7 × 109 cfu/ml
| Time point (hours after infectious feed) and mouse number | No. of infected fleas fed on naive mouse (total no. fed of total no. exposed to mouse) | Median (range) bacterial load per infected flea fed on sentinel mouse (cfu per flea) | Transmission from flea to mouse | Estimated transmission efficiency range, %‡ |
|---|---|---|---|---|
| 3 h | ND†§ | |||
| 1 | 14 (ND* of 14) | 2.38 × 105 (5.15 × 104 to 1.12 × 106) | No | ND† |
| 2 | 12 (ND* of 12) | 3.45 × 105 (1.12 × 105 to 6.50 × 105) | No | ND† |
| 3 | 13 (ND* of 13) | 3.10 × 105 (5.60 × 104 to 6.10 × 105) | No | ND† |
| 4 | 9 (ND* of 9) | 2.50 × 105 (1.40 × 104 to 1.60 × 106) | No | ND† |
| 5 | 10 (ND* of 10) | 2.78 × 105 (6.95 × 104 to 8.30 × 105) | No | ND† |
| 24 h | 10.0 (9.3–10.6) | |||
| 1 | 11 (12 of 12) | 1.69 × 106 (2.65 × 105 to 3.91 × 106) | Yes | 9.1–100.0 |
| 2 | 9 (10 of 11) | 4.45 × 105 (2.44 × 105 to 2.86 × 106) | Yes | 11.1–100.0 |
| 3 | 10 (10 of 11) | 1.11 × 106 (5.04 × 104 to 2.44 × 106) | Yes | 11.1–100.0 |
| 4 | 10 (10 of 11) | 1.63 × 106 (3.25 × 105 to 3.12 × 106) | Yes | 11.1–100.0 |
| 5 | 10 (10 of 10) | 4.95 × 105 (4.25 × 102 to 3.67 × 106) | Yes | 10.0–100.0 |
| 6 | 10 (10 of 10) | 1.31 × 106 (1.15 × 104 to 5.13 × 106) | Yes¶ | 10.0–100.0 |
| 48 h | 8.7 (7.7–9.6) | |||
| 1 | 11 (11 of 13) | 5.80 × 105 (8.75 × 102 to 3.26 × 106) | Yes | 9.1–100.0 |
| 2 | 13 (13 of 13) | 8.85 × 105 (1.15 × 104 to 1.61 × 106) | Yes | 7.1–100.0 |
| 3 | 13 (13 of 13) | 1.10 × 106 (1.00 × 103 to 2.64 × 106) | Yes | 7.1–100.0 |
| 4 | 10 (10 of 11) | 1.95 × 105 (9.25 × 102 to 3.05 × 106) | Yes | 10.0–100.0 |
| 5 | 11 (11 of 12) | 3.85 × 105 (1.75 × 104 to 4.47 × 106) | Yes | 9.1–100.0 |
| 6 | 12 (12 of 12) | 3.75 × 105 (1.00 × 103 to 4.96 × 106) | Yes | 8.3–100.0 |
| 72 h | 9.2 (8.0–10.4) | |||
| 1 | 9 (10 of 12) | 1.85 × 104 (3.00 × 102 to 4.20 × 105) | Yes | 11.1–100.0 |
| 2 | 11 (11 of 12) | 1.55 × 105 (2.80 × 104 to 1.76 × 106) | Yes | 9.1–100.0 |
| 3 | 12 (12 of 12) | 8.20 × 105 (2.60 × 104 to 1.19 × 106) | Yes | 8.3–100.0 |
| 4 | 12 (12 of 12) | 6.78 × 104 (7.5 × 101 to 1.76 × 106) | Yes | 8.3–100.0 |
| 5 | 12 (13 of 13) | 3.90 × 105 (3.00 × 103 to 8.70 × 105) | Yes | 8.3–100.0 |
| 6 | 10 (11 of 12) | 5.95 × 104 (4.00 × 103 to 1.41 × 106) | Yes | 10.0–100.0 |
| 96 h | 7.7 (3.5–11.8) | |||
| 1 | 12 (12 of 12) | 1.64 × 105 (4.30 × 104 to 5.25 × 105) | Yes | 8.3–100.0 |
| 2 | 11 (12 of 12) | 4.05 × 104 (1.00 × 103 to 5.65 × 105) | Yes | 9.1–100.0 |
| 3 | 12 (12 of 12) | 4.20 × 104 (2.50 × 103 to 2.50 × 105) | Yes | 8.3–100.0 |
| 4 | 10 (11 of 11) | 2.33 × 104 (2.50 × 101 to 1.07 × 105) | Yes | 10.0–100.0 |
| 5 | 9 (11 of 11) | 2.20 × 104 (5.00 × 101 to 1.90 × 105) | Yes¶ | 11.1–10.0 |
| 6 | 9 (9 of 10) | 3.65 × 104 (1.00 × 102 to 3.15 × 105) | No | 0.0–0.0 |
For each time point, the mean minimum transmission efficiency is shown in boldface. ND*, feeding success could not be determined for this time point because the bloodmeal from the mouse could not be differentiated from the previous infectious bloodmeal. ND†, number of fleas that fed could not be determined.
‡Mean minimum transmission efficiency (95% confidence interval) for each time point.
§Transmission observed previously in two of four mice exposed during pilot study. During a pilot study following methods explained within this report, two naïve mice were exposed to potentially infectious fleas 3 h after feeding on artificial feeders containing rabbit blood infected with CO96-3188 (7.6 × 108 and 3.63 × 108 cfu/ml) or infected rat blood (4.14 × 108 and 5.8 × 109 cfu/ml). Transmission was not observed for either of the mice exposed to fleas fed on infected rabbit blood, but both mice exposed to infected fleas fed on rat blood became infected.
¶Day 21 titers by passive hemagglutination and inhibition tests: 1:2,048 for 24 h mouse 6 and 1:512 for 96 h mouse 5.
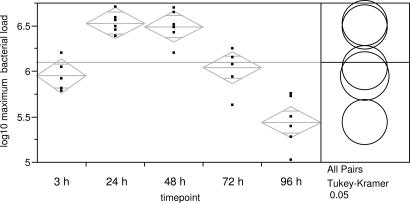
O. montana readily fed as early as 1 d after an infectious bloodmeal. Feeding success was similar across time points (24–96 h) and ranged from 83.3% to 100.0% per animal exposed (Table 1). Infection prevalence in fed fleas remained high from 3 to 96 h p.i. and ranged from 81.8% to 100% (Table 1). Mean maximum bacterial load (i.e., maximum value per flea pool averaged by time point) differed significantly among time points (ANOVA: F = 28.63, df = 4, 28, P < 0.0001; Fig. 1). Mean maximum bacterial load increased significantly from 3 to 24 h p.i., after which it reached a plateau and then declined rapidly (Fig. 1).
Fig. 1.
Mean maximum number of Y. pestis colony forming units in O. montana flea pools for 3, 24, 48, 72, and 96 h p.i. Data points are means of each replicate. Bacteremia in infectious blood meals ranged from 1.495 to 4.7 × 109 cfu/ml.
Flea density per host that is required to maintain transmission was estimated for O. montana based on the extrinsic incubation period and minimum estimates of transmission efficiency (Table 2). To maintain plague in a population (R0 = 1), the mean number of O. montana per host, m, was estimated as 5.4. During epizootic conditions (R0 ≥ 2) (18), this value would increase to ≥10.8. Such loads of O. montana are commonly found on Spermophilus spp. rodents in western North America (3, 23, 24). Furthermore, because values of transmission efficiency used in our model are based on mean minimum rates, the model likely overestimates the number of fleas required for enzootic or epizootic spread. In contrast, using previously published data on transmission efficiency examined after the period required for a Y. pestis-induced proventricular blockage to form (12), the mean number of O. montana per host exceeded natural infestation levels. Flea loads needed ranged from 60.4 during enzootic conditions to >120.8 during epizootics.
Table 2.
Terms used to determine flea loads required for epizootic spread derived experimentally or from published literature for O. montana
| Variable | Definition | Early-phase value | Post-early-phase value |
|---|---|---|---|
| a | Biting rate per day of infected flea | 0.92* | 0.92* |
| b | Probability of a flea becoming infected and infectious after feeding on a host with septicemia at the threshold or greater† | 0.10‡ | 0.015 |
| 1/r | Life expectancy (in days) of host after reaching threshold septicemia§ | 2 | 2 |
| pn | Probability of flea surviving the extrinsic incubation period | 1.0 | 0.6¶ |
Early-phase values for variables a, b, and pn are from the present study; the post-early-phase value for variable a is from the present study; and estimates of variables b and pn are from Engelthaler et al. (12).
*Of the 65 fleas fed for all replicates of the 24 h time point, 60 fed on the sentinel mouse.
†Based on the 3 h time point, all fleas that fed became infected; therefore, the probability of becoming infected after feeding on a host with septicemia at the threshold or greater equals 1. The probability of an infected flea being infectious was calculated as the proportion infected (1) multiplied by the minimum transmission rate (Table 1).
‡Mean minimum transmission efficiency observed for the 24 h time point.
§From ref. 18.
¶Assumes a mean extrinsic incubation period of 22.8 d (12) and from Fig. 6 assumes that 167 of 421 infected O. montana died by week 3 p.i.
Discussion
Previously, it was assumed that individual fleas, including O. montana, could not transmit reliably without a prolonged extrinsic incubation period. We have demonstrated that after a bacteremic bloodmeal containing 109 cfu/ml, O. montana appears to be immediately infectious, and transmission of the etiological agent of plague occurs efficiently without Y. pestis-induced proventricular block formation. These dynamics call for a reevaluation of the mechanisms responsible for the rapid spread of Y. pestis during epidemics and epizootics.
Given the frequency with which early-phase (24–96 h) transmission was observed in our study, one may wonder why it has not been given serious consideration as a mechanism driving rapidly spreading epizootics. Early studies reviewed by Burroughs (11) demonstrated that transmission occurs shortly after pathogen acquisition. However, because these experiments used flea batch sizes that far exceed natural infestation rates, the results were viewed as anomalous. Transmission by blocked fleas is a relatively efficient mechanism of transmission, and after its discovery (9), the primacy of this mechanism became dogma and alternative transmission modes were discredited (3, 6, 11, 25, 26). Although Bacot and Martin (9) speculated that partially blocked fleas might be efficient vectors and Burroughs (11) postulated that mechanical transmission was important during epizootics, subsequent experimental studies focused primarily on the window of time during which block formation was expected to occur [>5 d after a flea becomes infected (3, 11–13, 27)]. For fleas in the laboratory to survive the period required for blockage formation, which often takes many weeks, regular feeding is required. As a result of this necessity, several instances of apparent early-phase transmission were observed but considered unremarkable and outside the focus of the studies. Transmission was reported as early as 4 d p.i. for O. montana on at least three previous occasions (11, 12, 15), yet transmission efficiency before day 4 was not evaluated explicitly. Before the discovery of block formation in 1914, McCoy (28) found evidence that transmission of Y. pestis by O. montana occurred 1–5 d after flea infection, assuming that rodents die within 3–10 d after exposure (6, 9) and that fleas were infected at any time within the duration they spent on the infectious host. Based on the same assumptions, early-phase transmission can be inferred for X. cheopis and Malaraeus telchinum (11, 29), a result that was not emphasized and is often overlooked. Finally, transmission was observed as early as 1 d p.i. in the cat flea, Ctenocephalides felis (formerly Pulex felis), the dog flea, Ctenocephalides canis (formerly P. canis), and a common mouse flea, Leptopsylla segnis (formerly Typhlopsylla musculi) (10), and within 2 d in the cotton rat flea, Polygenis gwyni (30). Interestingly, Pulex irritans was observed to transmit as early as 3 d p.i. (10). This flea is a common human biter that was abundant in peridomestic environments during medieval times and has been proposed by some to have been an important vector among humans during the Black Death (2, 31–33). However, the role of this flea as a vector has been questioned based on its poor blocking capabilities (21, 33). Our results, coupled with the early observations of Verjbitski (10), suggest that early-phase transmission by P. irritans could explain the rapid spread of plague observed during the Black Death in regions of Europe where X. cheopis was rare or absent (32).
In our study, we focused on a time period during which fleas are not expected to block. With one notable exception (34), block formation is not typically seen before day 5 p.i. (3). Furthermore, none of the fleas in our study showed visible signs of blockage. Although we are confident that the mechanism by which these fleas transmitted is not by classical block formation, we have not identified the early-phase transmission mechanism. Transmission via infected flea feces has been demonstrated previously but discredited for several reasons: some species of flea do not defecate while feeding, feces tend to stick to rodent fur and rarely come into contact with the skin, and infectivity is believed to be short-lived (3, 6, 11). In our study, fecal contamination of the skin is unlikely. We rarely observed fecal matter within the feeding capsules, and when feces were present, they were removed from the capsules with an aspirator. Mechanical transmission, in which bacteria are introduced into the skin via contaminated mouthparts, seems unlikely. It is unclear how long the plague bacterium can survive on flea mouthparts. A single study indicated that Y. pestis is viable on flea mouthparts for only 3 h (26). In our study, we observed fleas in a feeding position on naïve mice 3 h p.i., but transmission did not occur in most cases where this was seen. Regurgitation of the infectious remnants from a previous bloodmeal seems the most likely scenario. The likelihood that regurgitating infectious bloodmeal remnants will result in transmission of Y. pestis should increase with the number of bacilli infecting the flea. For the time points with 100% of flea pools transmitting (24–72 h p.i.), mean maximum bacterial loads were above the previously cited transmission threshold of 106 bacilli per flea (12). For 3 and 96 h, maximum bacterial loads were significantly lower. Maximum bacterial loads were significantly higher at 3 h p.i. than 96 h, but transmission was rare at 3 h. Perhaps the only fleas that feed as early as 3 h after infectious feed are those that did not feed to repletion on the artificial feeders and thus contained light infections resulting in low transmission efficiency. Future studies may address the mechanism, but the objective of the current study was to demonstrate the efficiency of transmission in the absence of block formation and to explore how these dynamics could affect epizootic or epidemic spread.
The recognition of early-phase transmission, regardless of mechanism, as a potential key mode of spread of Y. pestis by fleas could explain previous inconsistencies between the rate of pathogen spread expected by classical transmission by blocked fleas and observed mortality rates of susceptible host populations (7), including humans during the Black Death (2, 8). To compare early-phase transmission efficiency by O. montana with blocked X. cheopis efficiency, we calculated the number of fleas per host needed to maintain Y. pestis in a susceptible host population and the number required for epizootic spread. Our estimates of 5.4 and 10.8 fleas per host during enzootic or epizootic periods, respectively, are similar to those reported for blocked X. cheopis transmission: 4.7 blocked fleas per host for maintenance and 9.4 for epizootics (18). These flea loads are well within the range reported from field collections of California ground squirrels and rock squirrels (3, 23, 24) and, because they were determined by using mean minimum transmission efficiency, probably represent overestimates.
The length of time required from pathogen acquisition until a flea can transmit is believed to be the most important factor affecting vector efficiency. Decreasing the extrinsic incubation period increases the likelihood of an infected flea surviving to become infectious and increases its potential to infect susceptible hosts over a shorter span of time (3). We have demonstrated that O. montana are infectious as early as 3 h p.i. and remain infectious for as long as blocked X. cheopis, which typically require 12–16 d to form a block and become infectious (3, 11, 12), and which, if they remain blocked, typically die <5 d later (3, 12). In our study, bacterial loads in O. montana decreased from 72 to 96 h, and this corresponded with a decline in transmission efficiency, which is believed to remain low from 5 to 18 d p.i. (12). Compensating for this decrease in bacterial loads, O. montana may infect a new host causing a severe septicemia that could result in the death of the host and provide a bloodmeal containing a sufficient quantity of bacteria to once again render the flea infectious and continue the cycle of transmission. This early-phase transmission scenario, with the possibility of boosting bacterial loads upon subsequent feeding on a septicemic host, matches the criterion (e.g., infectious for 2–3 wk) defined previously for a short-term reservoir that is capable of driving an epizootic. It is noteworthy that blocked fleas were incapable of playing this role because the infectious window was too short (7). Similar to transmission by blocked rat fleas, the high host bacteremia required to infect fleas for efficient early-phase transmission likely imposes selective pressure to maintain high virulence of Y. pestis (18). Relative to classical transmission by blocked fleas, the dynamics of early-phase transmission could result in faster rates of enzootic and epizootic spread of Y. pestis (3), perhaps as rapid as that reported during the Black Death. Given the diversity of flea species observed to transmit before block formation, the efficiency of early-phase transmission demonstrated for O. montana is probably not unique to this species. Thus, our finding of efficient early-phase transmission of Y. pestis by unblocked fleas calls for a paradigm shift in concepts of how Y. pestis is transmitted during rapidly spreading epizootics and requires future studies to elucidate the mechanism by which early-phase transmission occurs.
Methods
Species and Strains of Bacteria, Fleas, and Mice.
A fully virulent North American strain of Y. pestis (biovar orientalis), designated CO96-3188 [Pgm+, pMT1+, pCD1+, pPCP1+; LD50 of 10–100 cfu for mice (12)], was used to infect colony-reared adult female O. montana. During a pilot study described in a footnote to Table 1, fleas were infected with CO96-3188 by using the methods described below. To be certain that the strain used in our study was infectious to fleas and mice, a single flea infected with 3.4 × 103 bacilli was used to infect a Swiss–Webster mouse (Centers for Disease Control and Prevention/Division of Vector-Borne Infectious Diseases laboratory colony). Y. pestis was isolated on blood agar containing 6% sheep blood (SBA) from the liver of this mouse, and a stock culture containing >1.0 × 109 cfu/ml was created and maintained at −80°C in heart infusion broth (HIB) (Difco, Sparks, MD) supplemented with 10% glycerol for use in all subsequent feeding trials. The 102-kb chromosomal fragment termed the pigmentation locus, pgm, of Y. pestis, which contains genes important for virulence within the host or transmission by the flea, is frequently deleted en bloc at a frequency of 1.0 × 10−5 (35, 36). To confirm that the bacteria used to start the culture and the bacteria recovered from infected mice contained pgm, a total of 100 μl of HIB containing CO96-3188 was streaked onto Congo red agar (Scientific Resources Program, Atlanta, GA) and incubated at 25°C for ≈48 h (37, 38). A pgm-positive phenotype was observed in bacteria used for feeder inoculations as well as in plague bacilli isolated from infected fleas and recovered from infected mouse tissues (data not shown).
The ability of O. montana to acquire Y. pestis by feeding on infected blood in artificial feeders and subsequently infect naïve 6-wk-old female Swiss–Webster mice was evaluated at five time points p.i. (3, 24, 48, 72, and 96 h). To confirm that CO96-3188 used to infect fleas maintained virulence in Swiss–Webster mice, two mice per flea transmission replicate were inoculated s.c. with 104 to 105 bacilli of this strain suspended in 0.85% NaCl. Mice were monitored daily and euthanized when signs of Y. pestis exposure were evident (e.g., slow response to stimuli, shivering, ruffled fur). Presumptive positive infections were determined by direct fluorescence assays with fluorescein-conjugated rabbit polyclonal antibodies (Centers for Disease Control and Prevention/Division of Vector-Borne Infectious Diseases) targeting the F1 antigen to stain slide preparations of liver and spleen smears. Infections were confirmed by specific bacteriophage lysis of Y. pestis bacilli isolated from the liver or spleen of infected mice (39). This study was approved by the Centers for Disease Control and Prevention Division of the Vector-Borne Infectious Diseases Institutional Animal Care and Use Committee.
Infection of Fleas with Artificial Feeders Containing Y. pestis-Infected Blood.
Artificial feeders were used to infect fleas. This method is preferable to feeding on septicemic mice because the concentration of bacteria in the feeders can be controlled. A previous study (12) indicated that to reliably infect O. montana, bacteremia in Swiss–Webster mice must be greater than or equal to 108 cfu/ml. However, the window of time that mice are infected at this threshold level is often short and difficult to predict. To infect blood for use in feeders, CO96-3188 was grown overnight in HIB at 28°C and 150 rpm to OD620 nm = 2, and subsequently added to defibrinated Sprague–Dawley strain rat blood (Bioreclamation, Jericho, NY) to a final concentration of 1.0–3.0 × 109 cfu/ml. This level of bacteremia was expected to yield fleas with bacterial loads high enough to allow for transmission to susceptible rodents (12) and is within the range observed in infected hosts (40). The number of bacteria contained in stock cultures and in blood used for the feeders was determined by plating serial dilutions on SBA and counting colony forming units after 48 h.
Batches of colony-reared adult female fleas (Table 1) were starved for 4 d before being offered an infected blood meal by using artificial feeders covered by a freshly prepared mouse skin (41). Each replicate included fleas to be held for 3, 24, 48, 72, or 96 h before the transmission feeds on naïve mice. The fleas remained in the feeders for 1 h. After removal, they were immobilized by chilling on ice and examined by light microscopy to remove those that failed to obtain a blood meal. Fed fleas, which contained obvious red blood in the proventriculus or midgut, were considered to have ingested blood and were kept at ≈20°C and 75–80% relative humidity before transmission trials at 3, 24, 48, 72, and 96 h p.i. Blood from each feeder was serially diluted and plated in duplicate on SBA medium to determine the concentration of bacteria.
Transmission of Bacteria by Fleas to Naïve Mice.
Groups of 10–13 potentially infected fed fleas, as determined by the presence of a blood meal, were placed in feeding capsules on individual anesthetized naïve mice at 3, 24, 48, 72, and 96 h p.i. The rationale for using mass feeds rather than feeds by individual fleas was that previous studies indicated very low (2%) transmission rates (11, 12). Flea pool sizes for our experiments were selected to reflect infestation rates observed for this species in nature (3). Mice were anesthetized with ketamine (Vedco, St. Joseph, MO)–Rompun (Xylazine; Anased, Bedford, OH) before adhering the feeding capsule with a wax-resin mixture to the posterior dorsal surface of the shaved animal. Before exposure to potentially infected fleas, ≈60 μl of blood was drawn from the retroorbital sinus or by cheek punch of the mouse, and blood was applied to a filter paper strip (42). For blood samples from mice that later seroconverted, these strips were tested later by passive hemagglutination and inhibition assay to confirm that the mice had not been exposed previously to Y. pestis and were not immune to the pathogen (39).
Fleas were allowed to feed for 1 h and were then removed from the capsules, immobilized by chilling, examined by microscopy, divided into groups that fed or did not feed on the mouse, and frozen at −80°C in individual 1.5-ml microcentrifuge tubes. Because it takes >3 h but <24 h for the color of the initial blood meal to change from red to black, we were unable to quantify flea-feeding success for the 3 h time point. Thus, the ability to transmit at the 3 h time point could be determined but transmission efficiency could not. After exposure to mice, prevalence of Y. pestis infection in the fed fleas was determined by homogenization of individual fleas in a mixture containing 90 μl of HIB and 10 μl of glycerol, culturing on SBA plates, and counting colony-forming units (see below).
To prevent airborne transmission or transmission by direct contact, each mouse exposed to potentially infectious fleas was housed individually in a standard filter-top mouse cage. Naïve mice that were never exposed to fleas were group-housed in the same holding room in standard mouse cages with stainless-steel grill tops rather than filter tops; none of these control mice became symptomatic. Transmission from flea to mouse was demonstrated if the exposed mouse showed clinical symptoms of plague followed by presumptive identification of Y. pestis in the liver or spleen by direct fluorescence antibody test targeting the F1 antigen, and was confirmed by culture isolation and subsequent bacteriophage lysis. Alternatively, Y. pestis exposure in surviving mice was determined by measuring antibody titers to the F1 antigen. Antibody titers >1:10 at 21 d p.i. by passive hemagglutination and inhibition tests in whole serum were considered positive (39).
Evidence That Fleas Fed on Mice Were Exposed to Y. pestis.
To confirm that fleas fed on naïve mice at each time point were infected with Y. pestis, fed fleas suspended in 90 μl of HIB and 10 μl of glycerol were macerated in 1.5-ml microcentrifuge tubes, serially diluted in PBS, and plated in duplicate on SBA plates. Cultures were allowed to grow for 48 h at 25–28°C, and bacterial load was determined from counts of colony-forming units. Again, because feeding success could not be determined at 3 h, all fleas from such pools were tested for infection and bacterial loads quantified.
Statistical Analysis and Estimate of Flea Loads Required to Maintain Plague in a Susceptible Host Population.
The range in flea transmission efficiency was determined as follows. Minimum transmission efficiency was calculated as 1 divided by the number of infected fleas fed per mouse. Maximum efficiency was equal to 100% if the mouse was infected and 0% if it was not. Mean maximum bacterial load for infected fleas fed per animal at each time point was compared by using ANOVA with Tukey–Kramer post hoc pairwise comparisons based on log10-transformed values. Differences among or between flea groups were considered statistically significant at P < 0.05. All statistical comparisons were run by using JMP statistical software (SAS Institute, Cary, NC).
The number of O. montana per host required for enzootic or epizootic plague transmission was determined by modifying a recent plague model of Lorange et al. (18). The model assumes that host density is sufficiently high for every infected flea to find a susceptible host on which to feed and that the flea attempts to feed on the new host at least twice. The resulting model predicts the force of infection in a susceptible population or the number of fleas required per host to maintain an infection in a population as
where R0 = 1 during enzootic conditions and R0 ≥ 2 during plague epizootics, m is the flea density per host, a is the biting rate of infected fleas, b is the probability of a flea acquiring an infection after feeding on a septicemic host and transmitting the infection during a subsequent feeding on a susceptible host, pn is the probability of the flea surviving the extrinsic incubation period (defined as the duration of time from which a flea is infected until it can transmit), and 1/r defines the life expectancy of the host after it reaches threshold septicemia. In the model presented by Lorange et al. (18), the value b represents the probability of a flea forming a block after feeding on a host with septicemia at the threshold level or greater, and makes the assumption that all blocked fleas are infectious. However, blocking rates cannot be equated with transmission rates (12, 27, 43). Thus, under such a scenario, the number of fleas per host required under enzootic and epizootic conditions may be underestimated.
Acknowledgments
We thank L. G. Carter, R. M. Pappert, S. K. Urich, and R. A. Vera-Tudela for technical and logistical support and L. Eisen, C. B. Beard, J. F. Piesman, R. Rosenberg, and C. T. Webb for helpful discussions. A.P.W. was supported by National Science Foundation Emerging Infectious Disease Program Grant EID-0327052 and Shortgrass Steppe Long-Term Ecological Research Project Grant DEB-0217631.
Abbreviations
- HIB
heart infusion broth
- p.i.
postinfection
- SBA
blood agar containing 6% sheep blood.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Barnes AM. Symp Zool Soc Lond. 1982;50:237–270. [Google Scholar]
- 2.Drancourt M, Houhamdi L, Raoult D. Lancet Infect Dis. 2006;6:234–241. doi: 10.1016/S1473-3099(06)70438-8. [DOI] [PubMed] [Google Scholar]
- 3.Eskey CR, Haas VH. Plague in the Western Part of the United States. Washington, DC: US Government Printing Office; 1940. [Google Scholar]
- 4.Gage KL, Kosoy MY. Annu Rev Entomol. 2005;50:505–528. doi: 10.1146/annurev.ento.50.071803.130337. [DOI] [PubMed] [Google Scholar]
- 5.Kartman L, Prince FM, Quan SF, Stark HE. Ann NY Acad Sci. 1958;70:668–711. doi: 10.1111/j.1749-6632.1958.tb35421.x. [DOI] [PubMed] [Google Scholar]
- 6.Pollitzer R. Plague, World Health Organization Monograph Series. Geneva: WHO; 1954. No 22. [Google Scholar]
- 7.Webb CT, Brooks CP, Gage KL, Antolin MF. Proc Natl Acad Sci USA. 2006;103:6236–6241. doi: 10.1073/pnas.0510090103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood JW, Ferrell RJ, Dewitte-Avina SN. Hum Biol. 2003;75:427–448. doi: 10.1353/hub.2003.0067. [DOI] [PubMed] [Google Scholar]
- 9.Bacot AW, Martin CJ. J Hyg. 1914;13(Suppl 3):423–439. [PMC free article] [PubMed] [Google Scholar]
- 10.Verjbitski DT. J Hyg. 1908;8:162–208. doi: 10.1017/s0022172400003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burroughs AL. J Hyg. 1947;43:371–396. doi: 10.1017/s0022172400014042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelthaler DM, Hinnebusch BJ, Rittner CM, Gage KL. Am J Trop Med Hyg. 2000;62:552–560. doi: 10.4269/ajtmh.2000.62.552. [DOI] [PubMed] [Google Scholar]
- 13.Kartman L, Prince FM. Am J Trop Med Hyg. 1956;5:1058–1070. doi: 10.4269/ajtmh.1956.5.1058. [DOI] [PubMed] [Google Scholar]
- 14.Kartman L, Prince FM, Quan SF. Am J Trop Med Hyg. 1958;7:317–322. doi: 10.4269/ajtmh.1958.7.317. [DOI] [PubMed] [Google Scholar]
- 15.Wheeler CM, Douglas JR. J Infect Dis. 1945;77:1–12. [Google Scholar]
- 16.Hinnebusch BJ, Gage KL, Schwan TG. Am J Trop Med Hyg. 1998;58:562–569. doi: 10.4269/ajtmh.1998.58.562. [DOI] [PubMed] [Google Scholar]
- 17.Krasnov BR, Shenbrot GI, Mouillot D, Khokhlova IS, Poulin R. Oecologia. 2006;149:474–481. doi: 10.1007/s00442-006-0455-7. [DOI] [PubMed] [Google Scholar]
- 18.Lorange EA, Race BL, Sebbane F, Hinnebusch BJ. J Infect Dis. 2005;191:1907–1912. doi: 10.1086/429931. [DOI] [PubMed] [Google Scholar]
- 19.Rust JH, Harrison DN, Marshall JD, Cavanaugh DC. J Wildl Dis. 1972;8:127–133. doi: 10.7589/0090-3558-8.2.127. [DOI] [PubMed] [Google Scholar]
- 20.Lewis RE. J Vector Ecol. 2002;27:184–206. [PubMed] [Google Scholar]
- 21.Hirst LF. The Conquest of Plague: A Study of the Evolution of Epidemiology. Oxford: Clarendon; 1953. [Google Scholar]
- 22.Hinnebusch BJ, Perry RD, Schwan TG. Science. 1996;273:367–371. doi: 10.1126/science.273.5273.367. [DOI] [PubMed] [Google Scholar]
- 23.Quinones BE. The Potential for Human Plague Acquired from Rock Squirrels in the Fort Collins Area. Fort Collins, CO: Colorado State University; 1988. p. 67 pp. [Google Scholar]
- 24.Ryckman RE. J Med Entomol. 1971;8:668–670. doi: 10.1093/jmedent/8.6.668. [DOI] [PubMed] [Google Scholar]
- 25.Bibikova VA. Cesk Parazitol. 1965;12:41–46. [Google Scholar]
- 26.Bibikova VA. Annu Rev Entomol. 1977;22:23–32. doi: 10.1146/annurev.en.22.010177.000323. [DOI] [PubMed] [Google Scholar]
- 27.Kartman L, Quan SF, McManus AG. Exp Parasitol. 1956;5:435–440. doi: 10.1016/s0014-4894(56)80003-9. [DOI] [PubMed] [Google Scholar]
- 28.McCoy GW. Publ Health Rep. 1910;25:465. [Google Scholar]
- 29.Quan SF, Burroughs AL, Holdenried R, Meyer KF. Estratto Dagli Atti VI Congr Int Microbiol. 1953;5:1–4. [Google Scholar]
- 30.Holdenried R. J Infect Dis. 1952;90:131–140. doi: 10.1093/infdis/90.2.131. [DOI] [PubMed] [Google Scholar]
- 31.Ell SR. Bull Hist Med. 1980;54:497–510. [PubMed] [Google Scholar]
- 32.Beaucournu JC. Bull Soc Franc Parasitol. 1995;13:233–252. [Google Scholar]
- 33.Blanc G, Baltazard M. C R Acad Sci. 1941;213:813–816. [Google Scholar]
- 34.Gubareva NP, Akiev AK, Zemelman BM, Abdurakhmanov GA. Parazitologiya. 1976;10:315–319. [PubMed] [Google Scholar]
- 35.Fetherston JD, Schuetze P, Perry RD. Mol Microbiol. 1992;6:2693–2704. doi: 10.1111/j.1365-2958.1992.tb01446.x. [DOI] [PubMed] [Google Scholar]
- 36.Perry RD, Fetherston JD. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burrows TW, Jackson S. Br J Exp Pathol. 1956;37:570–576. [PMC free article] [PubMed] [Google Scholar]
- 38.Surgalla MJ, Beesley ED. Appl Microbiol. 1969;18:834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu MC. Laboratory Manual of Plague Diagnostic Tests. Geneva: Centers for Disease Control and Prevention/WHO; 2000. p. 129 pp. [Google Scholar]
- 40.Sebbane F, Gardner D, Long D, Gowen BB, Hinnebusch BJ. Am J Pathol. 2005;166:1427–1439. doi: 10.1016/S0002-9440(10)62360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wade SE, Georgi JR. J Med Entomol. 1988;25:186–190. doi: 10.1093/jmedent/25.3.186. [DOI] [PubMed] [Google Scholar]
- 42.Wolff KL, Hudson BW. Appl Microbiol. 1974;28:323–325. doi: 10.1128/am.28.2.323-325.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kartman L. Trans R Soc Trop Med Hyg. 1969;63:71–75. doi: 10.1016/0035-9203(69)90068-6. [DOI] [PubMed] [Google Scholar]