Abstract
Interferon (IFN)-γ plays an important role in the innate immune response against intracellular bacterial pathogens. It is commonly thought that natural killer cells are the primary source of this cytokine that is involved in activating antibacterial effects in infected cells and polarizing CD4+ T cells toward the Th1 subset. However, here we show that both effector and memory CD8+ T cells have the potential to secrete IFN-γ in response to interleukin (IL)-12 and IL-18 in the absence of cognate antigen. We demonstrate that memory CD8+ T cells specific for the ovalbumin protein secrete IFN-γ rapidly after infection with wild-type Listeria monocytogenes (LM). Furthermore, small numbers of ovalbumin-specific, memory CD8+ T cells can reduce spleen and liver bacterial counts in IFN-γ–deficient mice 3 d after LM infection. Up-regulation of the receptors for IL-12 and IL-18 provides a mechanism for the ability of memory CD8+ T cells to respond in this antigen nonspecific manner. Thus, CD8+ T cells play an important role in the innate immune response against intracellular pathogens by rapidly secreting IFN-γ in response to IL-12 and IL-18.
Keywords: innate immunity, T lymphocytes, cytokines, Listeria monocytogenes, IFN-γ
Introduction
The immune response against pathogenic microorganisms involves two components: a rapid, antigen nonspecific, innate response and a delayed, acquired response specific for the antigens displayed by the invading microbe. Studies using the intracellular bacterium Listeria monocytogenes (LM) have been useful in establishing the kinetics and mechanisms of both the innate and adaptive immune responses against intracellular pathogens in general. The innate immune response to LM is a complex network involving multiple cell types, cytokines, and bactericidal effector mechanisms (1, 2). The adaptive immune response to LM is T cell–mediated and required for complete resolution of the bacterium as evidenced by studies using T cell–deficient mice (3).
In the experimental murine model of LM infection, circulating monocytes and resident macrophages, such as Kupffer cells, play an important role in innate immunity by ingesting and destroying LM (1). The generation of reactive nitrogen and oxygen intermediates by infected macrophages and neutrophils results in direct killing of LM (4–6), although mechanisms independent of these intermediates also can play a role in host resistance (7–9). In support of the role of phagocytic cells and their effector functions, depletion of macrophages (10) or neutrophils (11) results in impaired innate resistance against LM.
Multiple cells of the immune system play a role in the innate response by reducing the bacterial load directly and secreting cytokines such as IL-1, IL-6, TNF-α, IFN-γ, and others. IL-1 is produced by LM-infected macrophages, and blockade of the IL-1 signaling pathway leads to an increased LM burden in mice (12). The mechanism involved in IL-1–mediated protection from LM infection is probably recruitment and activation of both neutrophils and macrophages (13). Likewise, IL-6 is rapidly secreted after LM infection (14). Removing this cytokine by depletion or gene targeting results in increased susceptibility to LM due to inefficient neutrophil recruitment (15, 16). A recent report has shown increased LM susceptibility in lymphotoxin β receptor–deficient mice (17). Although TNF-α and IFN-γ production were not affected in these mice, nitric oxide production by macrophages was impaired.
Studies using mice deficient in either TNF-α or its receptor identify this cytokine as being important in innate immunity to LM (18, 19). TNF-α functions not only to induce IFN-γ production (20) but also to directly activate macrophages independent of IFN-γ (21). IL-12 and IL-18, produced mainly by activated macrophages, are both important mediators in the immune response against LM, and their primary function is to induce IFN-γ secretion from responding immune cells (20–23). However, an IFN-γ–independent role in controlling LM has also been proposed for IL-18 (24).
IFN-γ is produced by multiple cell types including NK cells (25), NK-T cells (26), macrophages (27), B cells (28), dendritic cells (29), and γδ T cells (30). Evidence that IFN-γ is critically important in the innate immune response comes from experiments using mice deficient in either the cytokine or its receptor. These animals rapidly succumb when infected with low doses of LM (31, 32). SCID mice, which lack T and B lymphocytes, show increased susceptibility to LM when they are depleted of either IFN-γ or IL-12 (21, 33). Providing IFN-γ to IL-12–depleted SCID mice can reverse this effect (21). One mechanism for the increased susceptibility of animals lacking the IFN-γ receptor, or presumably the cytokine itself, is impaired macrophage activity (34).
It is generally thought that NK cells play an important role in the control of LM infection due to secretion of IFN-γ induced by IL-12 and IL-18 (25). In contrast, one study found that depleting NK cells led to decreased LM burdens in B6 mice (35). However, the IFN-γ–secreting activity of NK cells or any other cell type has never been directly tested for its ability to provide innate protection against LM infection. One cell type that can contribute to IFN-γ secretion during the innate immune response is CD8+ T cells. We and others have shown that CD44hi CD8+ T cells respond to cytokines by secreting IFN-γ rapidly after infection with intracellular bacteria (36, 37). Since this population of CD8+ T cells is as numerous as NK cells in normal mice, we directly tested their activity in vivo. This report demonstrates that memory CD8+ T cells provide protection against LM infection in IFN-γ–deficient animals in an antigen nonspecific manner.
Materials and Methods
Mice.
C57BL/6J (B6), C57BL/6.PL-Thy1a/Cy (B6.Thy1.1), B6.129S7-Ifng tm1Ts (IFN-γ−/−), and OT-I TCR transgenic mice were bred and maintained at the University of Texas Southwestern Medical Center animal facility under the approval of the Institutional Animal Care and Use Committee.
Bacteria and Viruses.
For infection of cell lines and mice, log phase cultures of LM 10403 serotype 1 or LM-expressing full-length ovalbumin protein (LM/OVA), provided by Dr. Hao Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) (38), were washed twice and diluted in PBS to the desired concentration. LM or LM/OVA were injected in the lateral tail vein at the indicated dosage.
Vesicular stomatitis virus (VSV)–expressing full-length ovalbumin protein (VSV/OVA) was provided by Dr. Leo Lefrancois (University of Connecticut Health Center, Farmington, CT) (39). Vaccinia virus (VV)–expressing full-length ovalbumin protein (VV/OVA) was provided by Dr. Jack Bennink (National Institutes of Health, Bethesda, MD) (40). VSV/OVA and VV/OVA were injected in the lateral tail vein at a dosage of 2 × 106 PFU for a primary response.
Cell Lines, Cell Culture, and Reagents.
The J774 macrophage line (H-2d) and the J774 macrophage line stably transfected with H-2Kb (J774:Kb) were grown in complete DMEM (Life Technologies) supplemented with 10% FCS (Atlanta Biologicals). For overnight in vitro experiments, the culture of mouse splenocytes was performed in complete RPMI (Life Technologies) supplemented with 10% FCS and 10 ng/ml recombinant human IL-2 (provided by Dr. Michael Bennett, UTSW). In experiments designed to test the direct ex vivo activity of T cells, splenocytes were cultured for 3 h in complete RPMI supplemented with 10% FCS (without added cytokines or antigens). Recombinant murine IL-12 (5 ng/ml final concentration) and IL-18 (10 ng/ml final concentration) were from Peprotech Inc. Blocking antibodies against murine IL-12 and IL-18 (1 μg/ml final concentration) were purchased from Peprotech Inc. and Medical and Biological Laboratories Co., Ltd., respectively. Carboxyfluorescein diacetate, succinimidyl ester (CFSE) labeling of splenocytes was performed at a final concentration of 1 μM and was obtained from Molecular Probes. The OVA-derived peptide SIINFEKL was synthesized by the UTSW peptide synthesis facility.
Antibodies and Cell Staining.
For cell staining experiments, the following antibodies from BD Biosciences were used: anti-CD8α (53–6.7), anti-CD44 (IM7), anti-CD90.2 (Thy1.2) (53–2.1), anti-CD94 (18d3), and anti–IFN-γ (XMG1.2). Secondary streptavidin-conjugated reagents were used to reveal biotinylated primary or secondary antibodies. Intracellular staining, data acquisition, and data analysis were performed as described previously (36). Staining for the presence of the IL-18Rα subunit was accomplished using an anti–IL-18Rα antibody (R&D Systems) followed by biotinylated anti–goat IgG (Jackson ImmunoResearch Laboratories), which was then revealed with streptavidin-conjugated PE. Identification of endogenous OVA-specific T cells was performed by first incubating the splenocytes with a blocking CD8α antibody (CT-CD8α) from Caltag Laboratories. After washing, the splenocytes were incubated at 4°C for 1 h with CD8α FITC and a Kb-SIINFEKL tetramer coupled to PE that was purchased from the Protein Chemistry Core Laboratory at Baylor College of Medicine.
In Vitro Stimulations.
Red blood cell–depleted splenocytes were cultured in 24-well plates at a concentration of 3 × 106/well. J774 or J774:Kb macrophages cultured in antibiotic-free media were infected at a multiplicity of infection of 5:1 for 1 h. The macrophages were then washed three times with PBS and cultured for 3 h in complete DMEM supplemented with 10% FCS and gentamycin (100 μg/ml). The infected macrophages were then harvested and plated at 3 × 105/well with the splenocytes.
T Cell Transfers and In Vivo Stimulations.
Splenocytes from OT-I TCR transgenic mice were passed over nylon wool columns to enrich for T cells. ∼3 × 106 cells were then injected i.v. into the lateral tail vein of B6.Thy1.1 recipient mice, rested for 1–3 d, and challenged with the indicated pathogen as described above. For the experiments analyzing OT-I T cells that were transferred and not challenged, 107 OT-I T cells were transferred into B6.Thy1.1 hosts, and the mice were killed 1–3 d later for analysis.
Harvest of Peripheral Organs.
Mice were perfused before harvesting the organs according to a published protocol (41). Briefly, mice were perfused with PBS containing 75 U/ml heparin (Sigma-Aldrich). Lung tissue was minced and incubated with constant stirring for 30 min at 37°C in HBSS containing 1.3 mM EDTA (Sigma-Aldrich). The lung tissue was next treated with 150 U/ml collagenase (Sigma-Aldrich) in RPMI containing 5% FCS for 1 h at 37°C. The resulting suspension was pelleted, resuspended in 44% Percoll (Amersham Biosciences), layered on 67.5% Percoll, and centrifuged at 600 g at 4°C for 15 min. Liver tissue was homogenized, pelleted, resuspended in 35% Percoll containing 200 U/ml heparin, layered on 67.5% Percoll, and centrifuged at 600 g at 4°C for 15 min. Lung and liver lymphocytes at the gradient interface were harvested and washed twice before use.
Real-time RT-PCR.
For the naive OT-I T cell population used for real-time RT-PCR, splenocytes from naive OT-I mice were purified on a nylon wool column to enrich for T cells and then stained for CD8, Vα2, and CD44. After washing, the splenocyte population was sorted on the basis of CD8+, Vα2+, and CD44lo using a MoFlo™ high speed sorter (Cytomation, Inc.). The effector OT-I T cell population was obtained from B6.Thy1.1 mice that were transferred with OT-I T cells and then primed with LM/OVA. 7 d postinfection, the mice were killed and the splenocytes were purified on a nylon wool column. The resulting cells were stained for CD8 and Thy1.2 and sorted for expression of these molecules. For the memory OT-I T cell population, the same protocol was used as for the effector population, except the mice were killed at >4 wk postinfection. For each of the sorted OT-I T cell populations, the purity of the cells was >95% as determined by flow cytometry after sorting.
RNA was purified from the sorted naive, effector, and memory OT-I T cell populations using QIAGEN RNeasy columns. The protocol provided by the manufacturer was followed with the addition of the on column DNA digestion using the RNase-free DNase set from QIAGEN in order to remove any residual, contaminating DNA. cDNA was then synthesized from the RNA using the oligo-dT method and TaqMan™ reverse transcription reagents from Applied Biosystems.
Real-time RT-PCR was performed on each of the three cDNA populations to amplify GAPDH (as a control to normalize for the quantity of cDNA), IL-12Rβ1, IL-12Rβ2, IL-18Rα, and IL-18Rβ. The primers used are listed from 5′ to 3′: GAPDH sense, TGCACCACCAACTGCTTAG and GAPDH antisense, GGATGCAGGGATGATGTTC; IL-12Rβ1 sense, GGCAACATGACATCCATGCA and IL-12Rβ1 antisense, GTGTGTCACCATCTTGGCAGGATC; IL-12Rβ2 sense, CACTATCAGGTGACGTTACA and IL-12Rβ2 antisense, TGCAG-AAGCGCCTTTTGAGTTGGT; IL-18Rα sense, GTGCACAGGAATGAAACAGC and IL-18Rα antisense, ATTTAAGGTCCAATTGCGACGA; and IL-18Rβ sense, GGAGTGGGAAATGTCAGTAT and IL-18Rβ antisense, CCGTGCC-GAGAAGGATGTAT. An Applied Biosystems GeneAmp 5700 thermocycler was used in conjunction with the SYBR Green™ PCR Master Mix, also from Applied Biosystems. Each sample was run in triplicate, and the data were analyzed using the 2-ΔΔC T method (42) in order to determine the relative gene expression of each of the IL-12 and IL-18 receptors.
Online Supplemental Material.
Fig. S1 shows the ability of effector P14 TCR transgenic T cells generated in vivo to respond in vitro to either a combination of IL-12 and IL-18 or their cognate peptide. After overnight culture of splenocytes containing effector P14 T cells with the stimuli shown, the T cells secrete IFN-γ. This indicates that in addition to OT-I T cells, transgenic CD8+ T cells with another specificity are responsive to IL-12 and IL-18. Fig. S2 depicts the response of memory OT-I T cells generated in vivo to both LPS and Escherichia coli. After culturing splenocytes containing memory OT-I T cells in vitro for 3 h in the presence of GolgiPlug and the indicated stimuli, the OT-I T cells secrete IFN-γ. This result indicates that memory T cells have the ability to secrete IFN-γ in response to pathogens other than LM. Fig. S3 compares the ability of sorted naive or memory OT-I T cells to provide protection from LM infection in IFN-γ2/− mice. The memory OT-I T cells provide protection from a LM infection as shown by the lowered LM colony counts in both the spleen and liver. The naive OT-I T cells do not provide protection from LM in either the spleen or the liver when they are transferred into IFN-γ2/− mice. Figs. S1–S3 are available at http://www.jem.org/cgi/content/full/jem.20031051/DC1.
Results
Responses of Effector CD8+ T Cells to IL-12 and IL-18.
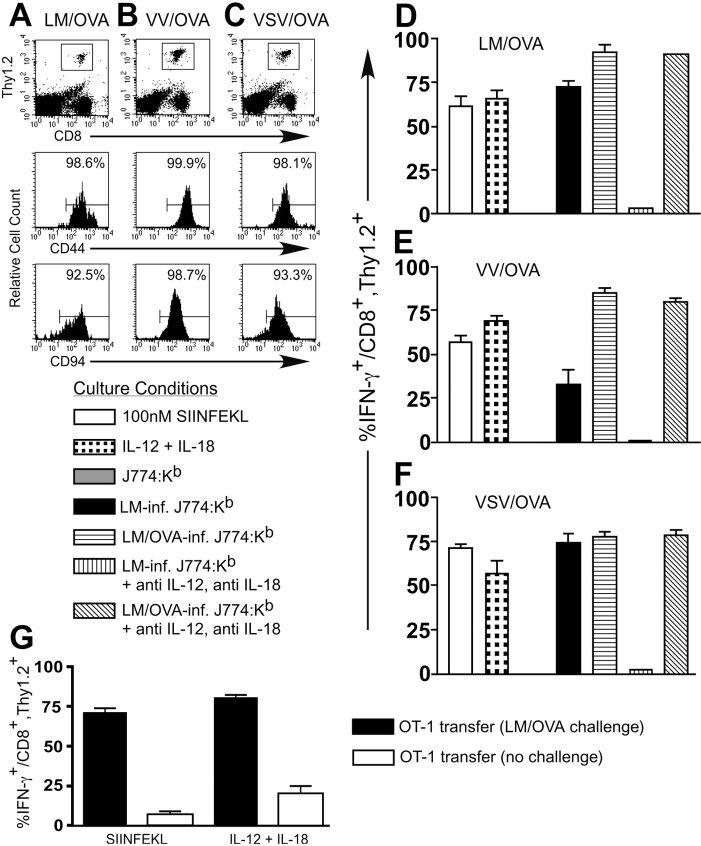
We demonstrated previously that a population of CD8+ T cells isolated from naive mice had the ability to respond to LM-infected macrophages by secreting IFN-γ (36). To further investigate the potential of CD8+ T cells to respond in vivo to LM, we used an adoptive transfer protocol where T cell–enriched splenocytes isolated from OT-I TCR transgenic mice were transferred into B6.Thy1.1 congenic hosts. The mice were then challenged with a strain of LM expressing the ovalbumin protein (LM/OVA) to induce the activation of the OT-I T cells. 7 d after infection, the CD8+, Thy1.2+ population identifies the transferred OT-I T cells, which express both Vα2 and Vβ5 and also stain positive using a Kb-SIINFEKL tetramer (Fig. 1 A and unpublished data). The OT-I population of T cells express the CD44 and CD94 molecules indicative of an activated phenotype (Fig. 1 A), and splenocytes from these mice are cytolytic against targets pulsed with the SIINFEKL peptide (unpublished data). When splenocytes from these OT-I–transferred, LM/OVA-primed mice were cultured overnight with SIINFEKL or a combination of IL-12 and IL-18, intracellular IFN-γ is detected in the majority of the CD8+, Thy1.2+ T cells (Fig. 1 D). In addition, stimulation of the CD8+, Thy1.2+ T cells to secrete IFN-γ with LM/OVA-infected J774:Kb macrophages could not be blocked by antibodies against IL-12 and IL-18. In contrast, IFN-γ secretion induced by LM/OVA-infected J774, LM-infected J774, or LM-infected J774:Kb was blocked by antibodies against IL-12 and IL-18.
Figure 1.
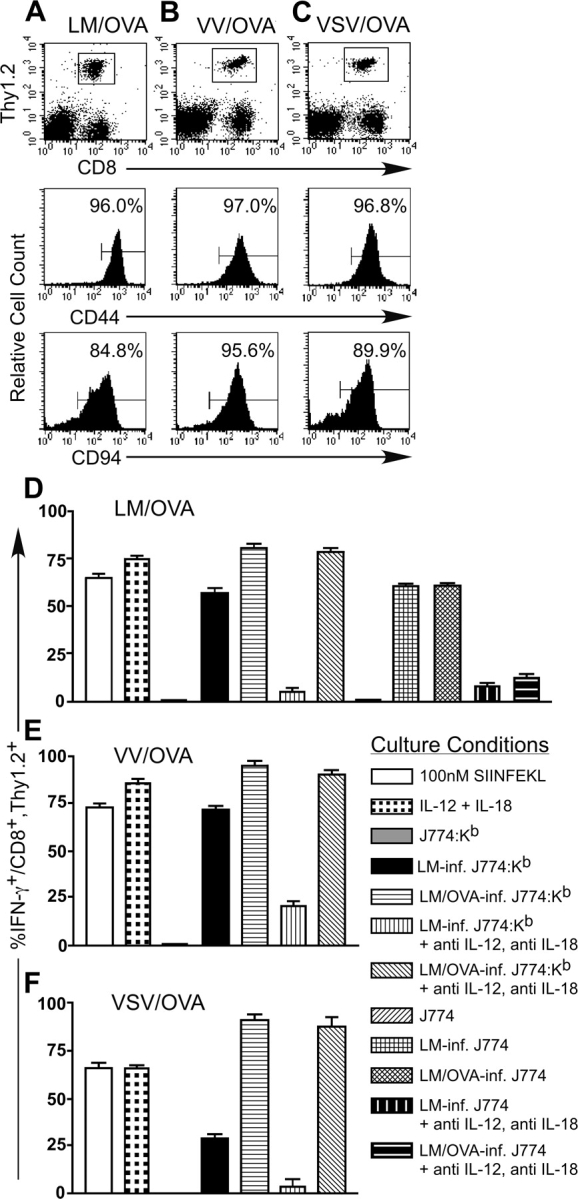
Effector OT-I T cells secrete IFN-γ in response to TCR stimulation or a combination of IL-12 and IL-18 in vitro. 3 × 106 nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 mice that were then infected with ∼2,000 LM/OVA (A), 2 × 106 PFU VV/OVA (B), or 2 × 106 PFU VSV/OVA (C). 7 d after infection, the mice were killed and splenocytes were stained to identify the OT-I T cells and their activation/memory status. After gating on the CD8+, Thy1.2+ population of OT-I T cells, the histograms indicate the percentage of cells positive for CD44 or CD94. Bulk splenocytes from the LM/OVA (D), VV/OVA (E), or VSV/OVA (F) effector mice were incubated overnight with the indicated culture conditions. GolgiPlug™ was added for the final 4 h of culture, and the cells were subsequently harvested and stained for expression of CD8, Thy1.2, and intracellular IFN-γ. The data are the average of two mice and are representative of at least two independent experiments.
To examine the effect of priming OT-I T cells with other pathogens, we infected B6.Thy1.1 mice that had been transferred with T cell–enriched OT-I splenocytes with VV-expressing ovalbumin (VV/OVA) or VSV-expressing ovalbumin (VSV/OVA). 7 d after infection, we analyzed the cell surface phenotype of the CD8+, Thy1.2+ T cells to confirm that they had responded to antigen (Fig. 1, B and C). After overnight stimulation, the OT-I T cells primed with either VV/OVA or VSV/OVA are able to respond to IL-12 and IL-18 by secreting IFN-γ (Fig. 1, E and F). These effector T cells respond to LM-infected J774:Kb by secreting IFN-γ even though the cognate antigen (OVA) is not provided. Antibodies against IL-12 and IL-18 block the IFN-γ secretion induced by LM-infected J774:Kb but not LM/OVA-infected J774:Kb. Similar responsiveness to IL-12 and IL-18 was observed using P14 TCR transgenic CD8+ T cells specific for lymphocytic choriomeningitis virus–derived glycoprotein 33–41 as an effector population generated in vivo (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20031051/DC1).
Responses of Memory CD8+ T Cells to IL-12 and IL-18.
To further our understanding of the ability of CD8+ T cells to respond to IL-12 and IL-18, we used the same OT-I transfer protocol as above but allowed the mice to rest for >4 wk before analysis. During this time period, the OT-I T cells respond to the antigen and then return to a resting but memory state. B6.Thy1.1 mice transferred with OT-I T cells that were challenged with LM/OVA (Fig. 2 A), VV/OVA (Fig. 2 B), or VSV/OVA (Fig. 2 C) were analyzed for expression of memory and activation markers. The expression of CD44 and CD94 on each of the transferred populations indicates that the OT-I T cells have a memory phenotype. In addition, none of the memory OT-I T cell populations expressed the activation markers CD25 or CD69 (not depicted). After overnight culture of splenocytes obtained from these animals, the OT-I T cells responded equally well to IL-12 and IL-18 or the cognate peptide in terms of their IFN-γ response. Importantly, regardless of the OVA-expressing pathogen used to generate memory OT-I T cells, LM-infected J774:Kb stimulators induced IFN-γ secretion, and this effect was blocked by antibodies against IL-12 and IL-18 (Fig. 2, D–F).
Figure 2.
Memory OT-I T cells secrete IFN-γ in response to TCR stimulation or a combination of IL-12 and IL-18 in vitro. 3 × 106 nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 mice that were then infected with ∼2,000 LM/OVA (A), 2 × 106 PFU VV/OVA (B), or 2 × 106 PFU VSV/OVA (C). The mice were then allowed to rest for >4 wk in order to generate stable memory populations of OT-I T cells. The mice were killed, and splenocytes were stained to identify the OT-I T cells and their activation/memory status. After gating on the CD8+, Thy1.2+ population of OT-I T cells, the histograms indicate the percentage of cells positive for CD44 or CD94. Bulk splenocytes from the LM/OVA (D), VV/OVA (E), or VSV/OVA (F) memory mice were incubated overnight with the indicated culture conditions. GolgiPlug™ was added for the final 4 h of culture, and the cells were subsequently harvested and stained for expression of CD8, Thy1.2, and intracellular IFN-γ. The results shown are the average of two mice and are representative of at least two independent experiments. (G) OT-I T cells that were transferred into B6.Thy1.1 mice and not challenged were compared with similar mice that were challenged with LM/OVA and then rested for >4 wk. After overnight stimulation with 10 nM SIINFEKL or a combination of IL-12 and IL-18, the cells were stained for CD8, Thy1.2, and intracellular IFN-γ. The results from two mice per group were averaged, and the experiment was performed three times with similar results.
Our previous work has shown that a very small percentage of OT-I T cells stimulated directly ex vivo from an OT-I mouse was able to secrete IFN-γ (36). To analyze a naive population of transferred CD8+ T cells, T cell–enriched OT-I splenocytes were injected into B6.Thy1.1 hosts, and their potential to secrete IFN-γ without challenging the mice in vivo was determined. Indeed, a small percentage of the transferred OT-I T cells was able to secrete IFN-γ in response to either IL-12 and IL-18 or SIINFEKL (Fig. 2 G). The IFN-γ–secreting OT-I T cells altered their cell surface phenotype compared with nontransferred OT-I T cells with respect to CD44, CD94, and other memory markers (unpublished data). These changes may explain why some transferred OT-I T cells are slightly more responsive to IL-12 and IL-18 compared with the nontransferred population of OT-I T cells. However, the nonprimed, transferred OT-I T cell population contained a much lower percentage of IL-12– and IL-18–responsive T cells compared with a memory population of OT-I T cells. Furthermore, when we transferred OT-I T cells into B6.Thy1.1 hosts and then attempted to prime with WT LM there was no proliferation of the OT-I T cells as measured by CFSE labeling experiments (unpublished data). Together, these data indicate that only effector and memory CD8+ T cells have the capacity to secrete IFN-γ in response to IL-12 and IL-18 and that this response is not mediated through the TCR.
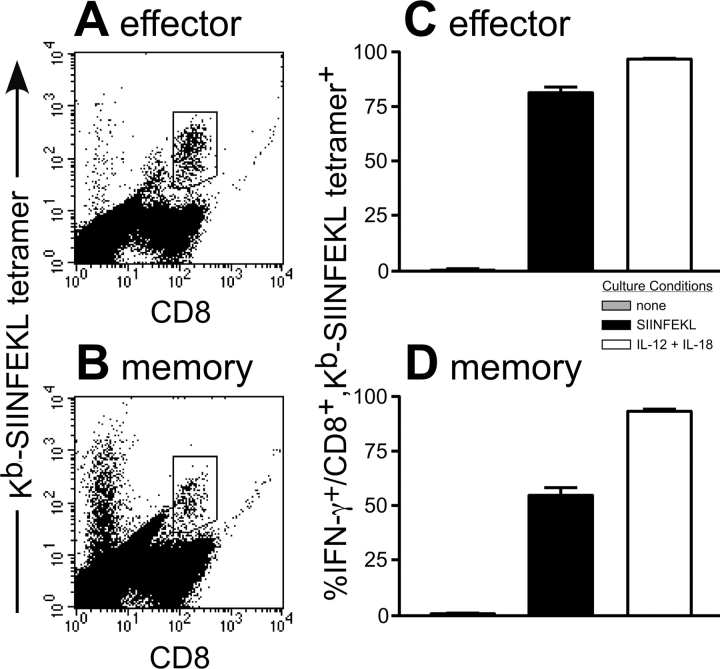
To analyze a population of antigen-specific, non-TCR transgenic CD8+ T cells, we injected B6 mice with LM/OVA and identified endogenous OVA-specific CD8+ T cells using Kb-SIINFEKL tetramers (Fig. 3, A and B) . Once again, both effector (7 d postinfection) and memory (>4 wk postinfection) OVA-specific CD8+ T cells secreted IFN-γ when stimulated overnight with a combination of IL-12 and IL-18 or their cognate peptide (Fig. 3, C and D). This result, along with our previous work using bulk populations of CD8+ T cells, (36) suggests that multiple populations of effector and memory CD8+ T cells respond to IL-12 and IL-18 by secreting IFN-γ.
Figure 3.
Endogenous effector and memory OVA-specific T cells secrete IFN-γ in response to TCR stimulation or a combination of IL-12 and IL-18 in vitro. (A and C) B6 mice were primed with LM/OVA and killed 7 d later. Bulk splenocytes from these mice were incubated overnight with the indicated culture conditions. GolgiPlug™ was added for the final 4 h of culture, and the cells were stained with the Kb-SIINFEKL tetramer and for CD8 and intracellular IFN-γ. (B and D) B6 mice were primed with LM/OVA and killed >4 wk later. Splenocytes were cultured and stained as in A and C. For each dot plot, one overnight culture with IL-12 and IL-18 is shown out of six representative mice. The results in C and D are the average of three mice for each group and were repeated with similar results.
Localization of Memory CD8+ T Cells in Peripheral Organs and Their Ability To Respond To IL-12 and IL-18.
Previous reports indicate that memory T cells preferentially relocate in peripheral organs (41, 43). When we compared the localization of naive (transferred, unprimed) OT-I T cells and memory (transferred, LM/OVA-primed) OT-I T cells, we found that the memory OT-I T cells preferentially relocated to nonlymphoid organs, whereas the naive OT-I T cells did not (Fig. 4, A and B) . Importantly, when we restimulated lymphocytes isolated from LNs, spleen, liver, and lung we found that memory OT-I T cells were able to respond to both SIINFEKL or a combination of IL-12 and IL-18 by secreting IFN-γ (Fig. 4 C). When this experiment was repeated, we used SIINFEKL-pulsed J774:Kb as stimulators to compensate for the low numbers of cells isolated from the lung, and this increased the percentage of IFN-γ–secreting OT-I T cells to ∼75% (unpublished data). These results indicate that not only do memory CD8+ T cells preferentially reside in peripheral organs but also that these T cells are capable of mounting an early, rapid response against a pathogen-induced infection.
Figure 4.
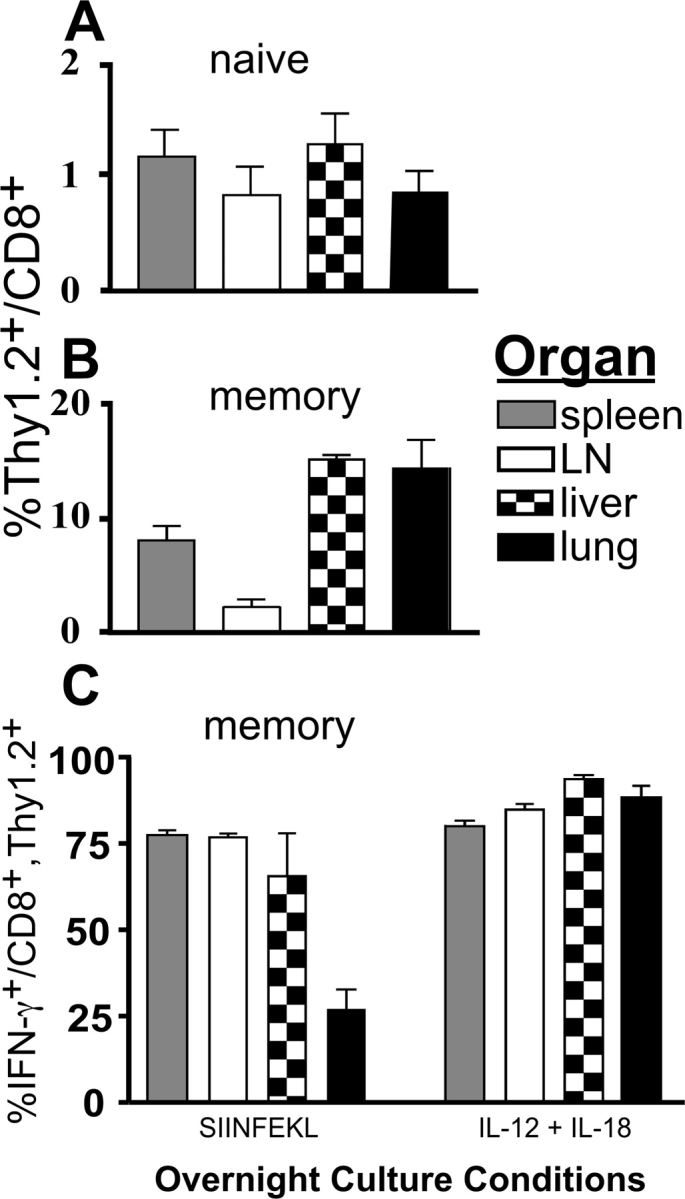
Memory OT-I T cells preferentially relocate to peripheral organs and respond to IL-12 and IL-18 by secreting IFN-γ. (A) 107 nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 hosts. (B and C) 3 × 106 nylon wool-purified OT-I T cells were transferred into B6.Thy1.1 hosts, challenged with ∼2,000 LM/OVA, and then rested for >4 wk. Graphs representing the percentage of Thy1.2+ (OT-I) cells out of the total CD8+ population within the indicated organs for the naive OT-I T cells (A) and the memory OT-I T cells (B) are shown. (C) After overnight stimulation with either 10 nM SIINFEKL or a combination of IL-12 and IL-18, the cells isolated from the indicated organs were incubated for 4 h with GolgiPlug™ and then stained for CD8, Thy1.2, and intracellular IFN-γ. Three mice were averaged per group, and the data are representative of three independent experiments.
Rapid In Vivo Production of IFN-γ from Memory CD8+ T Cells in Response To WT LM.
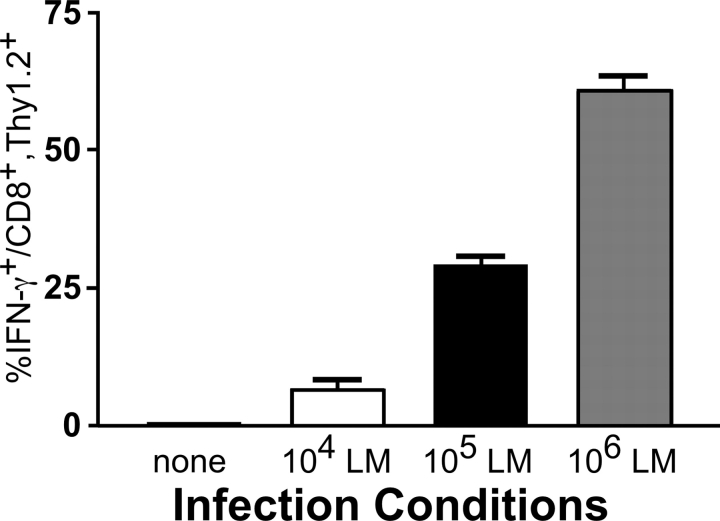
To test whether or not memory OT-I T cells respond to a WT LM infection in vivo, we rechallenged OT-I–transferred, VSV/OVA-primed memory mice for 16 h with differing doses of WT LM. The splenocytes were then cultured for 3 h in media with GolgiPlug™ and subsequently analyzed for IFN-γ production. Indeed, memory OT-I T cells responded to WT LM in a dose-dependent fashion by secreting IFN-γ (Fig. 5) . The probable reason for the requirement of high doses of WT LM to induce a substantial percentage of the OT-I T cells to secrete IFN-γ is that the assay was performed after only 16 h of infection. In other experiments, ∼50% of the memory OT-I T cells were able to secrete IFN-γ 3 d postinfection with the sublethal dose of ∼104 WT LM (unpublished data). OT-I–transferred, VSV/OVA-primed memory mice were also injected with IL-12 and IL-18. Once again, ∼50% of the OT-I T cells stained positive for intracellular IFN-γ (unpublished data). These results suggest that the combined actions of IL-12 and IL-18 are responsible for inducing rapid IFN-γ secretion in memory CD8+ T cells responding to WT LM. To expand these findings to another organism, we infected memory OT-I mice with E. coli and killed the mice 3 h later. ∼40% of the memory OT-I T cells isolated from the E. coli–infected mice secreted IFN-γ (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20031051/DC1). Together, these results indicate that memory CD8+ T cells have the ability to respond to multiple pathogens in an antigen-independent fashion.
Figure 5.
Memory OT-I T cells primed with VSV/OVA respond to an infection with WT LM by secreting IFN-γ. 3 × 106 nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 hosts. The mice were then challenged with 2 × 106 PFU VSV/OVA and rested for >4 wk to generate stable memory populations of OT-I T cells. The mice were rechallenged with varying doses of WT LM. 16 h after infection, the mice were killed and the splenocytes were cultured for 3 h in RPMI media containing GolgiPlug™ (no cytokines or antigens were added). The cells were subsequently harvested and stained for CD8, Thy1.2, and intracellular IFN-γ. The results shown are the average of two mice. Similar results were obtained using B6.Thy1.1 mice that were OT-I transferred, primed with VV/OVA, rested for >4 wk and rechallenged with WT LM.
Up-regulation of IL-12 and IL-18 Receptors in Effector and Memory CD8+ T Cells.
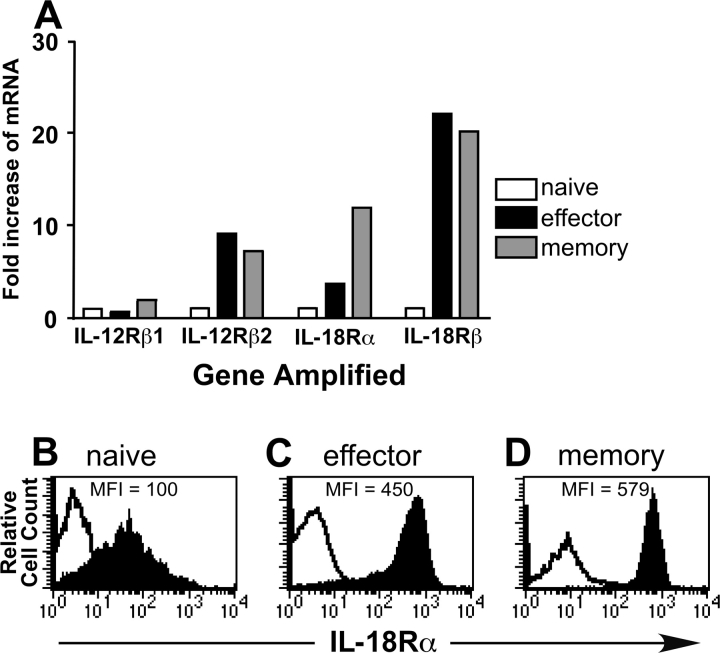
To establish a mechanism for the increased IFN-γ responsiveness of effector and memory OT-I T cells to a combination of IL-12 and IL-18 compared with their naive counterparts, we performed real-time RT-PCR on sorted populations of naive, effector, and memory OT-I T cells. Levels of IL-12Rβ2, IL-18Rα, and IL-18Rβ were all up-regulated in the effector and memory OT-I T cell populations (Fig. 6 A). To confirm these findings at the protein level, we analyzed naive, effector, and memory populations of OT-I T cells for expression of the IL-18Rα subunit. Although in each population of OT-I T cells the IL-18Rα subunit is expressed, the effector and memory populations express approximately a fivefold higher level than the naive population (Fig. 6, B–D). The flow cytometry data, which supports the RT-PCR data for the IL-18Rα subunit, suggests that the antibodies used for sorting to obtain the pure populations of OT-I T cells did not affect the gene expression of the IL-12 and IL-18 receptors. In addition, we observed that memory OT-I T cells generated by priming with either VV/OVA or VSV/OVA expressed high levels of the IL-18Rα subunit, explaining their increased responsiveness to IL-12 and IL-18 (unpublished data).
Figure 6.
Effector and memory OT-I T cells up-regulate IL-12Rβ2, IL-18Rα, and IL-18Rβ. (A) Naive, effector, and memory OT-I T cells were purified as described in Materials and Methods. mRNA was then purified, cDNA generated, and real-time RT-PCR performed. The results were normalized to GAPDH levels to control for quantity of cDNA in each sample. The levels of each of the receptors was set to a value of one for the naive OT-I population, and the results are presented as fold increase of mRNA over the naive OT-I population. Each reaction was performed in triplicate, and the data are the average of the triplicate. Levels of the IL-18Rα subunit were measured on populations of naive OT-I T cells (B), effector OT-I T cells (C), and memory OT-I T cells (D). For the naive population, Vα2+, CD8+ splenocytes from naive OT-I mice were gated on for the analysis of the IL-18Rα subunit. For the effector population, nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 hosts, which were then challenged with ∼2,000 LM/OVA. 7 d postinfection, splenocytes were stained, and the CD8+, Thy1.2+ population of OT-I T cells was gated on for analysis of the IL-18Rα subunit. The memory population was generated in the same manner as the effector population, except the mice were rested for >4 wk before analysis. The open histograms represent background staining in the absence of the primary antibody, whereas the filled histograms represent staining in the presence of the primary antibody. Each histogram is staining from one representative of four mice.
Differences in LM Susceptibility Between B6 and IFN-γ−/− Mice.
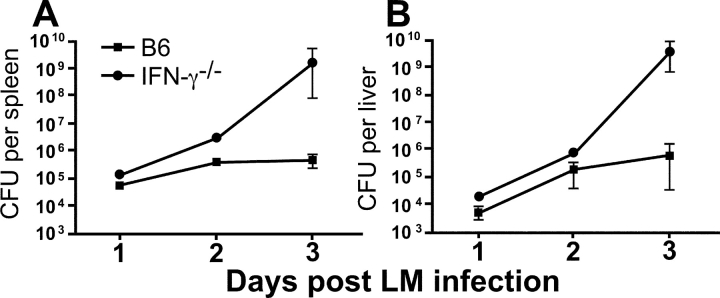
To investigate the early kinetics of the innate requirements for IFN-γ during an LM infection, we compared the susceptibility of B6 and IFN-γ−/− mice infected with ∼104 LM on days 1, 2, and 3 postinfection. The differences in LM susceptibility, as determined by LM counts in the spleen and liver, are only detectable between 2–3 d postinfection (Fig. 7) . These data indicate that IFN-γ is important during the innate immune response to LM, but the importance of this effector cytokine does not manifest itself immediately after infection.
Figure 7.
Innate susceptibility of B6 and IFN-γ2/2 mice to LM infection. B6 and IFN-γ−/− mice were infected with ∼104 LM. At days 1, 2, and 3 postinfection, the mice were killed and spleen (A) and liver (B) LM counts were determined by plating 10-fold dilutions of the homogenized organs on BHI plates. Three mice per group were averaged for days 1 and 2, whereas eight mice per group were averaged for day 3. Similar results were obtained in independent experiments performed on days 1, 2, and 4 postinfection.
Early Protection of IFN-γ−/− Mice from LM Infection by Memory CD8+ T Cells Independent of Antigen Specificity.
Our data suggest that antigen nonspecific secretion of IFN-γ in response to WT LM by memory CD8+ T cells may play a role in protective immunity against LM and that this response can be visualized within 16 h (Fig. 5). Furthermore, differences in LM counts mediated by IFN-γ can only be seen 2–3 d postinfection (Fig. 7). Therefore, we transferred memory OT-I T cells into IFN-γ−/− mice infected with ∼104 LM and determined spleen and liver LM counts 3 d postinfection. Spleen and liver LM counts for IFN-γ−/− mice on day 3 after infection show high numbers of bacterial colonies compared with WT B6 control animals, as expected (Fig. 8 A). In contrast, IFN-γ−/− mice that received 5 × 105 sorted memory OT-I T cells before LM infection show ∼2 logs of protection in both organs. We were able to detect CFSE staining of the transferred memory OT-I T cells 3 d postinfection (Fig. 8 B). As few as ∼5,000 IFN-γ–secreting OT-I T cells are able to reduce the LM counts in IFN-γ−/− mice almost to the levels of a B6 mouse. The small number of OT-I T cells recovered 3 d postinfection is most likely attributed to loss upon injection, localization to other organs, and the fact that not all of the OT-I T cells secrete IFN-γ at the time of the assay. Importantly, there are ∼290-fold fewer IFN-γ–secreting cells in the IFN-γ−/− mice transferred with memory OT-I T cells compared with the B6 mice (Fig. 8 C). It should be noted that this response does not represent cross-reactivity between the SIINFEKL epitope and LM since the transferred cells show no evidence of dilution of the CFSE label (Fig. 8 B). Our previous data indicate that naive OT-I T cells are not responsive to IL-12 and IL-18 and that they do not express high levels of the receptors for these cytokines (Figs. 2 and 6). In accordance with this data, when we transferred naive OT-I T cells into IFN-γ−/− mice they were unable to protect these animals from a WT LM infection (Fig. 8 D). These results are also demonstrated in an independent experiment where we transferred naive or memory OT-I T cells into IFN-γ–deficient mice and challenged both sets of mice with 104 WT LM (Fig. S3, available at http://www.jem.org/cgi/content/full/jem.20031051/DC1).
Figure 8.
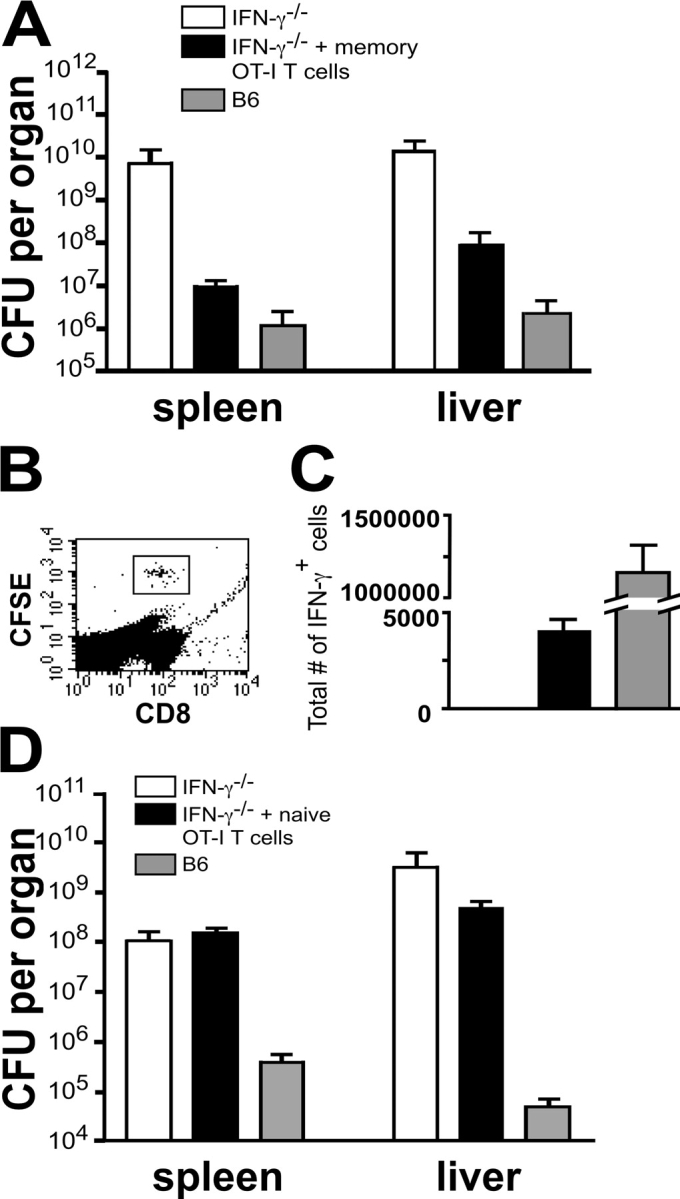
Memory OT-I T cells protect IFN-γ2/2 mice from a WT LM infection. 3 × 106 nylon wool-purified OT-I splenocytes were transferred into B6.Thy1.1 mice and then challenged with 2 × 106 PFU VV/OVA. The mice were then rested for >4 wk, killed, and the splenocytes were sorted for CD8+, Thy1.2+ OT-I T cells. 5 × 105 CFSE labeled memory OT-I T cells were injected into IFN-γ−/− recipients. (A) B6, IFN-γ−/−, and IFN-γ−/− mice transferred with memory OT-I T cells were then infected with ∼104 WT LM and killed 3 d later to determine spleen and liver LM counts. (B and C) Splenocytes from the above mice were cultured for 3 h in media containing GolgiPlug™ and then analyzed for CFSE, CD8, and intracellular IFN-γ expression. The number of IFN-γ–secreting OT-I T cells was calculated by multiplying the total number of splenocytes isolated from the infected mice by the percentage of CFSE-labeled, CD8+, IFN-γ+ cells determined by flow cytometry. The number of IFN-γ–secreting cells in the B6 mice was calculated by multiplying the total number of splenocytes isolated from the infected mice by the percentage of IFN-γ–secreting cells determined by flow cytometry. (D) Naive OT-I T cells were sorted for CD8+, Vα2+, and CD44lo expression. 5 × 105 CFSE-labeled naive OT-I T cells were injected into IFN-γ−/− recipients. B6, IFN-γ−/−, and IFN-γ−/− mice transferred with naive OT-I T cells were then infected with ∼104 WT LM and killed 3 d later to determine spleen and liver LM counts. The data shown are the average of three mice per group for A–C and two mice per group for D. Each experiment is one representative of two.
Discussion
CD8+ T cells play an important role in the control and elimination of LM and other intracellular pathogens. Signals propagated through the TCR upon binding to peptide–MHC complexes can induce a myriad of effects including proliferation, cytokine secretion, cytolysis of infected targets, and apoptosis of the T cells themselves (44). Generation of memory CD8+ T cells results in rapid responses upon reexposure to the same pathogen. In addition, memory CD8+ T cells have been shown to preferentially localize to peripheral nonlymphoid organs (41, 43). Recent reports in both human and murine systems have suggested that memory CD8+ T cells may belong to distinct subsets, termed central and effector, based upon their existence in either lymphoid or nonlymphoid organs (45, 46). Central memory CD8+ T cells are CD62Lhi and CCR7+ allowing homing to LNs, whereas effector memory CD8+ T cells are CD62Llo and CCR7− and do not have this homing property. Effector memory CD8+ T cells have been proposed to respond rapidly to a secondary infection by performing effector functions (46), although this has been questioned recently (45). Data presented here indicate that memory CD8+ T cells can respond rapidly in an antigen nonspecific manner by secreting IFN-γ (Figs. 2 and 5). We have isolated these memory CD8+ T cells from both lymphoid and nonlymphoid organs (Fig. 4). Therefore, they may belong to either subset of memory CD8+ T cells. Furthermore, we have shown previously that both CD62Lhi and CD62Llo CD8+ T cells have the capacity to secrete IFN-γ upon exposure to IL-12 and IL-18 (36). Importantly, the localization of memory CD8+ T cells to sites of infection such as the GI tract, lung, and liver make our findings relevant in terms of an immediate response against an infection.
IFN-γ is critical in immune responses against certain pathogens. For example, the published LD50 of IFN-γ–deficient Balb/c mice in response to LM is ∼10 (31). However, LM-specific CD8+ T cells generated in these mice can provide protective immunity to future LM infections. In addition, studies using SCID mice lacking T and B lymphocytes have demonstrated increased LM burdens upon depletion of IFN-γ (33). These results suggest that IFN-γ is required during the innate immune response to LM. Indeed, our data indicate that IFN-γ–deficient mice are highly susceptible to LM infections but that the differences in LM burden between B6 and IFN-γ−/− mice are not apparent until 2–3 d postinfection (Fig. 7). Although IFN-γ can have direct effects on LM growth by limiting escape from the phagosome (47), the major role of this cytokine may be in inducing macrophage activity or polarizing toward a Th1-type immune response (2). Exactly what role the IFN-γ that is secreted rapidly from antigen nonspecific memory CD8+ T cells plays was not determined in our studies. However, it is involved in controlling the early, rapid growth of LM that would otherwise occur in IFN-γ–deficient mice (Fig. 8).
Our experiments have used three different pathogens, all expressing the OVA protein, to induce effector and memory CD8+ T cells. In each case, the CD8+ T cells acquired the ability to secrete IFN-γ in response to IL-12 and IL-18 and up-regulated the expression of the IL-12 and IL-18 receptors (Fig. 6). Whether or not IL-12 and IL-18 production during the priming phase of CD8+ T cell activation is required for the increase in expression of the receptors for the cytokines is unclear. Signaling through the TCR upon binding to the cognate peptide–MHC complex may result in up-regulation of the IL-12 and IL-18 receptors in CD8+ T cells. It is likely that for each of the pathogens used the OVA antigen (SIINFEKL) is actually presented by DC through cross-priming (48). Recent work suggests that CD8+ DC are responsible for cross-priming CD8+ T cells and that this subset of DC produce high amounts of IL-12 (49, 50). DC of different types are also capable of secreting IL-18 (51). Therefore, it seems likely that IL-12 and IL-18 may be produced during the priming phase required to induce responsiveness of the CD8+ T cells to IL-12 and IL-18.
It is interesting to note that IL-12 and IL-18 did not induce any other effector functions, including proliferation, cytotoxicity, or secretion of TNF-α from effector or memory CD8+ T cells (unpublished data). Recent studies have demonstrated that IL-12 can have other effects on T cells, such as inducing the indirect proliferation of memory CD8+ T cells (52). IL-12 has also been shown to be necessary for complete activation of naive CD8+ T cells but not memory CD8+ T cells (53). IL-18 has been implicated in up-regulating the cytotoxic activities of CD8+ T cells (23). Our results indicate that the secretion of cytokine-induced IFN-γ by CD8+ T cells is under exquisite control. Once IL-12 and IL-18 are no longer produced, which should be rapid under conditions of a controllable infection, then IFN-γ will cease to be secreted from effector and memory CD8+ T cells.
The most striking result of this study is that so few memory CD8+ T cells can protect IFN-γ–deficient mice 3 d post LM infection (Fig. 8). Considering other cell types can respond rapidly to LM by secreting IFN-γ, there may be redundancy in this early response. Alternatively, the extra IFN-γ production resulting from multiple cell types may be required in order to establish a Th1 environment, aiding in the generation and effectiveness of the adaptive immune response. The ability of NK, NK-T, and γδ T cells to provide innate immune protection is a possibility that is being considered. In conclusion, the results presented here demonstrate that IFN-γ production from antigen nonspecific memory CD8+ T cells is sufficient in providing innate immune protection against LM. Furthermore, this study highlights the fact that CD8+ T cells can function in settings other than the adaptive immune response.
Acknowledgments
We would like to thank Angie Mobley in the Dallas Cell Analysis Facility for cell sorting and Charles Nguyen for assistance with the real-time RT-PCR.
This work was supported by National Institutes of Health grants AI37818 and AI45764 to J. Forman and a National Institutes of Health Postdoctoral Fellowship to R. Berg.
The online version of this paper contains supplemental material.
Abbreviations used in this paper: CFSE, carboxyfluorescein diacetate, succinimidyl ester; LM, Listeria monocytogenes; VSV, vesicular stomatitis virus; VV, vaccinia virus.
References
- 1.Portnoy, D.A. 1992. Innate immunity to a facultative intracellular bacterial pathogen. Curr. Opin. Immunol. 4:20–24. [DOI] [PubMed] [Google Scholar]
- 2.Unanue, E.R. 1997. Studies in listeriosis show the strong symbiosis between the innate cellular system and the T-cell response. Immunol. Rev. 158:11–25. [DOI] [PubMed] [Google Scholar]
- 3.Mombaerts, P., J. Arnoldi, F. Russ, S. Tonegawa, and S.H. Kaufmann. 1993. Different roles of alpha beta and gamma delta T cells in immunity against an intracellular bacterial pathogen. Nature. 365:53–56. [DOI] [PubMed] [Google Scholar]
- 4.MacMicking, J.D., C. Nathan, G. Hom, N. Chartrain, D.S. Fletcher, M. Trumbauer, K. Stevens, Q.W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial-infection and endotoxic-shock in mice lacking inducible nitric-oxide synthase. Cell. 81:641–650. [DOI] [PubMed] [Google Scholar]
- 5.Beckerman, K.P., H.W. Rogers, J.A. Corbett, R.D. Schreiber, M.L. McDaniel, and E.R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888–895. [PubMed] [Google Scholar]
- 6.Dinauer, M.C., M.B. Deck, and E.R. Unanue. 1997. Mice lacking reduced nicotinamide adenine dinucleotide phosphate oxidase activity show increased susceptibility to early infection with Listeria monocytogenes. J. Immunol. 158:5581–5583. [PubMed] [Google Scholar]
- 7.Endres, R., A. Luz, H. Schulze, H. Neubauer, A. Futterer, S.M. Holland, H. Wagner, and K. Pfeffer. 1997. Listeriosis in p47(phox−/−) and TRp55−/− mice: protection despite absence of ROI and susceptibility despite presence of RNI. Immunity. 7:419–432. [DOI] [PubMed] [Google Scholar]
- 8.Shiloh, M.U., J.D. MacMicking, S. Nicholson, J.E. Brause, S. Potter, M. Marino, F. Fang, M. Dinauer, and C. Nathan. 1999. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 10:29–38. [DOI] [PubMed] [Google Scholar]
- 9.Fehr, T., G. Schoedon, B. Odermatt, T. Holtschke, M. Schneemann, M.F. Bachmann, T.W. Mak, I. Horak, and R.M. Zinkernagel. 1997. Crucial role of interferon consensus sequence binding protein, but neither of interferon regulatory factor 1 nor of nitric oxide synthesis for protection against murine listeriosis. J. Exp. Med. 185:921–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen, H., S. Gordon, and R.J. North. 1989. Exacerbation of murine listeriosis by a monoclonal antibody specific for the type-3 complement receptor of myelomonocytic cells—absence of monocytes at infective foci allows Listeria to multiply in nonphagocytic cells. J. Exp. Med. 170:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlan, J.W., and R.J. North. 1994. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J. Exp. Med. 179:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Havell, E.A., L.L. Moldawer, D. Helfgott, P.L. Kilian, and P.B. Sehgal. 1992. Type-I Il-1 receptor blockade exacerbates murine listeriosis. J. Immunol. 148:1486–1492. [PubMed] [Google Scholar]
- 13.Rogers, H.W., C.S. Tripp, R.D. Schreiber, and E.R. Unanue. 1994. Endogenous Il-1 Is Required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093–2101. [PubMed] [Google Scholar]
- 14.Havell, E.A., and P.B. Sehgal. 1991. Tumor necrosis factor-independent Il-6 production during murine listeriosis. J. Immunol. 146:756–761. [PubMed] [Google Scholar]
- 15.Kopf, M., H. Baumann, G. Freer, M. Freudenberg, M. Lamers, T. Kishimoto, R. Zinkernagel, H. Bluethmann, and G. Kohler. 1994. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 368:339–342. [DOI] [PubMed] [Google Scholar]
- 16.Dalrymple, S.A., L.A. Lucian, R. Slattery, T. Mcneil, D.M. Aud, S. Fuchino, F. Lee, and R. Murray. 1995. Interleukin-6-deficient mice are highly susceptible to Listeria-monocytogenes infection—correlation with inefficient neutrophilia. Infect. Immun. 63:2262–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehlers, S., C. Holscher, S. Scheu, C. Tertilt, T. Hehlgans, J. Suwinski, R. Endres, and K. Pfeffer. 2003. The lymphotoxin beta receptor is critically involved in controlling infections with the intracellular pathogens Mycobacterium tuberculosis and Listeria monocytogenes. J. Immunol. 170:5210–5218. [DOI] [PubMed] [Google Scholar]
- 18.Pasparakis, M., L. Alexopoulou, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 364:798–802. [DOI] [PubMed] [Google Scholar]
- 20.Tripp, C.S., S.F. Wolf, and E.R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA. 90:3725–3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tripp, C.S., M.K. Gately, J. Hakimi, P. Ling, and E.R. Unanue. 1994. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J. Immunol. 152:1883–1887. [PubMed] [Google Scholar]
- 22.Okamura, H., H. Tsutsi, T. Komatsu, M. Yutsudo, A. Hakura, T. Tanimoto, K. Torigoe, T. Okura, Y. Nukada, and K. Hattori. 1995. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 378:88–91. [DOI] [PubMed] [Google Scholar]
- 23.Nakanishi, K., T. Yoshimoto, H. Tsutsui, and H. Okamura. 2001. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 12:53–72. [DOI] [PubMed] [Google Scholar]
- 24.Neighbors, M., X. Xu, F.J. Barrat, S.R. Ruuls, T. Churakova, R. Debets, J.F. Bazan, R.A. Kastelein, J.S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bancroft, G.J. 1993. The role of natural killer cells in innate resistance to infection. Curr. Opin. Immunol. 5:503–510. [DOI] [PubMed] [Google Scholar]
- 26.Leite-De-Moraes, M.C., A. Hameg, A. Arnould, F. Machavoine, Y. Koezuka, E. Schneider, A. Herbelin, and M. Dy. 1999. A distinct IL-18-induced pathway to fully activate NK T lymphocytes independently from TCR engagement. J. Immunol. 163:5871–5876. [PubMed] [Google Scholar]
- 27.Munder, M., M. Mallo, K. Eichmann, and M. Modolell. 1998. Murine macrophages secrete interferon gamma upon combined stimulation with interleukin (IL)-12 and IL-18: a novel pathway of autocrine macrophage activation. J. Exp. Med. 187:2103–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganapamo, F., V.A. Dennis, and M.T. Philipp. 2001. CD19(+) cells produce IFN-gamma in mice infected with Borrelia burgdorferi. Eur. J. Immunol. 31:3460–3468. [DOI] [PubMed] [Google Scholar]
- 29.Stober, D., R. Schirmbeck, and J. Reimann. 2001. IL-12/IL-18-dependent IFN-gamma release by murine dendritic cells. J. Immunol. 167:957–965. [DOI] [PubMed] [Google Scholar]
- 30.Ferrick, D.A., M.D. Schrenzel, T. Mulvania, B. Hsieh, W.G. Ferlin, and H. Lepper. 1995. Differential production of interferon-gamma and interleukin-4 in response to Th1-stimulating and Th2-stimulating pathogens by gamma-delta T-cells in-vivo. Nature. 373:255–257. [DOI] [PubMed] [Google Scholar]
- 31.Harty, J.T., and M.J. Bevan. 1995. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 3:109–117. [DOI] [PubMed] [Google Scholar]
- 32.Huang, S., W. Hendriks, A. Althage, S. Hemmi, H. Bluethmann, R. Kamijo, J. Vilcek, R.M. Zinkernagel, and M. Aguet. 1993. Immune response in mice that lack the interferon-gamma receptor. Science. 259:1742–1745. [DOI] [PubMed] [Google Scholar]
- 33.Bancroft, G.J., R.D. Schreiber, G.C. Bosma, M.J. Bosma, and E.R. Unanue. 1987. A T cell-independent mechanism of macrophage activation by interferon-gamma. J. Immunol. 139:1104–1107. [PubMed] [Google Scholar]
- 34.Dai, W.J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158:5297–5304. [PubMed] [Google Scholar]
- 35.Teixeira, H.C., and S.H.E. Kaufmann. 1994. Role of Nk1.1(+) cells in experimental listeriosis—Nk1(+) cells are early Ifn-gamma producers but impair resistance to Listeria-monocytogenes infection. J. Immunol. 152:1873–1882. [PubMed] [Google Scholar]
- 36.Berg, R.E., C.J. Cordes, and J. Forman. 2002. Contribution of CD8+ T cells to innate immunity: IFN-gamma secretion induced by IL-12 and IL-18. Eur. J. Immunol. 32:2807–2816. [DOI] [PubMed] [Google Scholar]
- 37.Lertmemongkolchai, G., G. Cai, C.A. Hunter, and G.J. Bancroft. 2001. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J. Immunol. 166:1097–1105. [DOI] [PubMed] [Google Scholar]
- 38.Pope, C., S.K. Kim, A. Marzo, D. Masopust, K. Williams, J. Jiang, H. Shen, and L. Lefrancois. 2001. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166:3402–3409. [DOI] [PubMed] [Google Scholar]
- 39.Kim, S.K., D.S. Reed, S. Olson, M.J. Schnell, J.K. Rose, P.A. Morton, and L. Lefrancois. 1998. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA. 95:10814–10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Restifo, N.P., I. Bacik, K.R. Irvine, J.W. Yewdell, B.J. McCabe, R.W. Anderson, L.C. Eisenlohr, S.A. Rosenberg, and J.R. Bennink. 1995. Antigen processing in vivo and the elicitation of primary CTL responses. J. Immunol. 154:4414–4422. [PMC free article] [PubMed] [Google Scholar]
- 41.Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 42.Livak, K.J., and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 25:402–408. [DOI] [PubMed] [Google Scholar]
- 43.Marshall, D.R., S.J. Turner, G.T. Belz, S. Wingo, S. Andreansky, M.Y. Sangster, J.M. Riberdy, T.B. Liu, M. Tan, and P.C. Doherty. 2001. Measuring the diaspora for virus-specific CD8(+) T cells. Proc. Natl. Acad. Sci. USA. 98:6313–6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harty, J.T., A.R. Tvinnereim, and D.W. White. 2000. CD8(+) T cell effector mechanisms in resistance to infection. Annu. Rev. Immunol. 18:275–308. [DOI] [PubMed] [Google Scholar]
- 45.Wherry, E.J., V. Teichgraber, T.C. Becker, D. Masopust, S.M. Kaech, R. Antia, U.H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 4:225–234. [DOI] [PubMed] [Google Scholar]
- 46.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 47.Portnoy, D.A., R.D. Schreiber, P. Connelly, and L.G. Tilney. 1989. Gamma-interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heath, W.R., and F.R. Carbone. 2001. Cross-presentation, dendrttic cells, tolerance and immunity. Annu. Rev. Immunol. 19:47–64. [DOI] [PubMed] [Google Scholar]
- 49.den Haan, J.M.M., S.M. Lehar, and M.J. Bevan. 2000. CD8(+) but not CD8(−) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192:1685–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maldonado-Lopez, R., T. De Smedt, P. Michel, J. Godfroid, B. Pajak, C. Heirman, K. Thielemans, O. Leo, J. Urbain, and M. Moser. 1999. CD8 alpha(+) and CD8 alpha(−) subclasses of dendritic cells direct the development of distinct T helper cells in vivo. J. Exp. Med. 189:587–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stoll, S., H. Jonuleit, E. Schmitt, G. Muller, H. Yamauchi, M. Kurimoto, J. Knop, and A.H. Enk. 1998. Production of functional IL-18 by different subtypes of murine and human dendritic cells (DC): DC-derived IL-18 enhances IL-12-dependent Th1 development. Eur. J. Immunol. 28:3231–3239. [DOI] [PubMed] [Google Scholar]
- 52.Tough, D.F., X. Zhang, and J. Sprent. 2001. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J. Immunol. 166:6007–6011. [DOI] [PubMed] [Google Scholar]
- 53.Curtsinger, J.M., D.C. Lins, and M.F. Mescher. 2003. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J. Exp. Med. 197:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]